Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Rita Xu and Version 1 by Anna Barra Caracciolo.
Hydrocarbons occur in fossil fuels such as crude oil and consist mainly of hydrogen and carbon. They are natural chemicals, crude oil refining results in commercial products with new physico-chemical properties, which can increase their complexity and toxicity, and hamper their degradation. The presence of biodiverse natural microbial communities is a prerequisite for an effective homeostatic response to the various hydrocarbons, that contaminate ecosystems.
- microbial communities
- regulation ecosystem services
- aliphatic hydrocarbons
- polycyclic aromatics hydrocarbons
1. Introduction
Economic activities are strongly dependent on the use of fossil fuels, which consist of hydrocarbon-containing material formed naturally in the Earth’s crust from dead plant and animal residues. Hydrocarbons provide energy for civil and industrial purposes, and for transport [1]. Owing to the growth of the human population, the worldwide demand for hydrocarbons is increasing, and petrochemical industries, accidental oil spills, disconnection between oil wells and combustion processes (e.g., industry emissions), abandoned refining sites, and vehicle combustion have been constantly polluting air, water, and soil [2,3,4][2][3][4]. The risk of accidental leaks in ecosystems has increased exponentially with the growing global demand for oil and it has been estimated that every year, ca. 1.3 million liters of oil reach natural environments [5]. Hydrocarbons are the organic contaminants most commonly found in ecosystems [1,6][1][6], and it is fundamental to evaluate their environmental fate and effects in different matrices.
Hydrocarbons are chemicals naturally occurring in crude oil and consisting mainly of hydrogen and carbon. Crude oil is a dark, viscous, and easily flammable complex liquid mixture of hydrocarbons (83–87%), comprising variable amounts of hydrogen (10–14%), oxygen (0.05–1.5%), sulfur (0.005–6.0%), nitrogen (0.1–0.2%), and metals (<1000 mg/L) such as nickel, iron, and copper. The specific composition depends on the oil field’s geological age, location, and depth. After crude oil refining, the resulting products have new physico-chemical properties, which increase their complexity and can hamper their biodegradation [7].
Microorganisms play key roles in natural ecosystem functioning, such as primary production, organic matter decomposition, nutrient cycling, and biodegradation of contaminants, including hydrocarbons, thus contributing in different ways to soil and water purification processes. The maintenance of these regulating ecosystem services is linked to bacterial diversity and metabolic versatility, which makes it potentially possible to biodegrade a huge variety of aliphatic and aromatic hydrocarbons.
2. Aliphatic Hydrocarbons and Polycyclic Aromatic Hydrocarbons
Hydrocarbons can be divided in accordance with their chemical structure into: aliphatic hydrocarbons or saturated hydrocarbons, aromatic hydrocarbons (monocyclic aromatic and polycyclic aromatic hydrocarbons); and heteroatomic compounds (saturated and aromatic ones), including resins and asphaltenes.
Aliphatic hydrocarbons (AH) (Figure 1A), also known as paraffins, are mainly present in deposits of natural gas and oil formed by plant and animal decomposition. AH have no double bonds (general formula CnH2n) and represent the highest percentage of the constituents of crude oil. The lack of functional groups makes them strongly apolar, so they have low water solubility and are poorly reactive at room temperature [8]. In accordance with their structure, they are classified as alkanes (which have a linear structure) and cycloalkanes (which have a condensed ring structure); they can be linear or branched [9].
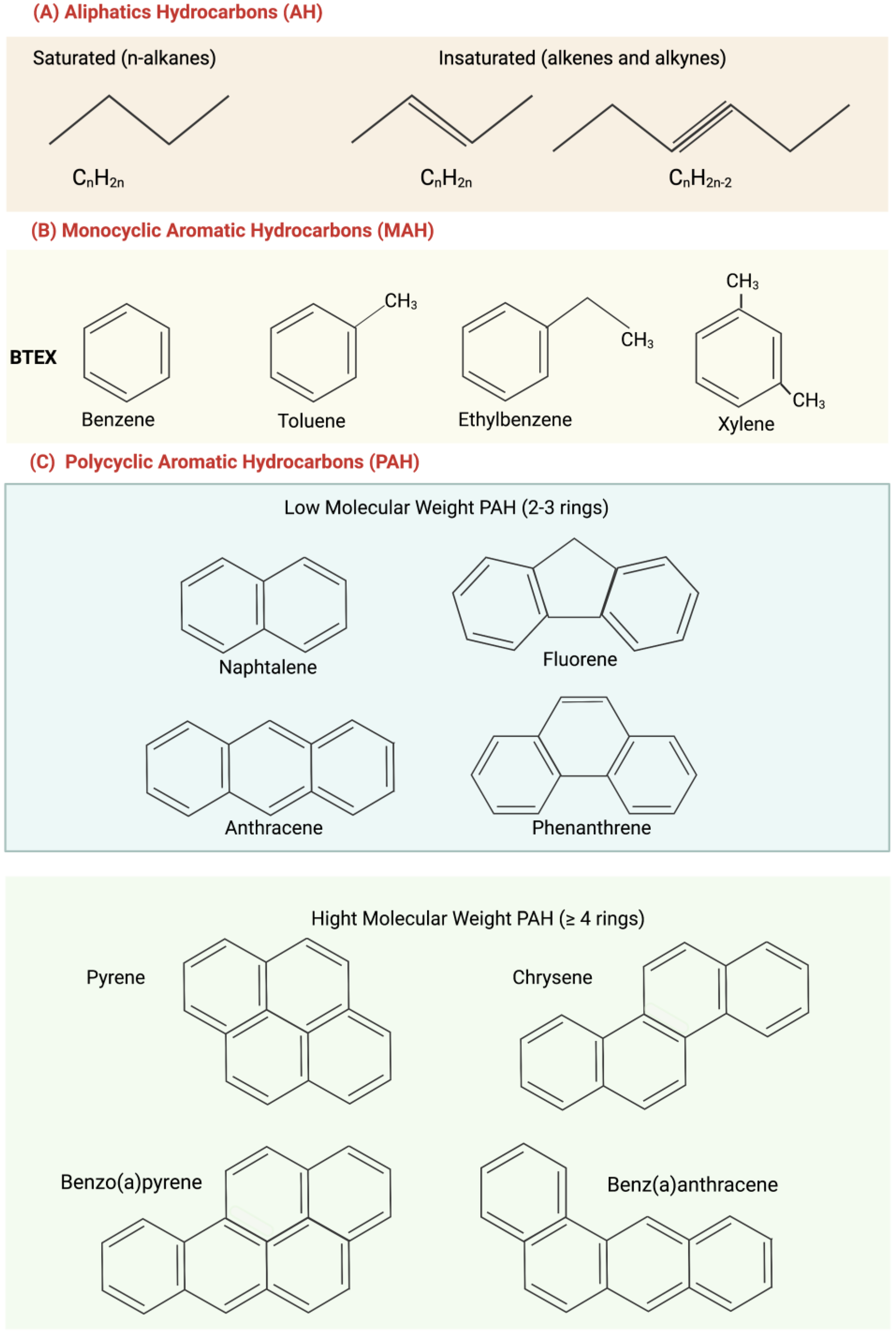
Figure 1. Structure of main hydrocarbons: (A) Aliphatic hydrocarbons (AH); (B) monocyclic aromatic hydrocarbons (MAH); (C) polycyclic aromatic hydrocarbons (PAH), subdivided into low molecular weight (in blue) and high molecular weight (in green).
Aromatic hydrocarbons have one or more benzene rings and are generally replaced with different aliphatic hydrocarbons. They are mainly divided into monocyclic aromatic hydrocarbons (MAH), such as benzene, toluene, ethylbenzene, and xylene, which together constitute BTEX (widely studied and making up 2–20% of oil) (Figure 1B) and polycyclic aromatic hydrocarbons (PAH), (Figure 1C).
PAHs are a group of lipophilic organic pollutants that derive from biological processes or are formed as products of incomplete combustion from natural (forest fires and shrubs) or anthropic (vehicle emissions, domestic heating, and cigarette smoke) sources. Owing to their ubiquitous presence and persistence in air, water, and soil, as well as their toxicity for both humans and biota, they are compounds of environmental concern [10,11][10][11].
PAHs with 2–3 benzene rings, such as naphthalene, fluorene, anthracene, and phenanthrene, are low-molecular-weight ones. PAHs with four or more benzene rings, such as pyrene, chrysene, benzo(a)pyrene, and benz(a)anthracene, are high molecular weight ones (Figure 1C). Low-molecular-weight PAHs are gases and tend to escape into the atmosphere, while those with a higher molecular weight are liquids or in a solid state at room temperature and often have a separate phase in water [8]. PAH solubility in aqueous solution decreases as the number of benzene rings increases, and this increases their environmental persistence. A high persistence increases the possibility of a compound making its toxicity felt.
It has recently been recognized that life quality is connected to that of the environment, as expressed by the new concept of ‘One Health’ proposed by the World Health Organization [12]. The One Health approach means that human health is connected to that of animals and the environment. For this reason, the ubiquitous occurrence (water, air, soil, and sediment) of hydrocarbons is a serious threat to both human and environmental health [13].
3. Environmental Fate and Toxic Effects
Hydrocarbons interact with both the abiotic and biotic components of ecosystems. Hydrocarbons can be divided into specific fractions, equivalent to the number of carbon atoms. These fractions can also be described for their physical, chemical, and toxicological characteristics. Fraction 1 (F1) contains hydrocarbons with a carbon number between 6 and 10 (C6–C10) and is classified as volatiles. Fraction 2 (F2) represents the semi-volatile hydrocarbons with at least 10 carbon atoms (C10–C16). Fraction 3 (F3) includes hydrocarbons with a carbon number between 16 and 34 (C16–C34) and are considered non-volatile. Fraction 4 (F4) refers to the hydrocarbon class with the lowest volatility and solubility, and more than 35 carbon atoms (>C35). Toxicity generally increases with the molecular weight [7,9][7][9]. For example, polycyclic aromatic hydrocarbons with a high molecular weight and boiling point have higher toxicities [7].
Due to their complex characteristics, the lightest and most volatile hydrocarbon fractions are released into the atmosphere, the amphipathic and hydrophilic fractions dissolve in water, and the lipophilic ones tend to bind to soil/sediment particles and organic matter [7].
The toxicity of a hydrocarbon also depends on its bioavailability, which in turn is influenced by its physical and chemical characteristics. Once a bioavailable chemical is absorbed by an organism, it interacts with cellular receptor sites and can cause lethal or sub-lethal effects. The effects depend on the concentration and duration (short or long) of exposure [7]. Lethality occurs from short-term exposure due to acute oil spills in aquatic environments in which hydrophobic compounds destroy the central nervous systems of organisms through their location in neuron cell membranes [7].
Sub-lethal toxic effects due to hydrocarbon exposure can cause lesions (internal or external), defects in early development, anoxia, and changes in molecular and behavioral functions related to nutrition and reproduction. Exposure to petroleum hydrocarbon products can cause acute effects such as changes in the nervous, ocular, and respiratory systems. Along with migraines and headaches, people living in oil-polluted areas also experience nausea, upper respiratory tract infections, and nose and eye irritations [5].
PAHs can potentially not only affect the nervous, immune, and excretory systems but also cause tumors and mutations [7,9][7][9]. Chronic exposure to PAHs can cause cancer in humans, and the cataractogenic properties of PAHs (i.e., alterations at the dermal and ocular levels) have been well documented [14]. Exposure to PAHs can occur through inhalation, ingestion (through contaminated food or drink), or dermally (direct contact). Once introduced into the body, these molecules rapidly diffuse due to their high fat solubility, which makes them able to cross cell membranes and deposit themselves in adipose tissue, the kidneys, and the liver. Exposure to these substances causes blood damage, and probable immunosuppression. Depending on the route of exposure, PAHs (particularly those characterized by 3–7 aromatic rings) cause lung, digestive, or skin cancers. The carcinogenic potential derives from the metabolism of these substances in the liver, which leads to a biotransformation into reactive intermediates capable of binding to DNA and RNA with consequent damage to genetic information. It has been empirically demonstrated that PAHs interact metabolically, resulting in synergistic and additive effects [15].
The toxicity of hydrocarbons affects humans, plants, animals, and microorganisms, with consequences for ecosystem biodiversity and functioning. Environmental contamination by hydrocarbons and derivatives has caused the local extinction of many species of plants and animals [5].
Hydrocarbons are freshwater, wastewater, and seawater contaminants. Because they contain a large number of hydrophobic components, which are adsorbed onto particulates and sediments in aquatic ecosystems, they can become bioavailable for benthic species. In fact, invertebrates, fish, eggs laid by fish, and filter feeders such as bivalves are in contact with these contaminants by filtration of suspended petroleum [7].
Hydrocarbons can hamper water and oxygen transfer through porous spaces in sediments and soil, influencing their permeability, moisture content, pH, nutrient availability (e.g., nitrate, phosphate, and sodium), and redox condition [3]. This is the case of higher molecular weight PAHs, which thanks to their low desorption from soil and low water solubility can form a surface layer that can prevent both bioaccessibility and vegetation development for several decades [16]. Damaging effects also occur on plant species directly exposed to hydrocarbons because hydrocarbons prevent light access and make plants unable to acquire nutrients and water, reducing primary and agricultural productivity [5,17][5][17].
Hydrocarbons can also be released into deeper waters from soil by percolation or atmospheric subsidence, bringing them into contact with underground ecosystems [18].
Hydrocarbons can also eliminate or inhibit microbial species, altering their corresponding ecosystem functions [17]. The high selective pressure exerted by hydrocarbons can reduce their diversity, producing rapid shifts in microbial populations [19]. The elimination or alteration of microbial species with key roles in biogeochemical cycles and primary production can also affect higher trophic levels of the food chain due to biomagnification [17]. Bacteria in soil, e.g., nitrifying bacteria, are susceptible to hydrocarbon exposure, which inhibits their enzyme ammonium monooxygenase, through competitive binding with low-weight hydrocarbons [7], with consequences for soil fertility.
Hydrocarbons act as a selective force, influencing the structure and functioning of natural microbial communities. Some microbial species are negatively affected by these toxic compounds [7]. However, others can respond to their presence with various mechanisms and remove them. Hydrocarbon biodegradation is possible if a high microbial metabolic diversity is present in a contaminated environment and the quantity of hydrocarbons is not so great as to prevent or inhibit the activity of the microbes [17].
4. Microbial Degradation of Hydrocarbons
Methane, the simplest hydrocarbon, can be degraded by a highly specialized group of bacteria, the methanotrophic one, which uses it as a carbon and energy source. However, these bacteria are unable to grow on hydrocarbons containing more carbon atoms [21][20]. The complex high-molecular-weight pyrene is highly hydrophobic, has low water solubility, and tends to sorb to soil organic carbon; these characteristics give it low bioavailability and make it recalcitrant to biodegradation [22][21]. Pyrene degradation was reported first in 1988, involving a Mycobacterium strain capable of mineralizing and using it as its sole carbon and energy source [23][22]. Subsequently, several pyrene degraders have also been identified, belonging to the genera Sphingomonas, Mycobacterium, Rhodococcus, Bacillus, Burkholderia, Cycloclasticus, Pseudomonas, and Stenotrophomonas, with degradation percentages in soil ranging from 32% to 96% with variable times (4–42 days) [22,24][21][23]. If large quantities of oil (hydrocarbon mixture) are released into the environment, the volatile hydrocarbon fraction evaporates rapidly, and longer aliphatic chains and aromatic compounds remain. Because oil is insoluble and less dense than water, it floats on the surface and forms blotches. Hydrocarbon-oxidizing bacteria need to adhere to insoluble petroleum droplets, on which a large number of cells can be observed. The activities of these organisms lead to the degradation of oil and the breakdown of oily patches [25][24]. Numerous studies have shown that several bacterial communities in contact with hydrocarbons rapidly shift to bacterial species able to degrade and utilize hydrocarbon compounds as sources of carbon and energy (hydrocarbonoclastic bacteria) [19]. Hydrocarbonoclastic bacteria have evolved adaptive mechanisms to tolerate the presence of hydrocarbons, such as the ability to emulsify and metabolize them, activation of DNA repairing mechanisms, production of the molecules involved in the mechanisms of quorum sensing and biofilm, and regulation of efflux pumps and pores to control the concentration of hydrocarbons inside a cell [19]. Biodegradation of a hydrocarbon involves adhesion to the substrate and production of compounds such as biosurfactants/bioemulsionants, biopolymers, solvents, gases, and acids for making them bioavailable [9]. In particular, biosurfactants are high- or low-molecular-weight amphiphilic molecules that can be synthesized by numerous microorganisms. In prokaryotes, the ability to produce these compounds is often matched with the ability to grow on insoluble substrates such as hydrocarbons; the production and function of these compounds are in fact closely related to the mechanisms of absorption (uptake) of such substances inside bacterial cells. These molecules often have the property of detoxifying the substrate [8]. Posada-Baquero et al. [26][25] showed that the sunflower rhizosphere, in the presence of a biosurfactant (rhamnolipid), favored the desorption of PAHs in a creosote-contaminated soil. Moreover, the enhancement of slow desorption of PAHs resulted in faster biodegradation in a slurry biodegradation experiment. Chebbi et al. (2017) [27][26] isolated a Pseudomonas strain (W10) from a diesel-contaminated soil through cultures enriched with phenanthrene. This strain showed potential growth in the presence of a broad group of hydrocarbons, including aliphatic, monocyclic aromatic, and polycyclic aromatic hydrocarbons. This was due to the strain’s ability to synthesize a biosurfactant, which made hydrocarbons readily available for their degradation. In another study [28][27], Pseudomonas aeruginosa was able to degrade n-alkanes (C16 and C19) and polycyclic aromatic hydrocarbons (PAHs) such as fluorene, phenanthrene, and pyrene. Other authors [29][28] isolated Enterobacter cloacae strains with a high capacity to emulsify hydrocarbons, lower surface tension, and increase the rate of degradation of these contaminants. The genus Delftia has also been identified for hydrocarbon degradation. For example, Delftia sp. NL1, isolated from an oil field in Algeria, degraded more than 66.76% of diesel in 7 days, producing glycolipids that acted as biosurfactants and improving the accessibility and bioavailability of the insoluble hydrocarbon fraction [30][29]. Another biosurfactant producer, the Achromobacter (AC15) bacterium, was isolated and was able to use high concentrations of pyrene as its sole carbon and energy source. It degraded (in 14 days) about 40% of the pyrene supplied at an initial concentration of 300 mg/L because it produced bioemulsionants able to reduce the culture medium surface tension (from 67.2 to 33.2 mN/m) [31][30]. Bacteria belonging to the genus Acinetobacter also have a key role in PAH biodegradation, as reported in a recent study where the increase in their relative abundance was demonstrated in the presence of diesel, heavy metals, and PAHs [32][31]. The Acinetobacter genus is generally known for its efficiency in metabolizing various hydrocarbons such as monoaromatic compounds [33][32], and polyaromatic compounds such as naphthalene [34][33], acenaphthene, and acenaphthylene [11,35][11][34]. In some bacteria, a hydrocarbon presence can modulate their chemotaxis [7]. Chemotaxis is a behavioral response where bacteria perceive variations in the concentration of a specific chemical using chemoreceptors and respond to them by changing their position. Bacteria able to degrade hydrocarbons can control their position and migrate towards contaminated points [7,14][7][14]. For example, the motile and chemotactic bacterium Pseudomonas putida G7 has recently been found to sorb and cometabolize pyrene in a column experiment, mobilizing and making it bioavailable for biodegradation [36][35]. The Stenotrophomonas genus is known to be able to use hydrocarbons as the sole carbon and energy source. Elufisan et al. [37][36] isolated Stenotrophomonas sp. Pemsol from a soil contaminated with crude hydrocarbons and showed its capability to grow in the presence of five different PAHs (biphenyl, anthraquinone, phenanthrene, naphthalene, and phenanthridine) as the sole source of carbon and energy. The complete genome of this sequenced strain revealed the presence of 145 genes involved in PAH degradation. The same authors also reported that some genes associated with the catalytic metabolism of hydrocarbons can be acquired by other bacteria by horizontal gene transfer. In addition to Proteobacteria, another phylum including several hydrocarbon-degrading strains is Actinobacteria [19]. The strain Rhodococcus sp. P14 has been reported to be able to degrade high molecular weight PAHs (3, 4, or 5 aromatic ring molecules such as phenanthrene, pyrene, and benzo(a)pyrene), as well as aliphatic hydrocarbons. The overall fatty acid composition of the cell membrane of Rhodococcus sp. P14 was altered when the strain was grown in enrichment cultures with different types of hydrocarbons, leading to a general decrease in short-chain fatty acids and an increase in branched-chain fatty acids. This happens to make the surface of the bacterial cells more hydrophobic in order to facilitate non-water-soluble hydrocarbon use. It has also been observed that this bacterial strain is able to form a biofilm between the surface of an aqueous solution and that of insoluble hydrocarbons [38][37]. In another study, the degrading potential of the genus Rhodococcus was shown. Indeed, Rhodococcus sp. WAY2 was found to be specialized in the metabolism of short-chain alkanes [39][38]. Although microbial metabolism has the potential to naturally attenuate contaminated environments and biostimulate hydrocarbons (e.g., using surfactants, plants, or nanoparticles) or bioaugment them, it is more effective in their removal. For example, bioremediation of a petroleum hydrocarbon-contaminated soil collected from an abandoned plant in China was performed successfully in batch experiments combining an isolated indigenous bacterial consortium (Enterococcus, Vagococcus, and Sphingomonas) and the sophorolipid biosurfactant. The surfactant enhanced bioremediation with increasing concentrations of sophorolipid because it improved hydrocarbon bioaccessibility for microorganisms [51][39]. Bioremediation of hydrocarbons can also be significantly enhanced by using plants (bioassisted phytoremediation). Vasilyeva et al. [52][40] showed significant reductions in total petroleum hydrocarbon content in a contaminated crude oil soil (5–15% w/w) using a mixed adsorbent (ACD), composed of granular activated carbon and diatomite, in combination with a biopreparation (BP) consisting of hydrocarbon-degrading bacteria (Pseudomonas putida B-2187 and Rhodococcus erytropolis Ac-859). The ACD mixture also reduced the wash-out of polar petroleum metabolites (oxidized hydrocarbons) and the phytotoxicity of the lysimetric waters, especially in highly contaminated soils.References
- Giri, K.; Rai, J.P.N. Bacterial Metabolism of Petroleum Hydrocarbons. Biotechnology 2014, 11, 73–93.
- Giraldez, P.; Aboal, J.R.; Fernandez, A.; Di Guardo, A.; Terzaghi, E. Plant-air partition coefficients for thirteen urban conifer tree species: Estimating the best gas and particulate matter associated PAH removers. Environ. Pollut. 2022, 315, 120409.
- Devatha, C.P.; Vishnu Vishal, A.; Purna Chandra Rao, J. Investigation of physical and chemical characteristics on soil due to crude oil contamination and its remediation. Appl. Water Sci. 2019, 9, 1–10.
- Wang, M.; Garrido-Sanz, D.; Sansegundo-Lobato, P.; Redondo-Nieto, M.; Conlon, R.; Martin, M.; Mali, R.; Liu, X.; Dowling, D.N.; Rivilla, R.; et al. Soil Microbiome Structure and Function in Ecopiles Used to Remediate Petroleum-Contaminated Soil. Front. Environ. Sci. 2021, 9, 39.
- Nagkirti, P.; Shaikh, A.; Vasudevan, G.; Paliwal, V.; Dhakephalkar, P. Bioremediation of terrestrial oil spills. Feasibility Assessment. In Optimization and Applicability of Bioprocess; Springer: Singapore, 2017; pp. 141–173.
- Abdullah, S.R.S.; Al-Baldawi, I.A.; Almansoory, A.F.; Purwanti, I.F.; Al-Sbani, N.H.; Sharuddin, S.S.N. Plant-assisted remediation of hydrocarbons in water and soil: Application, mechanisms, challenges and opportunities. Chemosphere 2020, 247, 125932.
- Logeshwaran, P.; Megharaj, M.; Chadalavada, S.; Bowman, M.; Naidu, R. Petroleum hydrocarbons (PH) in groundwater aquifers; An overview of environmental fate, toxicity, microbial degradation and risk-based remediation approaches. Environ. Technol. Innov. 2018, 10, 175–193.
- Barbieri, P.; Bestetti, G.; Galli, E.; Zannoni, D. Microbiologia Ambientale ed Elementi di Biologia Microbica; Ambrosiana: Milano, Italy, 2012; pp. 218–388.
- Varjani, S.J. Microbial degradation of petroleum hydrocarbons. Bioresour. Technol. 2017, 223, 277–286.
- Huang, L.; Batterman, S.A. Multimedia model for polycyclic aromatic hydrocarbons (PAHs) and nitro-PAHs in Lake Michigan. Environ. Sci. Technol. 2014, 48, 13817–13825.
- Ghosal, D.; Ghosh, S.; Dutta, T.K.; Ahn, Y. Current State of Knowledge in Microbial Degradation of Polycyclic Aromatic Hydrocarbons (PAHs): A Review. Front. Microbiol. 2016, 7, 1369.
- Lebov, J.; Grieger, K.; Womack, D.; Zaccaro, D.; Whitehead, N.; Kowalcyk, B.; MacDonald, P.D.M. A framework for One Health research. One Health 2017, 3, 44–50.
- Nzila, A. Biodegradation of high-molecular-weight polycyclic aromatic hydrocarbons under anaerobic conditions: Overview of studies, proposed pathways and future perspectives. Environ. Pollut. 2018, 239, 788–802.
- Sudip, K.S.; Om, V.S.; Rakesh, K.J. Polycyclic aromatic hydrocarbons: Environmental pollution and bioremediation. Trends Biotechnol. 2002, 20, 243–248.
- Boström, C.-E.; Gerde, P.; Hanberg, A.; Jernström, B.; Johansson, C.; Kyrklund, T.; Rannug, A.; Törnqvist, M.; Victorin, K.; Westerholm, R.; et al. Cancer risk assessment, indicators, and guidelines for polycyclic aromatic hydrocarbons in the ambient air. Environ. Health Perspect. 2002, 110, 451–488.
- Kuppusamy, S.; Maddela, N.R.; Megharaj, M.; Venkateswarlu, K. Total Petroleum Hydrocarbons: Environmental Fate, Toxicity and Remediation; Springer: Cham, Switzerland, 2020.
- Truskewycz, A.; Gundry, T.D.; Khudur, L.S.; Kolobaric, A.; Taha, M.; Alburto-Medina, A.; Ball, A.S.; Shahsavari, E. Petroleum Hydrocarbons Contamination in Terrestrial Ecosystem- Fate and Microbial Responses. Molecules 2019, 24, 3400.
- Qiao, X.; Zheng, B.; Li, X.; Zhao, X.; Dionysiou, D.D.; Liu, Y. Influencing factors and health risk assessment of polycyclic aromatic hydrocarbons in groundwater in China. J. Hazard. Mater. 2021, 402, 123419.
- Ruiz, O.N.; Radwan, O.; Striebich, R.C. GC–MS hydrocarbon degradation profile data of Pseudomonas frederiksbergensis SI8, a bacterium capable of degrading aromatics at low temperatures. Data Brief 2021, 35, 106864.
- Dedysh, S.N.; Dunfield, P.F. Facultative methane oxidizers. In Taxonomy, Genomics and Ecophysiology of Hydrocarbon-Degrading Microbes; Springer: Cham, Switzerland, 2019; pp. 279–297.
- Gupta, G.; Kumar, V.; Pal, A.K. Microbial Degradation of High Molecular Weight Polycyclic Aromatic Hydrocarbons with Emphasis on Pyrene. Polycycl. Aromat. Compd. 2017, 39, 124–138.
- Heitkamp, M.A.; Franklin, W.; Cerniglia, C.E. Microbial metabolism of polycyclic aromatic hydrocarbons: Isolation and characterization of a pyrene-degrading bacterium. Appl. Environ. Microbiol. 1988, 54, 2549–2555.
- Peng, J.; Zhang, Y.; Su, J.; Qiu, Q.; Jia, Z.; Zhu, Y.G. Bacterial communities predominant in the degradation of 13C4-4, 5, 9, 10-pyrene during composting. Bioresour. Technol. 2013, 143, 608–614.
- Madigan, M.T.; Martinko, J.M.; Bender, K.S.; Buckley, D.H.; Stahl, D.A. Brock Biologia dei Microrganismi. In Microbiologia Generale, Ambientale e Industriale; Pearson: Milano/Torino, Italy, 2016; pp. 698–699.
- Posada-Baquero, R.; Jiménez-Volkerink, S.N.; García, J.L.; Vila, J.; Cantos, M.; Grifoll, M.; Ortega-Calvo, J.J. Rhizosphere-enhanced biosurfactant action on slowly desorbing PAHs in contaminated soil. Sci. Total Environ. 2020, 720, 137608.
- Chebbi, A.; Hentati, D.; Zaghden, H.; Baccar, N.; Rezgui, F.; Chalbi, M.; Sayadi, S.; Chamkha, M. Polycyclic aromatic hydrocarbon degradation and biosurfactant production by a newly isolated Pseudomonas sp. strain from used motor oil-contaminated soil. Int. Biodeterior. Biodegrad. 2017, 122, 128–140.
- Medic, A.; Lješevic, M.; Inui, H.; Beškoski, V.; Kojic, I.; Stojanovic, K.; Karadžic, I. Efficient biodegradation of petroleum n-alkanes and polycyclic aromatic hydrocarbons by polyextremophilic Pseudomonas aeruginosa san ai with multidegradative capacity. R. Soc. Chem. 2019, 10, 14060–14070.
- Ahmed, A.W.; Alzubaidi, F.S.; Hamza, S.J. Biodegradation of crude oil in contaminated water by local isolates of Enterobacter cloacae. Iraqi J. Sci. 2014, 55, 1025–1033.
- Lenchi, N.; Kebbouche-Gana, S.; Servais, P.; Gana, M.L.; Llirós, M. Diesel biodegradation capacities and biosurfactant production in saline-alkaline conditions by Delftia sp NL1, isolated from an Algerian oilfield. Geomicrobiol. J. 2020, 37, 454–466.
- Li, J.; Chen, W.; Zhou, W.; Wang, Y.; Deng, M.; Zhou, S. Synergistic degradation of pyrene by Pseudomonas aeruginosa PA06 and Achromobacter sp. AC15 with sodium citrate as the co- metabolic carbon source. Ecotoxicology 2020, 30, 1487–1498.
- Czarny, J.; Staninska-Pięta, A.; Piotrowska-Cyplik, W.; Juzwa, A.; Wolniewicz, R.; Marecik, Ł.; Ławniczak, Ł. Acinetobacter sp. as the key player in diesel oil degrading community exposed to PAHs and heavy metals. J. Hazard. Mater. 2020, 383, 121168.
- Van Hamme, J.D.; Singh, A.; Ward, O.P. Recent advances in petroleum microbiology. Microbiol. Mol. Biol. Rev. 2003, 67, 503–549.
- Thangaraj, K.; Kapley, A.; Purohit, H.J. Characterization of diverse Acinetobacter isolates for utilization of multiple aromatic compounds. Bioresour. Technol. 2008, 99, 2488–2494.
- Ghosal, D.; Dutta, A.; Chakraborty, J.; Basu, S.; Dutta, T.K. Characterization of the metabolic pathway involved in assimilation of acenaphthene in Acinetobacter sp. strain AGAT-W. Res. Microbiol. 2013, 164, 155–163.
- Rolando, L.; Vila, J.; Baquero, R.P.; Castilla-Alcantara, J.C.; Barra Caracciolo, A.; Ortega-Calvo, J.-J. Impact of bacterial motility on biosorption and cometabolism of pyrene in a porous medium. Sci. Total Environ. 2020, 717, 137210.
- Elufisan, T.O.; Rodríguez-Luna, I.C.; Oyedara, O.O.; Sánchez-Varela, A.; Hernández-Mendoza, A.; Dantán Gonzalez, E.; Paz-González, A.D.; Muhammad, K.; Rivera, G.; Villalobos-Lopez, M.A.; et al. The Polycyclic Aromatic Hydrocarbon (PAH) degradation activities and genome analysis of a novel strain Stenotrophomonas sp. Pemsol isolated from Mexico. Peer J. 2020, 8, e8102.
- Song, X.; Xu, Y.; Li, G.; Zhang, Y.; Huang, T.; Hu, Z. Isolation, characterization of Rhodococcus sp. P14 capable of degrading high-molecular-weight polycyclic aromatic hydrocarbons and aliphatic hydrocarbons. Mar. Pollut. Bull. 2011, 62, 2122–2128.
- Garrido-Sanz, D.; Sansegundo-Lobato, P.; Redondo-Nieto, M.; Suman, J.; Cajthaml, T.; Blanco-Romero, E.; Rivilla, R. Analysis of the biodegradative and adaptive potential of the novel polychlorinated biphenyl degrader Rhodococcus sp. WAY2 revealed by its complete genome sequence. Microb. Genom. 2020, 6, e000363.
- Feng, L.; Jiang, X.; Huang, Y.; Wen, D.; Fu, T.; Fu, R. Petroleum hydrocarbon-contaminated soil bioremediation assisted by isolated bacterial consortium and sophorolipid. Environ. Pollut. 2021, 273, 116476.
- Vasilyeva, G.; Kondrashina, V.; Strijakova, E.; Ortega-Calvo, J.-J. Adsorptive bioremediation of soil highly contaminated with crude oil. Sci. Total Environ. 2020, 706, 135739.
More
