You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Dean Liu and Version 1 by enze zhang.
Chemical compounds dissolved in insulating oil, as indicators can excellently monitor the paper aging condition, which has attracted increasing interest in areas of transformer condition monitoring and fault diagnosis. Because of their outstanding features, such as good correlation with the degree of polymerization of cellulose paper and the aid of non-destructive online monitoring, chemical indicators have been effectively used for transformer condition assessment.
- chemical indicators
- monitoring
- aging status
- transformer insulation
1. Study on Production Mechanism of Indicators from the Microscopic Perspective
Because of the benefits of clear microscopic modeling and precise calculation, molecular simulation is a computer-aided technology developed in the 1950s and 1960s that has been widely used in many fields such as physics, chemical engineering, material science, and life science. If molecular simulation technology is used, the degradation products of paper insulation and oil insulation are distinguished by analyzing the aging ways and methods of polymer compounds under different stresses. This can play an effective theoretical support role in monitoring the state of high-voltage insulation.
The first cellulose map was obtained in 1913 when molecular simulation was gradually introduced into the study of the aging mechanism of transformer oil-paper insulation [1]. Meyer et al. proposed an epoch-making model of cellulose molecular structure of insulating paper in 1928 [2]. Cellulose is the basic component of insulating paper. From the perspective of molecular microstructure, the aging of cellulose is related to the horizontal break of hydrogen bonds between molecules and within molecules, and the longitudinal break of molecular chain caused by the rupture of 1,4-β-glycosidic bonds. In short, the mechanical life of insulating paper is determined by the average length of the cellulose chains.
The main research method of the aging micro-mechanism of transformer insulating paper is to simulate the temperature rise of the molecular models of the crystalline and amorphous regions in cellulose [3][4]. The simulation technique can observe the chemical reaction paths and the products of each temperature section that cannot be obtained in the macroscopic experiment, which provides strong support and guidance for the results of thermal aging experiments. A large number of scholars compared the crystalline and amorphous regions and analyzed the intensity of molecular chain motion and the changes in the number of hydrogen bonds in the two regions at different temperatures. It was found that the cellulose molecules had stronger interaction forces in the crystalline region, the rehearsing density was well organized and the thermal stability was strong. Moreover, the molecular arrangement in the amorphous region is disordered and the interaction between molecules is small. The physical and chemical properties are susceptible to temperature and other environmental factors, which prompts the aging of insulating paper to begin with the amorphous region. The reaction molecular dynamic force field (ReaxFF) proposed by Van-Duin et al. [5] provided an effective tool for the study of hydrocarbon cracking. The reaction force field defines the interaction between atoms as a function of the bond level. It not only has the advantage of fast simulation speed but also can simulate the formation and breaking of chemical bonds during chemical reactions. This lays the groundwork for simulating chemical indicators during cellulose degradation. The current ReaxFF can handle systems with a million atoms and a time scale of 100 ns.
Reaction molecular dynamics simulation provides an effective way to study the degradation of oil-paper insulation from the atomic level and also provides a new idea for the diagnosis of transformer aging and faults. Figure 1 summarizes the thermal degradation products and reaction pathways of cellulose, hemicellulose, and lignin, which are the main compounds of insulating paper, by simulating the aging process of the transformer oil-paper insulation system [6]. In addition, the molecular dynamics simulation and quantum mechanics model combined with nuclear magnetic resonance (NMR) were used to determine the specific positions and degradation products of the major components of cellulose xylose [7][8] and glucose [9][10] after protonation.
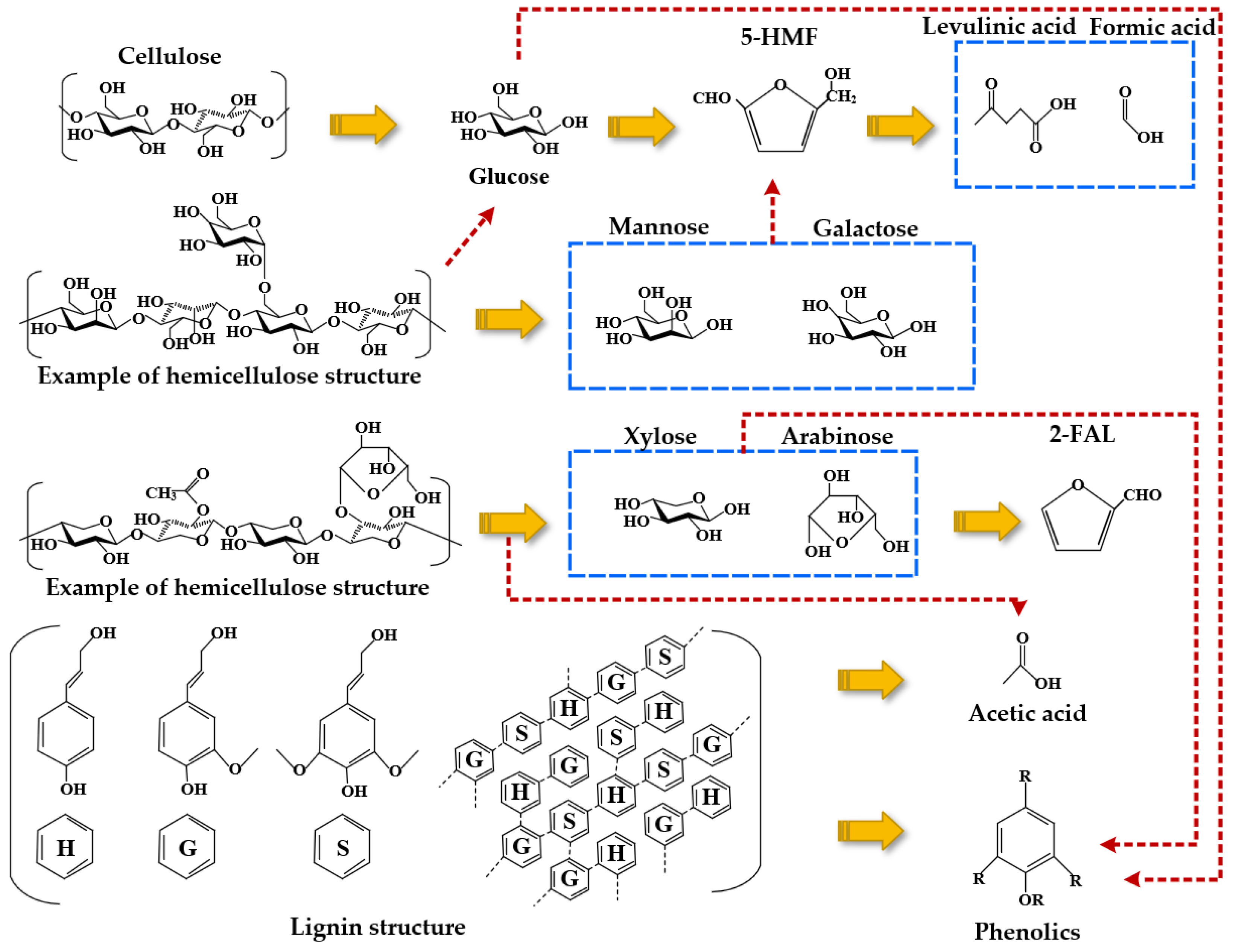
Figure 1. Main composition of insulating paper: cellulose, hemicellulose, and lignin degradation by-products.
At present, scholars have studied the effects of moisture, temperature, and oxygen content on the microscopic mechanism of oil-paper insulation. For example, Lundgaard [11] and Tian [12] et al. analyzed the microscopic mechanism of synergy between acid compounds and water molecules and the law of the diffusion behavior of organic acids based on molecular simulation. Davydov et al. [13] studied several factors affecting laboratory accelerated thermal aging. The study found that there was an exponential relationship between insulation life and temperature, but the proportion of moisture in the oil-paper was constantly changing.
In addition, Hohlein et al. [14] studied the effect of oxygen content and showed that the aging rate under aerobic conditions was three times that of anaerobic conditions. Liao et al. [15] simulated the diffusion motion mechanism of the compound model of dissolved gas in oil from the microscopic level. It is proved that water molecules can eventually diffuse into cellulose and form hydrogen bonds with glycosidic bonds, destroying the originally stable hydrogen bonding network of cellulose. Tanaka et al. [16] compared the arrangement of cellulose molecules with and without water. Under high temperatures, Li et al. [17] simulated the pyrolysis process and product changes of insulating oil and cellulose paper. After dehydration, cleavage, and polycondensation, the cellobiose repeat unit was completely decomposed into small molecular products, such as formic acid, CO2, and H2O. Zhang et al. [18] performed a pyrolysis simulation of cellobiose on the basis of the reaction force field and the Monte Carlo method and analyzed the generation pathway of the methanol indicator at the atomic level. Furthermore, it is consistent with the previous research results, indicating that methanol is suitable for the indication of early insulation aging, and later becomes unstable or even disappears.
2. Monitoring Technology of Chemical Indicators
2.1. Monitoring of Furan Compounds
In labs, almost all the furfural-relevant test data could be acquired under carefully controlled experiment conditions. (i) The most commonly used method for determining furfural is high-performance liquid chromatography (HPLC). Furfural and furan compounds are eluted from the column with acetonitrile-water mixed solvent after the sample is enriched by Solid Phase Extraction Cartridge (SPE), and the measurement results are accurate and reliable [19][20][21][22]. (ii) According to the characteristics of the larger solubility of furfural in water, furfural in insulating oil can also be purified and enriched by water as an extractant, with aniline acetate as the chromogenic agent. The red product of furfural and chromotropic agent in oil can have obvious characteristic absorption peaks at a specific wavelength, and then the content of furfural will be quantitatively determined by a spectrophotometer [23][24]. Of the above two test methods, although HPLC has high accuracy, it requires complicated and time-consuming pretreatment, and a lot of manpower and material resources to maintain. Spectrophotometry has strong anti-interference ability and a simple operation, but the accuracy is not very satisfactory.
In recent years, Abu-Siada [25] and Lei et al. [26] proposed an extraction-free furfural detection method based on ultraviolet-visible spectroscopy (UV-Vis). The absorption peak of furfural was separated from the spectrum of the components in the oil, and the influence of other oil compounds on furfural was reduced. The results show that the method has a good linear relationship between furfural concentration and characteristic absorbance. Chen et al. [27] also investigated the application of Confocal laser Raman spectroscopy (CLRS) in furfural concentration detection, identifying that the CLRS method was simpler and faster than HPLC, with a detection limit of 0.1 mg/L. The currently reported 5-HMF determination method is similar to the furfural detection method, and the common methods include gas chromatography and spectrophotometry [28][29].
Many countries have formulated relevant evaluation standards, which stipulate the threshold of furfural concentration in transformer oil. China power industry standards stipulate that for transformers that have been operating for 20 years, attention should be paid to furfural concentration greater than 1 mg/L. According to DL/T 596 “Preventive Test Regulations for Power Equipment”, when the furfural concentration in operating transformer oil is greater than 4 mg/L, it indicates that the aging condition of paper insulation has been relatively serious [30]. Xue et al. [31] tested the data of 77 step-up transformers with rated voltages ranging from 100 to 500 kV in the China State Grid, obtaining statistical data for transformers with varying operating years and furfural concentrations after regression analysis.
2.2. Monitoring of Carbon-Oxygen Gases
Carbon monoxide and carbon dioxide are good paper detection indicators. Studies have shown that the content of CO and CO2 is directly related to the degree of polymerization of insulating paper. Furthermore, the ratio of the contents of the two gases is an important feature for monitoring the degradation degree of cellulose [32][33][34][35][36]. Behjat et al. [37] made a detailed summary of the dissolved gas detection technology and analyzed the advantages and disadvantages of each method. At present, the detection methods for dissolved gas in oil are widely accepted as gas chromatography (GC), hydrogen on-line monitoring, and photo-acoustic spectroscopy (PSA).
According to Duval et al., if the CO2/CO ratio is lower than six and accompanied by a significant increase in ethylene, the paper degradation rate can be inferred to be higher [38]. Further research [39] revealed that the value of CO2/CO in oil for transformers in normal operation is typically greater than seven. When the value of CO2/CO is less than six, it may indicate that the existence of fault leads to rapid aging of the insulating paper. When the value of CO2/CO is less than two, it may indicate that there is a serious paper insulation fault in the transformer. The relevant guidelines of China provide the relationship between the total amount of carbon monoxide and carbon dioxide generated and the ratio of CO2/CO to the state of insulating materials [40]: (i) for open transformers, the carbon monoxide content generally does not exceed 300 mg/L. (ii) The carbon monoxide content in diaphragm transformer oil is usually higher than that in open transformers. When CO2/CO is greater than 0.5, the transformer may be abnormal. (iii) For nitrogen-type transformers, when it exceeds 0.2, the transformer may be abnormal. Mcshane et al. [41] found that when the content of CO + CO2 was about 1 mL/g, the average residue rate of polymerization degree was 50%. When the content of CO + CO2 was about 3 mL/g, the average residual rate of polymerization degree was about 30%.
2.3. Monitoring of Low Molecular Alcohols
Over time and the test of reality, methanol and ethanol have been identified as oil-soluble by-products generated by the aging of oil-immersed insulation materials in power transformers [42], and their existence provides reliable information for the diagnosis of transformer insulation. A simple, rapid, direct, and accurate detection method is essential, mainly including high-performance liquid chromatography (HPLC), gas chromatography (GC), gas chromatography-mass spectrometry (GC-MS), spectrophotometry, solid-phase micro-extraction (SPME) and headspace sampling-gas chromatography-mass spectrometry (HS-GC-MS). Although the spectrophotometric method is easy to operate and can obtain results quickly, the reproducibility is relatively poor and the accuracy is relatively low [43]. High-performance liquid chromatography (HPLC) and gas chromatography (GC) require time-consuming preparatory work, which reduces detection efficiency [43]. In addition, some scholars use the hydrogen flame ionization detector (FID), which has a high sensitivity to organic compounds, simple structure, and fast response. By comparing mass spectrometry detectors with hydrogen flame ion detectors, it was found that the mass spectrometry detectors were better and have a wider detection range [44]. Finally, a large number of outstanding scholars confirmed that the headspace sample equipped with a gas chromatography-mass spectrometer (HS-GC-MS) was the best detection method [44][45][46][47][48].
Table 1 displays the results of recent studies on the detection and quantitation limits of ethanol and methanol in insulating oil using various methods [49]. By including an internal standard in the sample, the influence of sampling error on quantitative results can be greatly reduced. The principle is to quantify the compound by the area ratio of the target component with the internal standard peak. Studies [46][49] found that deuterium ethanol (ethanol d-6) and 2-propanol can be used as an internal standard of methanol and ethanol. The two compounds are completely separated from the target product and are not produced by the aging of the insulating paper. It is also widely used in practice analysis because of its good stability. Matharage et al. [50] also applied HS-GC-MS to compare the accuracy of the internal and external standard methods for methanol measurement results, respectively. With the increasing innovation of technology, Fu et al. [51] recently proposed a new method for the determination of methanol based on an ultraviolet-visible spectrometer, which is based on the extraction of methanol from oil samples and oxidation with potassium permanganate. Finally, spectrometry can be used to determine methanol after the reaction of chromic acid with the oxidized product formaldehyde to form the purple compound.
Table 1. Detection limits and quantification limits of ethanol/methanol under different methods.
| Research Scholars | Method | EtOH/MeOH MDL (ng g−1) |
EtOH/MeOH ML (ng g−1) |
|---|---|---|---|
| J. Jalbert [46] | GC-MS | 3.6/4 | 13/14 |
| M. Bruzzoniti [44] |
GC-MS | 3.1/1.3 | 9.3/3.9 |
| M. Bruzzoniti [44] |
GC-FID | 26.8/12.1 | 79.6/36 |
| H. Molavi [49] | GC-MS | 155/144 | 495/458 |
| Z. Wang [50] | GC-MS | 21.1/19.5 | 67.1/62.0 |
2.4. Monitoring of Acid Compounds
There is no standard method for detecting hydrophilic low molecular acids in insulating paper at the moment. Literature [52] proposed direct titration with KOH isopropanol solution, but no specific test method flow was given. The method for determining the acid value in oil is relatively mature, with chemical titration being the most common method. The core idea is expressed by the mass (in mg) of KOH required to neutralize the acidic compounds contained in 1 g of oil [11], also known as the neutralization number, with units of 10−3. However, such as Lundgaard [11] and Lelekakis [53] et al. studied the effect of acid on the transformer insulation life and pointed out that the neutralization number cannot represent the strength and corrosiveness of the acids, nor can it distinguish the type of acids [54]. In addition, Ingebrigsten et al. [55] found through experiments that only 15% of the neutralization value of acid concentration in insulating oil is hydrophilic low molecular acid generated by the degradation of insulating paper.
2.5. Monitoring of Ketone Compounds
Wang et al. [56] used gas chromatography to determine propanol in insulating oil, which was simple, fast, and reliable because acetone has a low boiling point and is easy to gasify and separate. Following a series of tests, the minimum detection limit of this method was 0.036 mg/L, and the relative standard deviation was 4.2%, which fully met the requirements for determining acetone in transformer oil samples. After a one year inspection of the field transformer, it was verified that the measured acetone volume ranged from 0 to 1.44 mg/L. In recent years, Gu et al. [57] proposed a method of conical laser raman spectroscopy (CLRS) to detect the acetone concentration in transformer insulating oil. The characteristic peaks of acetone were analyzed by comparing acetone plus insulating oil samples, insulating oil samples, and acetone samples. Considering the degree of migration, superposition, and vibration mode, the Raman spectral peak at the high intensity of 780 cm−1 was used as the characteristic spectral peak to determine the quality and quantity of acetone. CLRS has a wider detection limit range than traditional methods and is a non-destructive detection method, providing a new technique for on-site analysis of acetone concentration in oil.
2.6. Monitoring of Sugar Compounds
An ion chromatograph equipped with a pulse amperometric detector is a better method for detecting sugar in oil [58]. It can solve well the shortcomings of low sensitivity and poor selectivity, and the early pretreatment is simple. Hu et al. [59] proposed an ultraviolet spectroscopy method for determining mixed sugar concentration. By establishing a calibration model of the wavelength spectrum and the content of the corresponding composition, the concentration of sugar in the unknown sample can be predicted by inputting the ultraviolet spectral information into the model. In addition, the method does not require the use of other chemical reagents and has high-level safety and reliability.
References
- Nishikawa, S.; Ono, S. Transmission of X-rays through fibrous, lamellar and granular substances. Tokyo Sugaku-Buturigakkwai Kizi Dai Ki 1913, 7, 131–138.
- Meyer, K.H.; Mark, H. Über den Bau des krystallisierten Anteils der Cellulose. Ber. Der Dtsch. Chem. Ges. 1928, 61, 593–614.
- Tang, C.; Zhang, S.; Xie, J.; Lv, C. Molecular simulation and experimental analysis of Al2O3-nanoparticle-modified insulation paper cellulose. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 1018–1026.
- Tang, C.; Zhang, S.; Wang, Q.; Wang, X.; Hao, J. Thermal stability of modified insulation paper cellulose based on molecular dynamics simulation. Energies 2017, 10, 397.
- Van-Duin, A.C.T.; Dasgupta, S.; Lorant, F.; Goddard, W.A. ReaxFF: A reactive force field for hydrocarbons. J. Phys. Chem. A 2001, 105, 9396–9409.
- Rasmussen, H.; Sørensen, H.R.; Meyer, A.S. Formation of degradation compounds from lignocellulosic biomass in the biorefinery: Sugar reaction mechanisms. Carbohydr. Res. 2014, 385, 45–57.
- Qian, X.; Nimlos, M.R.; Davis, M.; Johnson, D.K.; Himmel, M.E. Ab initio molecular dynamics simulations of β-D-glucose and β-D-xylose degradation mechanisms in acidic aqueous solution. Carbohydr. Res. 2005, 340, 2319–2327.
- Nimlos, M.R.; Qian, X.; Davis, M.; Himmel, M.E.; Johnson, D.K. Energetics of xylose decomposition as determined using quantum mechanics modelling. J. Phys. Chem. A 2006, 110, 11824–11838.
- Yang, G.; Pidko, E.A.; Hensen, E.J.M. Mechanism of Brønsted acid-catalyzed conversion of carbohydrates. J. Catal. 2012, 295, 122.
- Qian, X. Mechanisms and energetics for Brønsted acid-catalyzed glucose condensation, dehydration and isomerization reactions. Top. Catal. 2012, 55, 218–226.
- Lundgaard, L.E.; Hansenm, W.; Ingebrigtsen, S. Aging of mineral oil impregnated cellulose by acid catalysis. IEEE Trans. Dielectr. Electr. Insul. 2008, 15, 540–546.
- Tian, M. Molecular Simulation Study on the Influence of Moisture and Acid on the Microscopic Properties of Oil-Impregnated Insulation Paper. Master’s Thesis, Chongqing University, Chongqing, China, 2014. (In Chinese).
- Davydov, V.; Roizman, O. Transformer Operating Risk Assessment: Development of Models. In Proceedings of the Electric Power Research Institute EPRI, Palo Alto, CA, USA, 2005; pp. 13–27.
- Hohlein, I.; Kachler, A.J. Aging of cellulose at transformer service temperatures. Part 2. Influence of moisture and temperature on degree of polymerization and formation of furanic compounds in free-breathing systems. IEEE Electr. Insul. Mag. 2005, 21, 20–24.
- Liao, R.; Zhu, M.; Zhou, X.; Yang, L.; Yan, J.; Sun, C. Molecular dynamics simulation of the diffusion behavior of water molecules in oil and cellulose composite media. Acta Phys. Chim. Sin. 2011, 27, 815–824.
- Tanaka, F.; Fukui, N. The behavior of cellulose molecules in aqueous environments. Cellulose 2004, 11, 33–38.
- Li, J.; Chen, J.; Zhu, M.; Zhang, H. Research on pyrolysis mechanism of transformer oil-paper insulation based on reaction molecular dynamics simulation. Insul. Mater. 2019, 52, 79–85. (In Chinese)
- Zhang, Y.; Li, Y.; Zheng, H.; Zhu, M.; Liu, J.; Yang, T.; Zhang, C.; Li, Y. Microscopic reaction mechanism of the production of methanol during the thermal aging of cellulosic insulating paper. Cellulose 2020, 27, 2455–2467.
- Unsworth, J.; Mitchell, F. Degradation of electrical insulating paper monitored with high performance liquid chromatography. IEEE Trans. Dielectr. Electr. Insul. 1990, 25, 737–746.
- Hill, D.J.T.; Le, T.T.; Darveniza, M.; Saha, T. A study of the degradation of cellulosic insulation materials in a power transformer. Part III: Degradation products of cellulose insulation paper. Polym. Degrada. Stabil. 1996, 51, 211–218.
- Burton, P.J. Applications of liquid chromatography to the analysis of electrical insulating materials. In Proceedings of the CIGRE Conference, Paris, France, 1988; pp. 15–28.
- Vahidi, B.; Teymouri, A. Quality Confirmation Tests for Power Transformer Insulation Systems; Springer International Publishing: Berlin/Heidelberg, Germany, 2019.
- Abu-Siada, A. Correlation of furan concentration and spectral response of transformer oil-using expert systems. IET Sci. Meas. Technol. 2011, 5, 183–188.
- Wang, R.; Huang, X.; Wang, L. Facile electrochemical method and corresponding automated instrument for the detection of furfural in insulation oil. Talanta 2016, 148, 412–418.
- Abu-Siada, A.; Lai, S.P.; Islam, S. Remnant life estimation of power transformer using oil UV-Vis spectral response. In Proceedings of the IEEE/PES Power Systems Conference Exposition, Seattle, WA, USA, 15–18 March 2009; pp. 1–5.
- Peng, L.; Fu, Q.; Lin, M.; Zhao, Y.; Qian, Y.; Li, S. A novel furfural-detection-method for the aging prediction of paper insulation in power transformer. In Proceedings of the 2018 IEEE Conference Electrical Insulation and Dielectric Phenomena, Cancun, Mexico, 21–24 October 2018; pp. 630–633.
- Chen, W.; Gu, Z.; Zou, J.; Wan, F.; Xiang, Y. Analysis of furfural dissolved in transformer oil based on confocal laser Raman spectroscopy. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 915–921.
- Chun, C.; Xiao-Jian, M.A.; Pei-Lin, C. Spectrophotometric Determination of 5-Hydroxyfurfural and Furfural in the Hydrolyzed Liquor of Celulose. Phys. Test. Chem. Anal. 2008, 44, 223–225.
- Zhang, Y.; Song, Y.; Hu, X.; Liao, X.; Ni, Y.; Li, Q. Determination of 5-Hydroxymethylfurfural and Furfuralin Soft Beverages by HPLC. Adv. Mater. Res. 2012, 550–553, 1959–1966.
- Q/CSG 114002-2011; China Southern Power Grid Corporation Preventive Test Procedures for Power Equipment. China Southern Power Grid Co., Ltd.: Guangzhou, China, 2011. (In Chinese)
- Xue, C. Monitoring Paper Insulation Aging by Measuring Furfural Contents in Oil. In Proceedings of the Technical Report of State Electric Power Research Institute EPRI, August 1990.
- Tamura, R.; Anetai, H.; Ishii, T.; Kawamura, T. The diagnostic of aging deterioration of insulating paper. JIEE Proc. A 1981, 101, 30–36.
- Abu-Siada, A.; Islam, S. A new approach to identify power transformer criticality and asset management decision based on dissolved gas-in-oil analysis. IEEE Trans. Dielectr. Electr. Insul. 2012, 19, 1007–1012.
- Sun, C.; Ohodnicki, P.R.; Stewart, E.M. Chemical sensing strategies for real-time monitoring of transformer oil: A review. IEEE Sens. J. 2017, 17, 5786–5806.
- Scheirs, J.; Camino, G.; Tumiatti, W.; Avidano, M. Study of the mechanism of thermal degradation of cellulosic paper insulation in electrical transformer oil. Angew. Makromol. Chem. 1998, 259, 19–24.
- Jalbert, J.; Lessard, M.C. Cellulose chemical markers relationship with insulating paper post-mortem investigations. IEEE Trans. Dielectr. Electr. Insul. 2015, 22, 3550–3554.
- Behjat, V.; Emadifar, R.; Pourhossein, M.; Rao, U.M.; Fofana, I.; Najjar, R. Improved Monitoring and Diagnosis of Transformer Solid Insulation Using Pertinent Chemical Indicators. Energies 2021, 14, 3977.
- Emsley, A.M.; Stevens, G.C. Review of chemical indicators of degradation of cellulosic electrical paper insulation in oil-filled transformers. IEE Proc. Sci. Meas. Techol. 1994, 141, 324–334.
- Singh, S.; Bandyopadhyay, M.N. Dissolved gas analysis technique for incipient fault diagnosis in power transformers: A bibliographic survey. IEEE Electr. Insul. Mag. 2010, 26, 41–46.
- IE Commission. Mineral Oil-Filled Electrical Equipment in Service—Guidance on the Interpretation of Dissolved and Free Gases Analysis; IE Commission: Dublin, Ireland, 2015; IEC 60599-2015.
- Mcshane, C.P.; Rapp, K.J.; Corkran, J.L.; Gauger, J.A.; Luksich, J. Aging of paper insulation in natural ester dielectric fluid. In Proceedings of the IEEE/PES Transmission and Distribution Conference Exposition, Atlanta, GA, USA, 2 November 2001; pp. 675–679.
- Matharage, S.Y.; Liu, Q.; Wang, Z.D. Aging assessment of kraft paper insulation through methanol in oil measurement. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 1589–1596.
- Dettmer-Wilde, K.; Engewald, W. Practical Gas Chromatography; Springer: Berlin/Heidelberg, Germany, 2014.
- Bruzzoniti, M.C.; Maina, R.; De-Carlo, R.M.; Sarzanini, C.; Tumiatti, V. GC methods for the determination of methanol and ethanol in insulating mineral oils as markers of cellulose degradation in power transformers. Chromatographia 2014, 77, 1081–1089.
- Jalbert, J.; Gilbert, R.; Tétreault, P.; Morin, B.; Lessard-Déziel, L. Identification of a chemical indicator of the rupture of 1,4-β-glycosidic bonds of cellulose in an oil-impregnated insulating paper system. Cellulose 2007, 14, 295–309.
- Jalbert, J.; Duchesne, S.; Rodriguez-Celis, E.; Tétreault, P.; Collin, P. Robust and sensitive analysis of methanol and ethanol from cellulose degradation in mineral oils. J. Chromatogr. A 2012, 1256, 240–245.
- Schaut, A.; Eeckhoudt, S. Identification of Early-Stage Paper Degradation by Methanol; CIGRE: Paris, France, 2012; p. A2-107.
- Zheng, H.; Zhang, C.; Zhang, Y.; Liu, J.; Zhang, E.; Shi, Z.; Shao, G.; Shi, K.; Guo, J.; Zhang, C. Optimization of ethanol detection by automatic headspace method for cellulose insulation aging of oil-immersed transformers. Polymers 2020, 12, 1567.
- Molavi, H.; Yousefpour, A.; Mirmostafa, A.; Sabzi, A.; Hamedi, S.; Narimani, M.; Abdi, N. Static headspace GC/MS method for determination of methanol and ethanol contents, as the degradation markers of solid insulation systems of power transformers. Chromatographia 2017, 80, 1129–1135.
- Matharage, S.Y.; Liu, Q.; Davenport, E. Methanol Detection in Transformer Oils using Gas Chromatography and Ion Trap Mass Spectrometer. In Proceedings of the 2014 IEEE International Conference Dielectric Liquids, Bled, Slovenia, 29 June–3 July 2014; pp. 1–4.
- Fu, Q.; Peng, L.; Li, L.; Lin, M.; Zhao, Y.; Li, S.; Chen, C. Detection of Methanol in Power Transformer Oil Using Spectroscopy. J. Electr. Eng. Technol. 2019, 14, 861–867.
- Lundgaard, L.E.; Hansen, W.; Ingebrigtsen, S.; Linhjell, D.; Dahlund, M. Aging of Kraft paper by acid catalyzed hydrolysis. In Proceedings of the IEEE International Conference Dielectric Liquids, Coimbra, Portugal, 26 June–1 July 2005; pp. 381–384.
- Lelekakis, N.; Wijaya, J.; Martin, D.; Susa, D. The effect of acid accumulation in power-transformer oil on the aging rate of paper insulation. IEEE Electr. Insul. Mag. 2014, 30, 19–26.
- Kouassi, K.; Fofana, I.; Cissé, L.; Hadjadj, Y.; Yapi, K.M.L.; Diby, K.A. Impact of Low Molecular Weight Acids on Oil Impregnated Paper Insulation Degradation. Energies 2018, 11, 1465.
- Ingebrigtsen, S.; Dahlund, M.; Hansen, W.; Linhjell, D.; Lundgaard, L.E. Solubility of carboxylic acids in paper (Kraft)-oil insulationsystems. In Proceedings of the 2004 IEEE Conference Electrical Insulation and Dielectric Phenomena, Boulder, CO, USA, 20 October 2004; pp. 253–257.
- Wang, H.; Liu, X.; Wang, Z.; Xue, K.; Chen, G. Study on the analysis method of acetone volume fraction in power transformer oil. High Volt. Apparatus. 2008, 5, 395–398. (In Chinese)
- Gu, Z.; Chen, W.; Du, L.; Shi, H.; Wan, F. Application of Raman Spectroscopy for the Detection of Acetone Dissolved in Transformer Oil. J. Appl. Spectrosco. 2018, 85, 225–231.
- Lessard, M.G.; Masst, M. Prediction of remaining life of the paper insulation by the analysis of new oil-soluble compounds in power transformers. In Proceedings of the 2003 IEEE Conference Electrical Insulation and Dielectric Phenomena, Albuquerque, NM, USA, 19–22 October 2003; pp. 129–132.
- Hu, Z.; Wang, J.; Chai, X.; Kong, H. A new method for determining the content of mixed sugar by ultraviolet spectroscopy. Acta Chim. Sin. 2008, 5, 1233–1237. (In Chinese)
More
