You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 3 by Catherine Yang and Version 2 by Vrinda Gote.
mRNA vaccine molecules are large (104–106 Da) in size and are negatively charged. They are unable to pass through the lipid bilayer of cell membranes. Naked mRNA would be destroyed and degraded by the nucleases present in the bloodstream. In addition, naked mRNA is also attached and engulfed by immune cells in the tissue and the serum. Methods to deliver mRNA molecules into the cells include techniques such as gene gun, electroporation, and ex vivo transfection. The in vivo methods of delivering mRNA involves transfection of immune or non-immune cells using lipids or transfecting agents.
- mRNA vaccines
- mRNA structure
- mRNA vaccine immune response
- mRNA vaccines clinical trials
- lipid nanoparticles (LNPs)
- cationic lipids
- ionizable lipids
- PEGylated lipids
- lyophilized mRNA vaccines
- adjuvants
- antigen presentation
- self-amplifying mRNA vaccines
- saf
1. Lipid Nanoparticles (LNPs)
Although naked mRNA, liposomes, and polyplexes have shown clinical effectiveness in humans, LNPs for mRNA vaccines are the only drug delivery system that has demonstrated clinical effectiveness and has been approved for human use. The COVID-19 mRNA vaccines against SARS-CoV-2, developed by Moderna and Pfizer/BioNTech, employ LNPs to deliver the mRNA payload to the body. LNPs are currently the foremost non-viral delivery vector employed for gene therapy [33][1]. The clinical effectiveness of LNPs was first demonstrated when LNP-siRNA therapeutic Onpattro® (patisiran) was approved by the US FDA for hereditary transthyretin-mediated amyloidosis [34][2]. LNP formulations are the most successful, effective, and safe method of delivery of mRNA vaccines for human immunizations. LNPs offer numerous advantages for mRNA delivery to the site of action, including ease of formulation and scale-up, highly efficient transfection capacity, low toxicity profile, modularity, compactivity with different nucleic acid types and sizes, protection of mRNA from internal degradation, and increasing the half-life of mRNA vaccines [35][3]. LNPs are typically composed of four components, an ionizable cationic lipid, a helper phospholipid, cholesterol, and a PEGylated lipid. These lipids encapsulate the mRNA vaccine’s payload and protect the nucleic acid core from degradation [35][3].
2. Cationic and Ionizable Lipids
Cationic lipids were the first generation of lipids developed and utilized for mRNA vaccine delivery. These lipids contain a quaternary nitrogen atom imparting them a permanently positive charge. The positive charge of these lipids enables them to form ionic interactions with the negatively charged mRNA vaccines, forming a lipid complex called a lipoplex [4,36,37][4][5][6]. DOTMA and its synthetic analogue DOTAP were the first cationic lipids used to deliver mRNA vaccines in 1989 [38][7]. Cationic lipids such as DOTMA, DOPE, and DOGS have been widely used for mRNA delivery since then, including the commercially available and successful Lipofectin [4]. Lipofectin is a mixture of DOPE and DOTMA, and is one of the first LNP formulations, proving successful in the in vivo translation of mRNA [39][8].
The early cationic lipids demonstrated promising gene delivery in vitro, but they suffered from inadequate in vivo efficacy. The positive charge of the nitrogen head group and the non-biodegradable nature of the early cationic lipids were responsible for their ineffective delivery and efficacy in vitro [40][9]. Ionizable lipids, also called pH-dependent ionic lipids, are the second generation of cationic lipids containing a primary amine which imparts them a positive charge at or below physiological pH. The property of these lipids to have a neutral charge in the bloodstream at physiological pH helps in improving their safety as compared to the first-generation cationic lipids. They also extend the circulation time of the LNPs as compared to LNPs derived from cationic lipids. These were developed to overcome the shortcomings and safety issues such as immune activation and interaction with serum proteins of the first-generation cationic lipids [33][1]. DLin-MC3-DMA was the first US FDA-approved ionic lipid used in the first siRNA drug, Onpattro® [41][10]. The DLin-MC3-DMA ionic lipid was synthesized after a series of modifications on the first ionic lipid DODMA. DLinDMA was formed by replacing the oleyl tails of DODMA [42,43][11][12]. DLinDMA demonstrated superior ability as compared to DODMA in protective immunity against the respiratory syncytial virus (RSV) in vivo [44][13]. DLinDMA was further optimized to DLin-KC2-DMA, and further to DLin-MC3-DMA depending on a series of structure–activity relationship-based studies [45,46][14][15]. DLin-MC3-DMA is considered the first generation of ionizable lipids.
DLin-MC3-DMA or MC3 has a long plasma half-life of 72 h, increasing the duration of action of the siRNA [47][16]. The MC3 ionizable lipid was later shown to be effective in delivering mRNA along with siRNA [48,49,50,51,52,53,54][17][18][19][20][21][22][23]. The only shortcoming that MC has is its long half-life (72 h). This limits the chronic administration of vaccines with MC3. Thus, the next generation of ionizable lipids employed biodegradable functional groups which can facilitate fast clearance. The inclusion of ester moieties helped to increase the biodegradability of MC3 and increased its systemic clearance. Ester moieties are easy to install in a lipid, biodegradable, and chemically stable, which can be easily cleaved by the intracellular esterases. MC3 served as an important precursor and a starting point for the development of biodegradable ester ionizable lipids [55][24]. These include lipids such as Moderna’s proprietary lipids [56][25], Acuitas’ proprietary lipids [57][26], and others, including YSK12-C4 [58][27], CL4H6 [59][28], and L319 lipids, which are considered the second generation of ionizable lipids [47][16]. Ester-based biodegradable ionizable lipids have demonstrated higher potency in gene delivery as compared to the MC3 ionizable lipid. Moderna’s lipid 5 was found to have three-times-higher potency, and Acuitas’ lipid, ACL-0315 (the lipid used for the Pfizer/BioNTech COVID-19 vaccine), had six-times-higher potency as compared to MC3 lipid in delivering luciferase mRNA to animals US10166298B2.
The third-generation ionizable lipids are synthesized in an optimized manner, having a limited number of chemical synthesis steps, which increases the high-throughput production of the ionizable lipids [60][29]. 98N12-5 is the first example of a third-generation ionizable lipid [61][30]. Modifications and improvements to the 98N12-5 lipidoid lead to the invention of superior analogs, including C12-200 and C14-113 [62,63][31][32]. C14-113 lipidoids can specifically target cardiac muscles and, thus, can open new vistas to optimize and target gene therapies for enhancing cardiac function [63][32]. Li et al. reported TT3 as a potent lipidoid for delivering various mRNA molecules encoding for CRISPR/Cas9 [64][33], Factor IX [65][34], and SARS-CoV-2 [18][35]. In addition to the search for enhanced efficacy, a growing interest in improving the specificity of gene delivery to specific target cells or organs is underway. Targeted delivery for vaccines and immunotherapies to the immune cells and primary and secondary lymphoid organs is rapidly underway. Some examples of targeting agents include lipids containing polycyclic tails, including 11-A-M [66][36], and lipids containing cyclic imidazole head groups, such as 93-O17S [66][36], are specifically designed to target T cells. Moreover, the cyclic amine head group in lipid A18-Iso5-2DC18 has been demonstrated to bind to the stimulator of interferon genes (STING) protein. This results in dendritic cell maturation and can have antitumor efficacy by immune stimulation [67][37]. This can be a useful and desired characteristic for cancer immunotherapy using gene therapy [67][37]. Gene therapy utilizing third-generation ionizable lipids has also shown promise for multidrug-resistant bacterial infections. Cyclic vitamin C-derived ionizable lipids delivering an anti-microbial peptide and cathepsin B mRNA to macrophages, demonstrated that the therapy can eliminate multidrug-resistant bacteria and protect the mice from bacteria-induced sepsis [68][38]. LNPs are the most advanced and clinically approved delivery vehicles for mRNA [69][39].
3. PEG-Lipid
Among the ingredients, polyethylene glycol (PEG) is a hydrophilic material, well known for a wide range of applications in the cosmetic, food, and pharmaceutical industries. The PEGylated lipid component in LNPs is usually linked to an anchoring lipid. PEG was found to be an essential chemical in the formulation of LNPs to mitigate the uptake of nanoparticles by filter organs, also improving the colloidal stability of LNPs in biological fluids. Hence, circulation half-life and in vivo distribution of LNPs is enhanced. Usually, PEG-lipids account for minimal molar % among lipid constituents in LNPs (approximately 1.5%). However, they play a very pivotal role in affecting crucial parameters such as population size, polydispersity index, aggregation reduction, particle stability improvement, and encapsulation efficiency. The molecular weight of PEG and the carbon chain length of the anchor lipid can be exploited to fine-tune the time of circulation and uptake by immune cells, altering the efficiency [70][40]. Additionally, the PEG-lipid coat on LNPs acts as a steric hydrophilic barrier for preventing self-assembly and aggregation during storage. Therefore, the presence of PEG is helpful to stabilize the LNP and regulates size by limiting the lipid fusion. The amount of PEG is inversely proportional to the size of the LNP; higher the PEG content, the smaller the size of the LNP [71][41]. Generally, the molecular weight of PEG ranges between 350 and 3000 Da and the carbon chain of the anchored lipid lies between 13 and 18 carbon. Multiple literature reports indicated that a higher molecular weight of PEG and longer lipid chain increases the circulation time of nanoparticles and also reduces the uptake by immune cells. As the PEG-lipid dissociates from the LNP surface, it decreases the circulation time of the LNP, and provides more chances for delivering the mRNA cargo into target cells by an effect called “PEG-Dilemma”. In some instances, as the molar% of the PEG-lipid is maintained at 1.5%.The in vivo transfection level was found to be independent of the carbon chain length of the lipid. An added advantage of PEG-lipids relies on their capability of conjugating a specific ligand to the LNP, thus aiding in targeted drug delivery [72,73][42][43].
4. Helper Lipids
The main function of helper lipids in the formulation of LNPs lies in supporting their stability during storage and in vivo circulation. Chemically, these are glycerolipids and non-cationic in nature. Among the various helper lipids, sterols and phospholipids are the most widely used. Cholesterol is a natural component present in cell membranes. It is an exchangeable moiety that can be easily accumulated in the LNP. From a series of different studies, it has been indicated that cholesterol might be present on the surface, within the lipid bilayer, or even conjugated with the ionized lipid within its core. It is usually incorporated in LNP formulation, to maintain stability by filling gaps between lipids. The presence of cholesterol is needed to regulate the density, uptake, and fluidity of the lipid bilayer matrix within the LNP. Therefore, it controls the rigidity and integrity of the membrane, thereby preventing any leaks by the “condensing effect”. The hydrophobic tail, sterol ring flexibility, and polarity of hydroxy groups in cholesterol was reported to impact the efficacy of LNP delivery [74][44]. Cholesterol also contributes to improving the circulation half-life of LNPs by reducing the surface-bound protein. Moreover, it helps by fusing with the endosomal membrane during the cellular uptake of LNPs. It plays a vital role in lowering the temperature needed for transitioning from the lamellar phase to the hexagonal phase; therefore, the mRNA cargo from the LNP will be delivered to the cytosol [75][45].
The inclusion of phospholipids in LNP formulation can help with boosting encapsulation (together with cholesterol) and increasing cellular delivery. In general, the number of phospholipids in the LNP is considerably reduced, while increasing the cholesterol content for longer circulation times. Additionally, the inclusion of phospholipids promotes the entrapment efficiency and transfection potency of the LNP. It has been reported that increasing the molar percentage of phospholipids contributes to expediting the efficacy of delivery by LNPs. These phospholipids in Zwitter ionic form have been reported to play a pivotal role in the assembly of the LNP through the stabilization of electrostatic interactions between the cationic lipid, mRNA cargo, and surrounding water molecules. However, the actual role of phospholipids in the delivery of mRNA via LNPs is still ambiguous. Hence, it remains intriguing to further explore the actual role of phospholipids in enhancing the particle stability and delivery in vivo. Figure 41 describes the components of LNPs, including ionizable lipids, cholesterol, helper lipids, and PEGylated lipids [35][3].
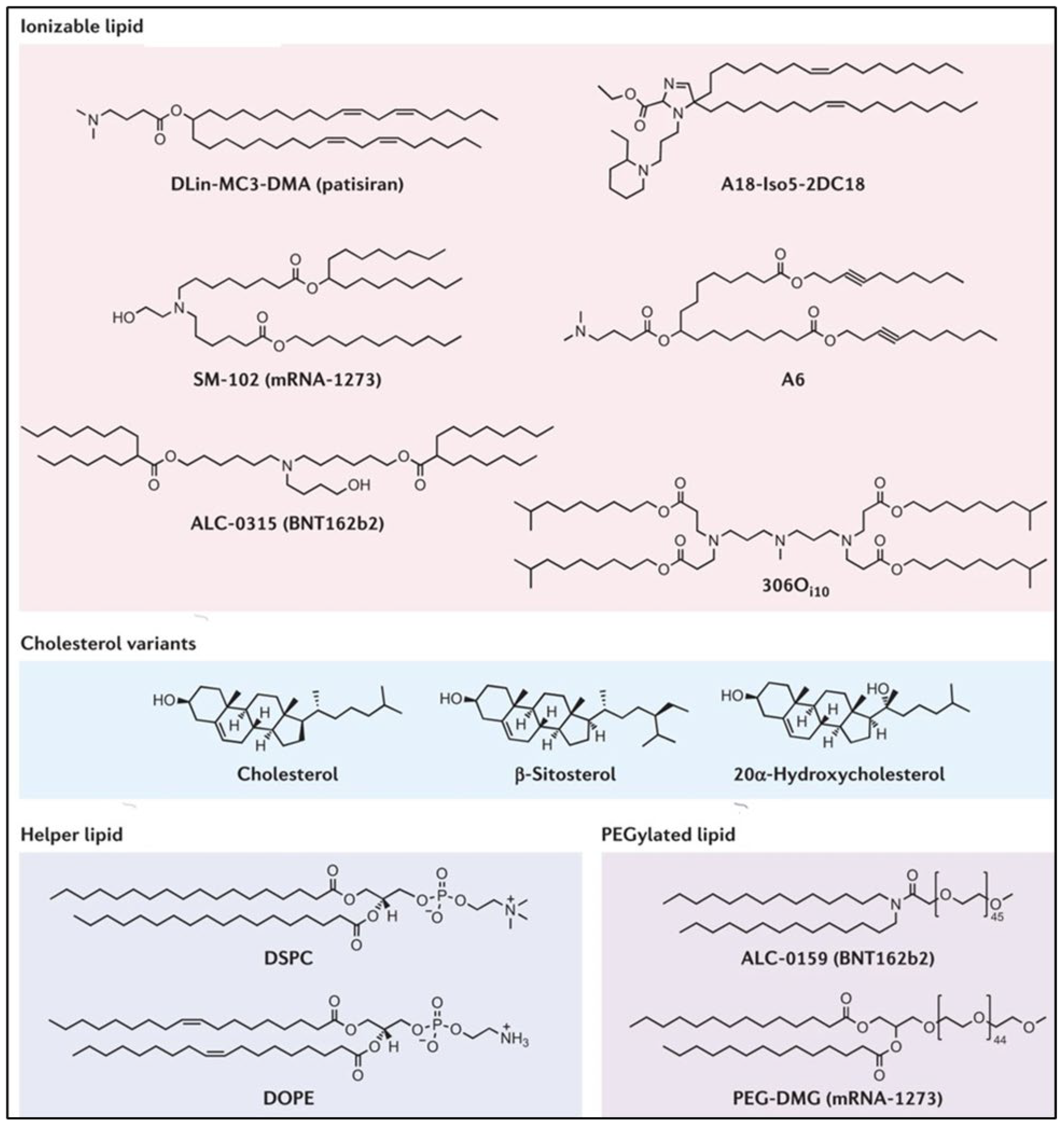
Figure 41. Components of lipid nanoparticles including ionizable lipids, cholesterol, helper lipids, and PEGylated lipids [35].
Components of lipid nanoparticles including ionizable lipids, cholesterol, helper lipids, and PEGylated lipids [3].
5. Physicochemical Properties Affecting mRNA-LNPs
LNPs possess many distinct characteristics, with a majority of being beneficial; ironically, a few characteristics grant some unwanted toxicities. Therefore, it is very critical to understand the physicochemical properties that affect the mRNA-loaded LNP.
Size and Surface area: Size and surface area dictates the LNP interaction process with the biological system, along with the distribution, elimination, internalization, degradation, and response. Decreasing the size corresponds to an increase in the surface area, thus making it more reactive towards the surrounding biological milieu. Essential biological activities including endocytosis and cellular uptake rely mostly on the particle size. Any size-dependent toxicity is based on the ability of LNPs in entering the biological system and modifying the macromolecules, thereby altering the essential biological functions. In the case of vaccines, high and efficient delivery is reported, while maintaining a particle size of ≅50 nm, irrespective of its chemical composition.
Charge: Charge plays a primary role in deciding the fate of biodistribution and efficacy of LNPs. The charge of the vector is very instrumental in transporting mRNA vaccines across biological membranes. Hence, negatively charged mRNA can develop electrostatic interactions with positively charged cationic lipids, leading to efficient encapsulation. In the end, the cationic liposome interacts with the anionic cell surface and endosomal membrane to release the mRNA cargo. The pKa (ability to attain positive charge) of cationic lipids has a significant effect on delivering the mRNA cargo; apparently, it is very important to understand its role. Although, there remains some uncertainty surrounding the actual pKa needed for gene delivery. A few reports indicated that the ideal pKa range for the delivery of LNPs via the IV route is in between 6.2 and 6.6. Charge modulation has effectively been researched for mitigating toxic manifestations, along with improving the delivery of mRNA from LNPs.
Shape and Structure: Both shape and internal structure are essential parameters that directly influence the cellular uptake and interaction with the biological environment. A few reports mentioned that the endocytosis of spherical nanoparticles is relatively easier in comparison to other shapes. Alternatively, non-spherical nanoparticles are more inclined to flow through capillaries. The exact mechanism of action underlying the shape and structure and its role in vivo remains obscure to date. Due to the involvement of many technological challenges, the actual mechanism of action stemming from shape and structure remains widely unexplored. Therefore, the research needs to be accelerated towards understanding their activity in deforming membranes and therapeutic efficiency.
Surface Composition: Efficient delivery and the biodistribution of LNPs can be influenced by the surface composition of delivering vectors. Well-known examples include the surface modification of LNPs by incorporating the PEG-Lipids by PEGylation. This process of PEGylation is known to alter nanocarrier trafficking and extend circulation half-life. Nevertheless, along with improving biodistribution and circulation, PEGylation can also result in reducing the uptake of LNPs by steric hinderance and limits interactions with the plasma membrane. Hence, the PEG-lipids detach into the serum and alleviate the steric hinderance to favor endosomal uptake [72,76][42][46].
- Cullis, P.R.; Hope, M.J. Lipid Nanoparticle Systems for Enabling Gene Therapies. Ther. 2017, 25, 1467–1475. https://doi.org/10.1016/J.YMTHE.2017.03.013.
- Rizk, M.; Tüzmen, Ş. Update on the Clinical Utility of an RNA Interference-Based Treatment: Focus on Patisiran. Pers. Med. 2017, 10, 267–278. https://doi.org/10.2147/PGPM.S87945.
- Chaudhary, N.; Weissman, D.; Whitehead, K.A. MRNA Vaccines for Infectious Diseases: Principles, Delivery and Clinical Translation. Rev. Drug Discov. 2021, 20, 817–838. https://doi.org/10.1038/s41573-021-00283-5.
- Felgner, P.L.; Gadek, T.R.; Holm, M.; Roman, R.; Chan, H.W.; Wenz, M.; Northrop, J.P.; Ringold, G.M.; Danielsen, M. Lipofection: A Highly Efficient, Lipid-Mediated DNA-Transfection Procedure. Natl. Acad. Sci. USA 1987, 84, 7413–7417. https://doi.org/10.1073/PNAS.84.21.7413.
- O’Sullivan, J.; Muñoz-Muñoz, J.; Turnbull, G.; Sim, N.; Penny, S.; Moschos, S. Beyond GalNAc! Drug Delivery Systems Comprising Complex Oligosaccharides for Targeted Use of Nucleic Acid Therapeutics. RSC Adv. 2022, 12, 20432. https://doi.org/10.1039/D2RA01999J.
- Malone, R.W.; Felgner, P.L.; Verma, I.M. Cationic Liposome-Mediated RNA Transfection. Natl. Acad. Sci. USA 1989, 86, 6077. https://doi.org/10.1073/PNAS.86.16.6077.
- Pardi, N.; Tuyishime, S.; Muramatsu, H.; Kariko, K.; Mui, B.L.; Tam, Y.K.; Madden, T.D.; Hope, M.J.; Weissman, D. Expression Kinetics of Nucleoside-Modified MRNA Delivered in Lipid Nanoparticles to Mice by Various Routes. Control. Release 2015, 217, 345–351. https://doi.org/10.1016/J.JCONREL.2015.08.007.
- Granot, Y.; Peer, D. Delivering the Right Message: Challenges and Opportunities in Lipid Nanoparticles-Mediated Modified MRNA Therapeutics-An Innate Immune System Standpoint. Immunol. 2017, 34, 68–77. https://doi.org/10.1016/J.SMIM.2017.08.015.
- Akinc, A.; Maier, M.A.; Manoharan, M.; Fitzgerald, K.; Jayaraman, M.; Barros, S.; Ansell, S.; Du, X.; Hope, M.J.; Madden, T.D.; et al. The Onpattro Story and the Clinical Translation of Nanomedicines Containing Nucleic Acid-Based Drugs. Nanotechnol. 2019, 14, 1084–1087. https://doi.org/10.1038/S41565-019-0591-Y.
- Heyes, J.; Palmer, L.; Bremner, K.; MacLachlan, I. Cationic Lipid Saturation Influences Intracellular Delivery of Encapsulated Nucleic Acids. Control. Release 2005, 107, 276–287. https://doi.org/10.1016/J.JCONREL.2005.06.014.
- Zimmermann, T.S.; Lee, A.C.H.; Akinc, A.; Bramlage, B.; Bumcrot, D.; Fedoruk, M.N.; Harborth, J.; Heyes, J.A.; Jeffs, L.B.; John, M.; et al. RNAi-Mediated Gene Silencing in Non-Human Primates. Nature 2006, 441, 111–114. https://doi.org/10.1038/NATURE04688.
- Bloom, K.; van den Berg, F.; Arbuthnot, P. Self-Amplifying RNA Vaccines for Infectious Diseases. Gene Ther. 2020, 28, 117–129. https://doi.org/10.1038/s41434-020-00204-y.
- Lin, P.J.C.; Tam, Y.Y.C.; Hafez, I.; Sandhu, A.; Chen, S.; Ciufolini, M.A.; Nabi, I.R.; Cullis, P.R. Influence of Cationic Lipid Composition on Uptake and Intracellular Processing of Lipid Nanoparticle Formulations of SiRNA. Nanomedicine 2013, 9, 233–246. https://doi.org/10.1016/J.NANO.2012.05.019.
- Semple, S.C.; Akinc, A.; Chen, J.; Sandhu, A.P.; Mui, B.L.; Cho, C.K.; Sah, D.W.Y.; Stebbing, D.; Crosley, E.J.; Yaworski, E.; et al. Rational Design of Cationic Lipids for SiRNA Delivery. Biotechnol. 2010, 28, 172–176. https://doi.org/10.1038/NBT.1602.
- Maier, M.A.; Jayaraman, M.; Matsuda, S.; Liu, J.; Barros, S.; Querbes, W.; Tam, Y.K.; Ansell, S.M.; Kumar, V.; Qin, J.; et al. Biodegradable Lipids Enabling Rapidly Eliminated Lipid Nanoparticles for Systemic Delivery of RNAi Therapeutics. Ther. 2013, 21, 1570–1578. https://doi.org/10.1038/MT.2013.124.
- Kim, J.; Jozic, A.; Sahay, G. Naturally Derived Membrane Lipids Impact Nanoparticle-Based Messenger RNA Delivery. Mol. Bioeng. 2020, 13, 463. https://doi.org/10.1007/S12195-020-00619-Y.
- Patel, S.; Ryals, R.C.; Weller, K.K.; Pennesi, M.E.; Sahay, G. Lipid Nanoparticles for Delivery of Messenger RNA to the Back of the Eye. Control. Release 2019, 303, 91–100. https://doi.org/10.1016/J.JCONREL.2019.04.015.
- Robinson, E.; MacDonald, K.D.; Slaughter, K.; McKinney, M.; Patel, S.; Sun, C.; Sahay, G. Lipid Nanoparticle-Delivered Chemically Modified MRNA Restores Chloride Secretion in Cystic Fibrosis. Ther. 2018, 26, 2034–2046. https://doi.org/10.1016/J.YMTHE.2018.05.014.
- Sedic, M.; Senn, J.J.; Lynn, A.; Laska, M.; Smith, M.; Platz, S.J.; Bolen, J.; Hoge, S.; Bulychev, A.; Jacquinet, E.; et al. Safety Evaluation of Lipid Nanoparticle-Formulated Modified MRNA in the Sprague-Dawley Rat and Cynomolgus Monkey. Pathol. 2018, 55, 341–354. https://doi.org/10.1177/0300985817738095.
- Veiga, N.; Goldsmith, M.; Granot, Y.; Rosenblum, D.; Dammes, N.; Kedmi, R.; Ramishetti, S.; Peer, D. Cell Specific Delivery of Modified MRNA Expressing Therapeutic Proteins to Leukocytes. Commun. 2018, 9, 4493. https://doi.org/10.1038/s41467-018-06936-1.
- Arteta, M.Y.; Kjellman, T.; Bartesaghi, S.; Wallin, S.; Wu, X.; Kvist, A.J.; Dabkowska, A.; Székely, N.; Radulescu, A.; Bergenholtz, J.; et al. Successful Reprogramming of Cellular Protein Production through MRNA Delivered by Functionalized Lipid Nanoparticles. Natl. Acad. Sci. USA 2018, 115, E3351–E3360. https://doi.org/10.1073/PNAS.1720542115/SUPPL_FILE/PNAS.201720542SI.PDF.
- Zhang, M.; Sun, J.; Li, M.; Jin, X. Modified MRNA-LNP Vaccines Confer Protection against Experimental DENV-2 Infection in Mice. Ther. Methods Clin. Dev. 2020, 18, 702. https://doi.org/10.1016/J.OMTM.2020.07.013.
- Gilham, D.; Lehner, R. Techniques to Measure Lipase and Esterase Activity in Vitro. Methods 2005, 36, 139–147. https://doi.org/10.1016/J.YMETH.2004.11.003.
- Sabnis, S.; Kumarasinghe, E.S.; Salerno, T.; Mihai, C.; Ketova, T.; Senn, J.J.; Lynn, A.; Bulychev, A.; McFadyen, I.; Chan, J.; et al. A Novel Amino Lipid Series for MRNA Delivery: Improved Endosomal Escape and Sustained Pharmacology and Safety in Non-Human Primates. Ther. 2018, 26, 1509–1519. https://doi.org/10.1016/J.YMTHE.2018.03.010.
- Tanaka, H.; Sakurai, Y.; Anindita, J.; Akita, H. Development of Lipid-like Materials for RNA Delivery Based on Intracellular Environment-Responsive Membrane Destabilization and Spontaneous Collapse. Drug Deliv. Rev. 2020, 154–155, 210–226. https://doi.org/10.1016/J.ADDR.2020.07.001.
- Sato, Y.; Hashiba, K.; Sasaki, K.; Maeki, M.; Tokeshi, M.; Harashima, H. Understanding Structure-Activity Relationships of PH-Sensitive Cationic Lipids Facilitates the Rational Identification of Promising Lipid Nanoparticles for Delivering SiRNAs in Vivo. Control. Release 2019, 295, 140–152. https://doi.org/10.1016/J.JCONREL.2019.01.001.
- Shobaki, N.; Sato, Y.; Suzuki, Y.; Okabe, N.; Harashima, H. Manipulating the Function of Tumor-Associated Macrophages by SiRNA-Loaded Lipid Nanoparticles for Cancer Immunotherapy. Control. Release 2020, 325, 235–248. https://doi.org/10.1016/J.JCONREL.2020.07.001.
- Mahon, K.P.; Love, K.T.; Whitehead, K.A.; Qin, J.; Akinc, A.; Leshchiner, E.; Leshchiner, I.; Langer, R.; Anderson, D.G. A Combinatorial Approach to Determine Functional Group Effects on Lipidoid-Mediated SiRNA Delivery. Chem. 2010, 21, 1448. https://doi.org/10.1021/BC100041R.
- Akinc, A.; Zumbuehl, A.; Goldberg, M.; Leshchiner, E.S.; Busini, V.; Hossain, N.; Bacallado, S.A.; Nguyen, D.N.; Fuller, J.; Alvarez, R.; et al. A Combinatorial Library of Lipid-like Materials for Delivery of RNAi Therapeutics. Biotechnol. 2008, 26, 561–569. https://doi.org/10.1038/NBT1402.
- Love, K.T.; Mahon, K.P.; Levins, C.G.; Whitehead, K.A.; Querbes, W.; Dorkin, J.R.; Qin, J.; Cantley, W.; Qin, L.L.; Racie, T.; et al. Lipid-like Materials for Low-Dose, in Vivo Gene Silencing. Natl. Acad. Sci. USA 2010, 107, 1864–1869. https://doi.org/10.1073/PNAS.0910603106/SUPPL_FILE/PNAS.0910603106_SI.PDF.
- Turnbull, I.C.; Eltoukhy, A.A.; Fish, K.M.; Nonnenmacher, M.; Ishikawa, K.; Chen, J.; Hajjar, R.J.; Anderson, D.G.; Costa, K.D. Myocardial Delivery of Lipidoid Nanoparticle Carrying ModRNA Induces Rapid and Transient Expression. Ther. 2016, 24, 66. https://doi.org/10.1038/MT.2015.193.
- Jiang, C.; Mei, M.; Li, B.; Zhu, X.; Zu, W.; Tian, Y.; Wang, Q.; Guo, Y.; Dong, Y.; Tan, X. A Non-Viral CRISPR/Cas9 Delivery System for Therapeutically Targeting HBV DNA and Pcsk9 in Vivo. Cell Res. 2017, 27, 440–443. https://doi.org/10.1038/cr.2017.16.
- Li, B.; Luo, X.; Deng, B.; Wang, J.; McComb, D.W.; Shi, Y.; Gaensler, K.M.L.; Tan, X.; Dunn, A.L.; Kerlin, B.A.; et al. An Orthogonal Array Optimization of Lipid-like Nanoparticles for MRNA Delivery in Vivo. Nano Lett. 2015, 15, 8099–8107. https://doi.org/10.1021/ACS.NANOLETT.5B03528.
- Lokugamage, M.P.; Sago, C.D.; Gan, Z.; Krupczak, B.R.; Dahlman, J.E.; Lokugamage, M.P.; Sago, C.D.; Gan, Z.; Krupczak, B.R.; Dahlman, J E; et al. Constrained Nanoparticles Deliver SiRNA and SgRNA to T Cells In Vivo without Targeting Ligands. Mater. 2019, 31, 1902251. https://doi.org/10.1002/ADMA.201902251.
- Miao, L.; Li, L.; Huang, Y.; Delcassian, D.; Chahal, J.; Han, J.; Shi, Y.; Sadtler, K.; Gao, W.; Lin, J.; et al. Delivery of MRNA Vaccines with Heterocyclic Lipids Increases Anti-Tumor Efficacy by STING-Mediated Immune Cell Activation. Biotechnol. 2019, 37, 1174–1185. https://doi.org/10.1038/S41587-019-0247-3.
- Hou, X.; Zhang, X.; Zhao, W.; Zeng, C.; Deng, B.; McComb, D.W.; Du, S.; Zhang, C.; Li, W.; Dong, Y. Vitamin Lipid Nanoparticles Enable Adoptive Macrophage Transfer for the Treatment of Multidrug-Resistant Bacterial Sepsis. Nanotechnol. 2020, 15, 41–46. https://doi.org/10.1038/S41565-019-0600-1.
- Ho, W.; Gao, M.; Li, F.; Li, Z.; Zhang, X.Q.; Xu, X. Next-Generation Vaccines: Nanoparticle-Mediated DNA and MRNA Delivery. Healthc. Mater. 2021, 10, 2001812. https://doi.org/10.1002/ADHM.202001812.
- Heyes, J.; Hall, K.; Tailor, V.; Lenz, R.; MacLachlan, I. Synthesis and Characterization of Novel Poly(Ethylene Glycol)-Lipid Conjugates Suitable for Use in Drug Delivery. Control. Release 2006, 112, 280–290. https://doi.org/10.1016/J.JCONREL.2006.02.012.
- Mui, B.L.; Tam, Y.K.; Jayaraman, M.; Ansell, S.M.; Du, X.; Tam, Y.Y.; Lin, P.J.; Chen, S.; Narayanannair, J.K.; Rajeev, K.G.; et al. Influence of Polyethylene Glycol Lipid Desorption Rates on Pharmacokinetics and Pharmacodynamics of SiRNA Lipid Nanoparticles. Ther. Nucleic Acids 2013, 2, e139. https://doi.org/10.1038/MTNA.2013.66.
- Fang, Y.; Xue, J.; Gao, S.; Lu, A.; Yang, D.; Jiang, H.; He, Y.; Shi, K. Cleavable PEGylation: A Strategy for Overcoming the “PEG Dilemma” in Efficient Drug Delivery. Drug Deliv. 2017, 24, 22–32. https://doi.org/10.1080/10717544.2017.1388451.
- Leung, A.K.K.; Tam, Y.Y.C.; Cullis, P.R. Lipid Nanoparticles for Short Interfering RNA Delivery. Genet. 2014, 88, 71–110. https://doi.org/10.1016/B978-0-12-800148-6.00004-3.
- Paunovska, K.; Da Silva Sanchez, A.J.; Sago, C.D.; Gan, Z.; Lokugamage, M.P.; Islam, F.Z.; Kalathoor, S.; Krupczak, B.R.; Dahlman, J.E. Nanoparticles Containing Oxidized Cholesterol Deliver MRNA to the Liver Microenvironment at Clinically Relevant Doses. Mater. 2019, 31, 1807748. https://doi.org/10.1002/ADMA.201807748.
- Patel, S.; Ashwanikumar, N.; Robinson, E.; Xia, Y.; Mihai, C.; Griffith, J.P.; Hou, S.; Esposito, A.A.; Ketova, T.; Welsher, K.; et al. Naturally-Occurring Cholesterol Analogues in Lipid Nanoparticles Induce Polymorphic Shape and Enhance Intracellular Delivery of MRNA. Commun. 2020, 11, 983. https://doi.org/10.1038/s41467-020-14527-2.
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a Strategy for Improving Nanoparticle-Based Drug and Gene Delivery. Drug Deliv. Rev. 2016, 99, 28. https://doi.org/10.1016/J.ADDR.2015.09.012.
References
- Cullis, P.R.; Hope, M.J. Lipid Nanoparticle Systems for Enabling Gene Therapies. Mol. Ther. 2017, 25, 1467–1475.
- Rizk, M.; Tüzmen, Ş. Update on the Clinical Utility of an RNA Interference-Based Treatment: Focus on Patisiran. Pharmgenomics Pers. Med. 2017, 10, 267–278.e16.
- Chaudhary, N.; Weissman, D.; Whitehead, K.A. MRNA Vaccines for Infectious Diseases: Principles, Delivery and Clinical Translation. Nat. Rev. Drug Discov. 2021, 20, 817–838.
- Martinon, F.; Krishnan, S.; Lenzen, G.; Magné, R.; Gomard, E.; Guillet, J.-G.; Lévy, J.-P.; Meulien, P. Induction of Virus-Specific Cytotoxic T Lymphocytes in Vivo by Liposome-Entrapped MRNA. Eur. J. Immunol. 1993, 23, 1719–1722.
- Felgner, P.L.; Gadek, T.R.; Holm, M.; Roman, R.; Chan, H.W.; Wenz, M.; Northrop, J.P.; Ringold, G.M.; Danielsen, M. Lipofection: A Highly Efficient, Lipid-Mediated DNA-Transfection Procedure. Proc. Natl. Acad. Sci. USA 1987, 84, 7413–7417.
- O’Sullivan, J.; Muñoz-Muñoz, J.; Turnbull, G.; Sim, N.; Penny, S.; Moschos, S. Beyond GalNAc! Drug Delivery Systems Comprising Complex Oligosaccharides for Targeted Use of Nucleic Acid Therapeutics. RSC Adv. 2022, 12, 20432.
- Malone, R.W.; Felgner, P.L.; Verma, I.M. Cationic Liposome-Mediated RNA Transfection. Proc. Natl. Acad. Sci. USA 1989, 86, 6077.
- Pardi, N.; Tuyishime, S.; Muramatsu, H.; Kariko, K.; Mui, B.L.; Tam, Y.K.; Madden, T.D.; Hope, M.J.; Weissman, D. Expression Kinetics of Nucleoside-Modified MRNA Delivered in Lipid Nanoparticles to Mice by Various Routes. J. Control. Release 2015, 217, 345–351.
- Granot, Y.; Peer, D. Delivering the Right Message: Challenges and Opportunities in Lipid Nanoparticles-Mediated Modified MRNA Therapeutics-An Innate Immune System Standpoint. Semin. Immunol. 2017, 34, 68–77.
- Akinc, A.; Maier, M.A.; Manoharan, M.; Fitzgerald, K.; Jayaraman, M.; Barros, S.; Ansell, S.; Du, X.; Hope, M.J.; Madden, T.D.; et al. The Onpattro Story and the Clinical Translation of Nanomedicines Containing Nucleic Acid-Based Drugs. Nat. Nanotechnol. 2019, 14, 1084–1087.
- Heyes, J.; Palmer, L.; Bremner, K.; MacLachlan, I. Cationic Lipid Saturation Influences Intracellular Delivery of Encapsulated Nucleic Acids. J. Control. Release 2005, 107, 276–287.
- Zimmermann, T.S.; Lee, A.C.H.; Akinc, A.; Bramlage, B.; Bumcrot, D.; Fedoruk, M.N.; Harborth, J.; Heyes, J.A.; Jeffs, L.B.; John, M.; et al. RNAi-Mediated Gene Silencing in Non-Human Primates. Nature 2006, 441, 111–114.
- Bloom, K.; van den Berg, F.; Arbuthnot, P. Self-Amplifying RNA Vaccines for Infectious Diseases. Gene Ther. 2020, 28, 117–129.
- Lin, P.J.C.; Tam, Y.Y.C.; Hafez, I.; Sandhu, A.; Chen, S.; Ciufolini, M.A.; Nabi, I.R.; Cullis, P.R. Influence of Cationic Lipid Composition on Uptake and Intracellular Processing of Lipid Nanoparticle Formulations of SiRNA. Nanomedicine 2013, 9, 233–246.
- Semple, S.C.; Akinc, A.; Chen, J.; Sandhu, A.P.; Mui, B.L.; Cho, C.K.; Sah, D.W.Y.; Stebbing, D.; Crosley, E.J.; Yaworski, E.; et al. Rational Design of Cationic Lipids for SiRNA Delivery. Nat. Biotechnol. 2010, 28, 172–176.
- Maier, M.A.; Jayaraman, M.; Matsuda, S.; Liu, J.; Barros, S.; Querbes, W.; Tam, Y.K.; Ansell, S.M.; Kumar, V.; Qin, J.; et al. Biodegradable Lipids Enabling Rapidly Eliminated Lipid Nanoparticles for Systemic Delivery of RNAi Therapeutics. Mol. Ther. 2013, 21, 1570–1578.
- Kim, J.; Jozic, A.; Sahay, G. Naturally Derived Membrane Lipids Impact Nanoparticle-Based Messenger RNA Delivery. Cell. Mol. Bioeng. 2020, 13, 463.
- Patel, S.; Ryals, R.C.; Weller, K.K.; Pennesi, M.E.; Sahay, G. Lipid Nanoparticles for Delivery of Messenger RNA to the Back of the Eye. J. Control. Release 2019, 303, 91–100.
- Robinson, E.; MacDonald, K.D.; Slaughter, K.; McKinney, M.; Patel, S.; Sun, C.; Sahay, G. Lipid Nanoparticle-Delivered Chemically Modified MRNA Restores Chloride Secretion in Cystic Fibrosis. Mol. Ther. 2018, 26, 2034–2046.
- Sedic, M.; Senn, J.J.; Lynn, A.; Laska, M.; Smith, M.; Platz, S.J.; Bolen, J.; Hoge, S.; Bulychev, A.; Jacquinet, E.; et al. Safety Evaluation of Lipid Nanoparticle-Formulated Modified MRNA in the Sprague-Dawley Rat and Cynomolgus Monkey. Vet. Pathol. 2018, 55, 341–354.
- Veiga, N.; Goldsmith, M.; Granot, Y.; Rosenblum, D.; Dammes, N.; Kedmi, R.; Ramishetti, S.; Peer, D. Cell Specific Delivery of Modified MRNA Expressing Therapeutic Proteins to Leukocytes. Nat. Commun. 2018, 9, 4493.
- Arteta, M.Y.; Kjellman, T.; Bartesaghi, S.; Wallin, S.; Wu, X.; Kvist, A.J.; Dabkowska, A.; Székely, N.; Radulescu, A.; Bergenholtz, J.; et al. Successful Reprogramming of Cellular Protein Production through MRNA Delivered by Functionalized Lipid Nanoparticles. Proc. Natl. Acad. Sci. USA 2018, 115, E3351–E3360.
- Zhang, M.; Sun, J.; Li, M.; Jin, X. Modified MRNA-LNP Vaccines Confer Protection against Experimental DENV-2 Infection in Mice. Mol. Ther. Methods Clin. Dev. 2020, 18, 702.
- Gilham, D.; Lehner, R. Techniques to Measure Lipase and Esterase Activity in Vitro. Methods 2005, 36, 139–147.
- Sabnis, S.; Kumarasinghe, E.S.; Salerno, T.; Mihai, C.; Ketova, T.; Senn, J.J.; Lynn, A.; Bulychev, A.; McFadyen, I.; Chan, J.; et al. A Novel Amino Lipid Series for MRNA Delivery: Improved Endosomal Escape and Sustained Pharmacology and Safety in Non-Human Primates. Mol. Ther. 2018, 26, 1509–1519.
- Tanaka, H.; Sakurai, Y.; Anindita, J.; Akita, H. Development of Lipid-like Materials for RNA Delivery Based on Intracellular Environment-Responsive Membrane Destabilization and Spontaneous Collapse. Adv. Drug Deliv. Rev. 2020, 154–155, 210–226.
- Sato, Y.; Hashiba, K.; Sasaki, K.; Maeki, M.; Tokeshi, M.; Harashima, H. Understanding Structure-Activity Relationships of PH-Sensitive Cationic Lipids Facilitates the Rational Identification of Promising Lipid Nanoparticles for Delivering SiRNAs in Vivo. J. Control. Release 2019, 295, 140–152.
- Shobaki, N.; Sato, Y.; Suzuki, Y.; Okabe, N.; Harashima, H. Manipulating the Function of Tumor-Associated Macrophages by SiRNA-Loaded Lipid Nanoparticles for Cancer Immunotherapy. J. Control. Release 2020, 325, 235–248.
- Mahon, K.P.; Love, K.T.; Whitehead, K.A.; Qin, J.; Akinc, A.; Leshchiner, E.; Leshchiner, I.; Langer, R.; Anderson, D.G. A Combinatorial Approach to Determine Functional Group Effects on Lipidoid-Mediated SiRNA Delivery. Bioconjug. Chem. 2010, 21, 1448.
- Akinc, A.; Zumbuehl, A.; Goldberg, M.; Leshchiner, E.S.; Busini, V.; Hossain, N.; Bacallado, S.A.; Nguyen, D.N.; Fuller, J.; Alvarez, R.; et al. A Combinatorial Library of Lipid-like Materials for Delivery of RNAi Therapeutics. Nat. Biotechnol. 2008, 26, 561–569.
- Love, K.T.; Mahon, K.P.; Levins, C.G.; Whitehead, K.A.; Querbes, W.; Dorkin, J.R.; Qin, J.; Cantley, W.; Qin, L.L.; Racie, T.; et al. Lipid-like Materials for Low-Dose, in Vivo Gene Silencing. Proc. Natl. Acad. Sci. USA 2010, 107, 1864–1869.
- Turnbull, I.C.; Eltoukhy, A.A.; Fish, K.M.; Nonnenmacher, M.; Ishikawa, K.; Chen, J.; Hajjar, R.J.; Anderson, D.G.; Costa, K.D. Myocardial Delivery of Lipidoid Nanoparticle Carrying ModRNA Induces Rapid and Transient Expression. Mol. Ther. 2016, 24, 66.
- Jiang, C.; Mei, M.; Li, B.; Zhu, X.; Zu, W.; Tian, Y.; Wang, Q.; Guo, Y.; Dong, Y.; Tan, X. A Non-Viral CRISPR/Cas9 Delivery System for Therapeutically Targeting HBV DNA and Pcsk9 in Vivo. Cell Res. 2017, 27, 440–443.
- Li, B.; Luo, X.; Deng, B.; Wang, J.; McComb, D.W.; Shi, Y.; Gaensler, K.M.L.; Tan, X.; Dunn, A.L.; Kerlin, B.A.; et al. An Orthogonal Array Optimization of Lipid-like Nanoparticles for MRNA Delivery in Vivo. Nano Lett. 2015, 15, 8099–8107.
- Zeng, C.; Hou, X.; Yan, J.; Zhang, C.; Li, W.; Zhao, W.; Du, S.; Dong, Y. Leveraging MRNA Sequences and Nanoparticles to Deliver SARS-CoV-2 Antigens In Vivo. Adv. Mater. 2020, 32, 2004452.
- Lokugamage, M.P.; Sago, C.D.; Gan, Z.; Krupczak, B.R.; Dahlman, J.E.; Lokugamage, M.P.; Sago, C.D.; Gan, Z.; Krupczak, B.R.; Dahlman, J.E.; et al. Constrained Nanoparticles Deliver SiRNA and SgRNA to T Cells In Vivo without Targeting Ligands. Adv. Mater. 2019, 31, 1902251.
- Miao, L.; Li, L.; Huang, Y.; Delcassian, D.; Chahal, J.; Han, J.; Shi, Y.; Sadtler, K.; Gao, W.; Lin, J.; et al. Delivery of MRNA Vaccines with Heterocyclic Lipids Increases Anti-Tumor Efficacy by STING-Mediated Immune Cell Activation. Nat. Biotechnol. 2019, 37, 1174–1185.
- Hou, X.; Zhang, X.; Zhao, W.; Zeng, C.; Deng, B.; McComb, D.W.; Du, S.; Zhang, C.; Li, W.; Dong, Y. Vitamin Lipid Nanoparticles Enable Adoptive Macrophage Transfer for the Treatment of Multidrug-Resistant Bacterial Sepsis. Nat. Nanotechnol. 2020, 15, 41–46.
- Ho, W.; Gao, M.; Li, F.; Li, Z.; Zhang, X.Q.; Xu, X. Next-Generation Vaccines: Nanoparticle-Mediated DNA and MRNA Delivery. Adv. Healthc. Mater. 2021, 10, 2001812.
- Heyes, J.; Hall, K.; Tailor, V.; Lenz, R.; MacLachlan, I. Synthesis and Characterization of Novel Poly(Ethylene Glycol)-Lipid Conjugates Suitable for Use in Drug Delivery. J. Control. Release 2006, 112, 280–290.
- Mui, B.L.; Tam, Y.K.; Jayaraman, M.; Ansell, S.M.; Du, X.; Tam, Y.Y.; Lin, P.J.; Chen, S.; Narayanannair, J.K.; Rajeev, K.G.; et al. Influence of Polyethylene Glycol Lipid Desorption Rates on Pharmacokinetics and Pharmacodynamics of SiRNA Lipid Nanoparticles. Mol. Ther. Nucleic Acids 2013, 2, e139.
- Fang, Y.; Xue, J.; Gao, S.; Lu, A.; Yang, D.; Jiang, H.; He, Y.; Shi, K. Cleavable PEGylation: A Strategy for Overcoming the “PEG Dilemma” in Efficient Drug Delivery. Drug Deliv. 2017, 24, 22–32.
- Leung, A.K.K.; Tam, Y.Y.C.; Cullis, P.R. Lipid Nanoparticles for Short Interfering RNA Delivery. Adv. Genet. 2014, 88, 71–110.
- Paunovska, K.; Da Silva Sanchez, A.J.; Sago, C.D.; Gan, Z.; Lokugamage, M.P.; Islam, F.Z.; Kalathoor, S.; Krupczak, B.R.; Dahlman, J.E. Nanoparticles Containing Oxidized Cholesterol Deliver MRNA to the Liver Microenvironment at Clinically Relevant Doses. Adv. Mater. 2019, 31, 1807748.
- Patel, S.; Ashwanikumar, N.; Robinson, E.; Xia, Y.; Mihai, C.; Griffith, J.P.; Hou, S.; Esposito, A.A.; Ketova, T.; Welsher, K.; et al. Naturally-Occurring Cholesterol Analogues in Lipid Nanoparticles Induce Polymorphic Shape and Enhance Intracellular Delivery of MRNA. Nat. Commun. 2020, 11, 983.
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a Strategy for Improving Nanoparticle-Based Drug and Gene Delivery. Adv. Drug Deliv. Rev. 2016, 99, 28.
More
