You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Rita Xu and Version 1 by Yuan Wang.
The “unprotected” metal and alloy nanoclusters (UMCs) prepared by the alkaline ethylene glycol method, which are stabilized with simple ions and solvent molecules, have the advantages of a small particle size, a narrow size distribution, good stability, highly efficient preparation, easy separation, surface modification and transfer between different phases. They can be composited with diverse materials to prepare catalytic systems with controllable structures, providing an effective means of studying the different factors’ effects on the catalytic properties separately. UMCs have been widely used in the development of high-performance catalysts for a variety of functional systems.
- metal nanocluster
- metal catalyst
- unprotected metal nanoclusters
- structure-property principle
- charge distribution state
- metal nanocluster atom spacing
- carbon dioxide conversion
- oxygen reduction reaction
- surfactant-free metal nanocluster
- structurally contro
1. Introduction
Metal nanoclusters refer to metal and alloy nanoparticles with a small size and a narrow size distribution. They are important elements of functional systems such as catalysts and sensors, playing important roles in the chemical industry, the development and utilization of sustainable energy, environmental protection, and basic research on catalysis science [1,2,3,4,5,6][1][2][3][4][5][6]. Many factors may influence the catalytic properties of metal nanocluster catalysts [7,8,9][7][8][9]. In the field of metal catalysts, it has long been a challenge to analyze the individual effects of the metal particle’s size, composition, support and modifier on the catalytic properties. This is related to another challenge in the field, namely the preparation of metal catalysts with controllable structures. In the traditional preparation methods of metal catalysts, such as the impregnation method, the abovementioned factors are often coupled with each other. To solve these problems, wresearchers proposed a solution, which is to use “unprotected” metal and alloy nanoclusters as building blocks to assemble or synthesize structurally controllable catalysts [10,11,12,13,14,15,16,17,18,19,20,21,22,23][10][11][12][13][14][15][16][17][18][19][20][21][22][23].
In 2000 [10], wresearchers reported the first “unprotected” or surfactant-free platinum group metal nanoclusters (Pt, Rh and Ru) with small particle sizes (dav = 1–3 nm) which were stabilized with adsorbed simple ions such as OH−, Cl−, etc. and solvent molecules, and they were prepared by a so-called alkaline ethylene glycol method (alkaline EG method). Many unprotected colloidal metal nanoclusters of the Pt group elements and their alloys (Pt, Ru, Rh [10[10][24],24], Ir [19,25][19][25], Os, PtRh [13] and PtRu [20[20][26],26], etc.) can be prepared by using this method.
Recently, wresearchers succeeded in the preparation of unprotected PtCu alloy nanoclusters [21]. This is an important addition to the family of unprotected alloy nanoclusters because there is a strong electronic interaction between the light transition metals and the Pt group metals, which is conducive to the regulation of the catalytic properties.
The unprotected metal nanoclusters prepared by using the alkaline EG method have the advantages of a small particle size, a narrow size distribution, good stability and highly efficient preparation. They can be separated from the preparation system by adding an aqueous solution of HCl to form a precipitate of the metal nanoclusters that could be re-dispersed in many organic solvents such as alcohol, ketone, THF, DMF and DMSO to form stable colloidal solutions without an obvious change in the metal particles’ size. The average particle size of the unprotected metal nanoclusters could be easily tuned within the range from 1.1 to 2.4 by changing the preparation conditions including the metal concentration and water content in the alkaline EG method [10]. Metal nanoclusters obtained by achieving the surface modification of the unprotected metal nanoclusters with an organic ligand such as PPh3, fatty amine or PVP could be transferred into some organic solvents or water to form stable colloidal solutions [10,27,28,29,30][10][27][28][29][30]. These special properties make them suitable for the development of many catalysts with unique properties.
Attenuated total reflectance infrared spectroscopy (ATR-IR) was used to study the species adsorbed onto the Pt and Ru unprotected metal nanoclusters prepared by using the alkaline EG method, which had been exposed to air. It was found that a small amount of CO was adsorbed on the surface of the as-prepared metal nanoclusters, and the signal intensity of the adsorbed CO in the IR spectrum of a colloidal solution of Pt nanoclusters in EG increased with the duration of the exposure to air. After being treated with an aqueous solution of HCl, the amount of CO adsorbed onto the Pt nanocluster increased obviously [24]. These experimental phenomena suggest that Pt and Ru nanoclusters could catalyze the oxidation of ethylene glycol by oxygen to produce CO adsorbed onto the unprotected Pt and Ru nanoclusters at room temperature, and the catalytic oxidation rate under an acidic condition is much faster than that under a basic condition.
Recently, Kunz and co-workers found that after drying, OH−-stabilized Pt nanoclusters without adsorbed CO, which were obtained by using a phase transfer method, could be dispersed in ethylene glycol to form a stable colloid solution without a change in the metal particle’s size [31]. This is an important property, because it enables unprotected metal nanoclusters to be preserved as a solid powder for a long time.
By depositing the unprotected metal and alloy nanoclusters onto different supports, and removing the adsorbed species that are different from the usual protective agents such as polymer, surfactant or strong organic ligand [32,33,34][32][33][34] by washing or oxidation under mild conditions, one can make the metal nanoclusters touch the surface of different supports to prepare different catalysts having the same size or composition of metal nanoclusters. This provides favorable conditions for separately studying the influence of each of the aforementioned factors on the catalytic properties. In the past two decades, this strategy has been widely applied and developed, and important achievements have been made in the preparation of structurally controllable catalysts and the exploration of the relationship between structure and catalytic performance [35,36,37,38,39,40,41,42,43,44,45,46][35][36][37][38][39][40][41][42][43][44][45][46]. Based on the unprotected metal and alloy nanoclusters, catalysts with a high activity level, high selectivity and excellent stability for a variety of important chemical reactions have been developed [14,16,22,47,48,49,50,51,52,53,54,55][14][16][22][47][48][49][50][51][52][53][54][55].
2. Applications of “Unprotected” Metal Nanoclusters
2.1. Application in Catalysis
2.1.1. Exploring the Structure–Function Relationship of Metal Nanocluster Catalysts
It is well known that the composition, size and surface structure of metal nanoclusters, as well as the environment surrounding the metal nanoclusters, have significant influences on the electronic and geometric structures of the metal nanoclusters, which determine the catalytic activity, selectivity and stability of the metal nanocluster-based catalysts [108,109,110,111][56][57][58][59]. Unprotected metal nanoclusters provide tractable tools for experimentally studying the effects of individual factors such as the metal composition, size, support, and the surface modification groups on the catalytic properties of metal nanocluster catalysts by assembling structure-controllable catalysts with unprotected metal or alloy nanoclusters that act as the building blocks [21,45,112,113,114,115][21][45][60][61][62][63]. A theoretical calculation based on the experimental results could further deepen the understanding of the relationship between the structure and performance of the catalytic sites of metal nanocluster catalysts [55,116][55][64]. Based on the relevant experimental and theoretical research results, described in this section, in this paper, weresearchers propose a principle of the influence of carriers, ligands and modifiers in metal nanocluster catalysts on the catalytic properties. The main points of the principle are as follows: Chemical bonding or electron transfer between carriers, ligands or modifiers and metal nanoclusters can alter the distribution of electrons (or charge) and the metal atom spacing of the metal nanoclusters in the catalysts. The changes have the following characteristics:- (1)
-
They change the extent of charge separation between the surface atomic layer and core of the metal nanocluster. The electron donation from the support or ligand will increase the charge separation extent, leaving the surface atomic layer with a more negative charge.
- (2)
-
They change the charge distribution state of the metal nanocluster surface atomic layer at the atomic (or subatomic) scale and make it more uneven compared with that of the naked metal nanocluster.
- (3)
-
They change the distance between some of the metal atoms.
- (4)
-
The extent of the change in the charge distribution or atomic spacing is related to the size of the metal nanocluster. Usually, small-sized metal nanoclusters exhibit more significant changes.
- (5)
-
Catalytic sites composed of surface metal atoms with different charge states or atomic spacings exhibit different adsorption energies for a reactant and reaction energy barrier, showing different catalytic activities and product selectivities. The species in the support surface (the ion, vacancy or chemical group) or the ligand adjacent to the metal nanocluster can form complex (or synergistic) catalytic sites with metal nanocluster surface atoms, at which the adsorption energies of the reactants and chemical reaction energy barriers are influenced by coordination polarization or hydrogen bonding, resulting in a synergistic catalytic effect.
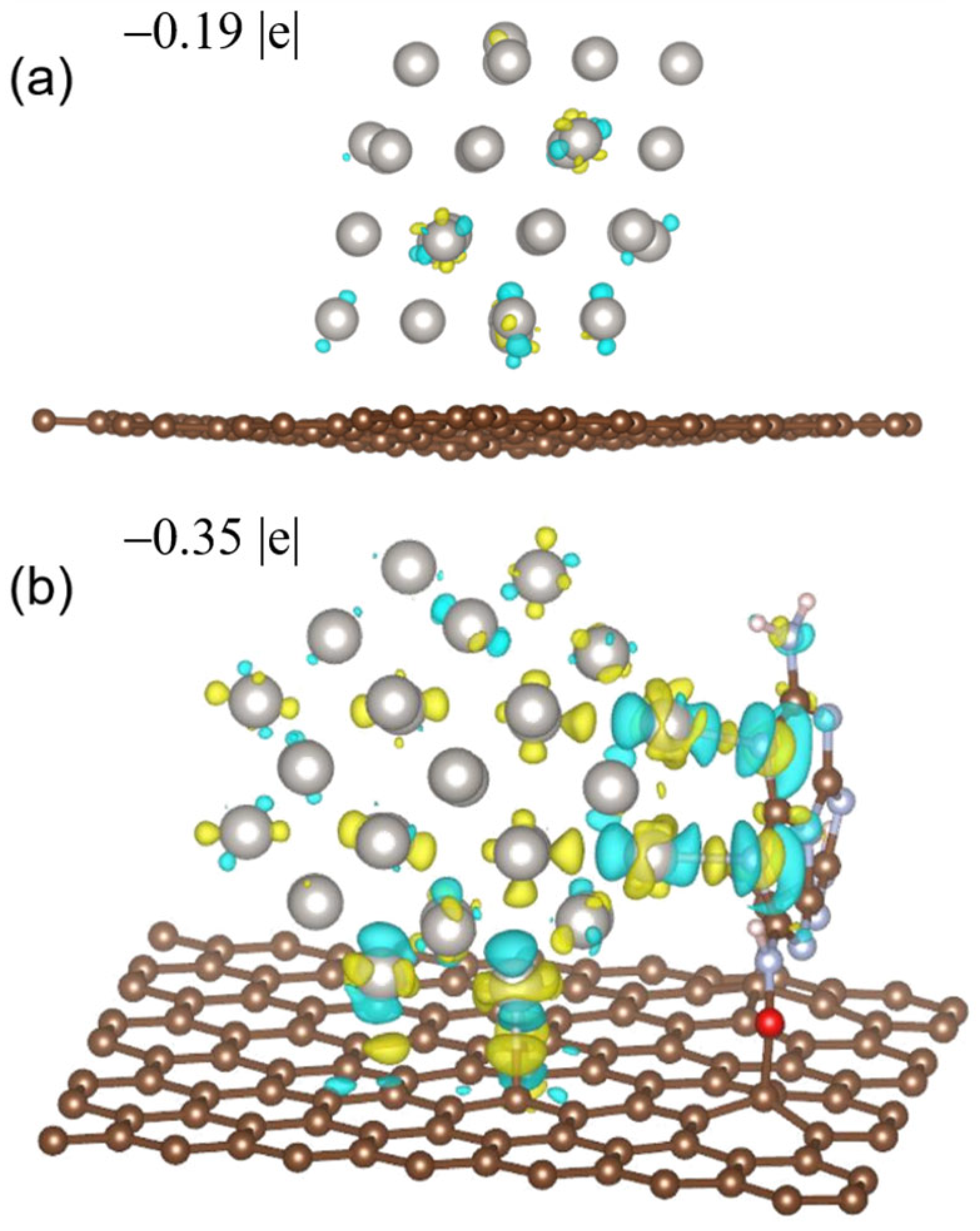 Figure 1. The charge density difference (isosurface unit = 4 × 10−3 e per Bohr3) and Bader charges of Pt40 NCs of (a) Pt40/C and (b) Pt40/MMC. The yellow and blue surface represent the charge increase and decrease, respectively. The Bader charges of the Pt atoms at the marked adsorption sites for OOH* and OH−* on Pt/C with an average value of −0.049 |e| are less than that on Pt/MMC (with an average value of −0.077 |e|). Reproduced with permission, Copyright 2022 Wiley-VCH GmbH [116][64].The high negative charge of the catalytic site weakens the adsorption of OH−*. On the other hand, the formation of a hydrogen bond between the intermediate OOH* and N atoms in MMC on some catalytic sites of Pt40/MMC increases the adsorption energy of OOH*. The obviously enhanced catalytic activity for ORR of Pt/MMC compared that of Pt/C is attributed to the decrease in OH−* adsorption energy and the increase in OOH* adsorption energy at some sites of Pt/MMC. The chemical bonds between the Pt nanoclusters and the MMC carriers explain the high durability of Pt/MMC. In 2004, wresearchers were the first to present that compared with the catalytic sites of the metal nanocluster themselves, the catalytic sites composed of metal nanocluster surface atoms and the adjacent surface oxygen vacancy of the metal oxide supports may exhibit much better catalytic properties [11], indicating a synergistic effect between the metal nanoclusters and adjacent surface oxygen vacancies. For a Ru/SnO2 catalyst assembled with unprotected Ru nanoclusters and tin oxide colloidal nanoparticles, with an average Ru particle size of 1.3 nm, the average catalytic activity (4.3 × 10−2 molo-CNB mol−1Ru s−1) for the selective hydrogenation of ortho-chloronitrobenzene (o-CNB) to ortho-chloroaniline (o-CAN) was much higher than that of the PVP-protected Ru nanoclusters prepared with the same unprotected Ru nanoclusters (6.9 × 10−3 molo-CNB mol−1Ru s−1). It was proposed that the oxygen vacancies or coordination-unsaturated Sn4+ or Sn2+ species at the support surface surrounding the Ru metal nanoclusters may activate the polar NO2 groups of o-CNB and coordinate with the NH2 groups of the produced o-CAN molecules, thereby significantly promoting the hydrogenation of o-CNB and depressing the dehalogenation of o-CAN. The selectivity for o-CAN of Ru/SnO2 reached 99.9%, which is much higher than that of the Ru/SiO2 prepared with the same unprotected Ru nanoclusters (95%) after complete o-CNB conversion, which is due to the hydrodechlorination rate being much lower for Ru/SnO2. Soon after, this concept was successfully applied to develop highly efficient catalysts for the selective hydrogenation of halonitrobenzenes to corresponding haloanilines by depositing unprotected Pt metal nanoclusters onto iron oxide supports [12,16,19][12][16][19]. It was found that during the activation of the catalysts, Pt nanoclusters could catalyze the partial reduction of iron oxide, thus forming oxygen vacancies surrounding the Pt nanoclusters. The partially reduced iron-oxide supported Pt nanocluster catalyst showed much high catalytic activity and selectivity for o-CAN than the Pt/C catalyst with the same size of Pt nanoclusters did, and the catalytic dehalogenation side reaction of was completely suppressed at 100% conversion of the substrates over the Pt/iron oxide catalysts. The interaction between the Pt nanoclusters and the surface oxygen vacancies resulted in an increase in the electron binding energy of Pt 4f7/2 in the partially reduced Pt/γ-Fe2O3 (Pt/γ-Fe2O3-PR), which is 0.5 eV higher than that of the same sized Pt nanoclusters protected by PVP, as measured using an in situ XPS spectrometer, indicating the electron transfer from Pt nanoclusters to iron cations in the catalyst [16]. The high selectivity for haloanilines in the hydrogenation of halonitrobenzenes was attributed to the electron-deficient state of the Pt nanoclusters supported on the partially reduced iron oxide supports, which may have weakened the extent of electron feedback from the Pt particles to the aromatic ring in o-CAN and suppressed the hydrodechlorination of the haloanilines. Pt/γ-Fe2O3, Pt/α-Fe2O3, Ir/γ-Fe2O3, Rh/γ-Fe2O3 and Ru/γ-Fe2O3 were prepared by depositing the corresponding unprotected platinum group metal (PGM) NCs onto the iron oxide supports and treating the solid products with H2 at 373 K. A universal rule was revealed for the first time through IR-CO probe and CO chemisorption measurements on these samples, namely, the PGM NCs supported on partially reduced iron oxides have an extremely weak affinity for CO [16,19][16][19]. This should be derived from the electron transfer from PGMs NCs to the surface oxygen vacancies of the iron oxides and weaken the degree of electron back-donation from the nanocluster surface atoms to CO. This discovery is very helpful for understanding the unique catalytic properties of these catalysts in many reactions. WResearchers succeeded in the preparation of unprotected PtCu alloy nanoclusters with solid solution structures and controllable Pt-to-Cu ratio (1:3-9:1) using a modified alkaline glycol method by introducing acetate ions into the reaction system [21,118][21][65]. MMC-supported alloy nanocluster catalysts (PtCu/MMC) with an average diameter of ca. 2 nm and different Pt/Cu ratios were prepared by assembling the PtCu alloy nanoclusters and MMC, which were used as catalysts for ORR. The alloy catalyst with a Pt/Cu ratio of 3:1 showed not only the highest MA (1.59A mg−1Pt @0.9 V), but it also showed the highest specific activity (SA) (3.98 mA cm−2Pt @0.9 V) among the tested catalysts. As the particle sizes of the alloy nanoclusters in the catalysts are almost the same, the effect of the Pt/Cu ratio on the activity should be mainly derived from the electronic properties of the nanoclusters. As revealed by the in situ XPS measurements, the electron transfer from Cu to Pt occurred in the alloy nanoclusters, which may be the reason why PtCu alloy catalysts exhibit enhanced catalytic activity for ORR compared to that of Pt/MMC. From a further comparison of the catalytic activation of Pt3Cu1/MMC, Pt3Cu1/C and Pt/C, it was found that the alloying effect increased the MA by 1.4 times, while the promoting effect of MMC increased the MA by 3.2 times. WResearchers prepared a PtRu/NCNHs composite using N-doped carbon nanohorns (NCNHs) and unprotected PtRu NCs with an average PtRu particle size of 1.9 nm, which was used as a catalyst for the electrochemical oxidation of methanol [20]. The MA of PtRu/NCNHs (850 mA mg−1PtRu @0.9 V) was 2.5 and 1.7 times larger than those of a commercial PtRu/C catalyst and a homemade PtRu/Vulcan carbon catalyst, respectively. The SA of PtRu/NCNHs (1.35 mA cm−2PtRu @0.9 V) was 1.8 times larger than that of PtRu/Vulcan carbon. The TEM, ICP and XPS results showed that the three catalysts had similar PtRu particle sizes and Pt/Ru atomic ratios. The Pt 4f and Ru 3p electron binding energies of PtRu/NCNHs had a negative shift of 0.2 eV compared with the corresponding values of PtRu/Vulcan, which was attributed to metal–support interactions, indicating the electron transfer from NCNHs to PtRu NCs in the PtRu/NCNHs catalyst. Based on the proposed principle described above, the improved catalytic activity for the oxidation of CH3OH and carbonaceous intermediates of PtRu/NCNHs compared to that of PtRu/Vulcan carbon might be derived from the unique structure of complex catalytic sites in the catalyst. In order to reveal the ligand effects, wresearchers investigated the effect of the organic ligands on the Pt 4f electron binding energies of small Pt nanoclusters [27]. The same sized Pt nanoclusters (dav = 1.3 nm, size distribution of between 0.8 and 2.8 nm) modified with C12H25NH2, C12H25SH, PPh3, polyvinylpyrrolidone (PVP) or polyvinyl alcohol (PVA) were prepared by the surface modification of the unprotected Pt nanoclusters. The 4f7/2 level electron binding energies of Pt in the prepared C12H25NH2-, PVP-, PVA-, PPh3- and C12H25SH-protected Pt nanoclusters increased by 0.5, 0.5, 0.5, 0.6 and 0.8 eV than that of bulk Pt, respectively, as measured by XPS. Since the weak interaction between the PVA and Pt nanoclusters cannot affect the core-level electron binding energy of the Pt core to an observable extent, the increment in the Pt 4f binding energy of the PVA-protected Pt nanocluster was mainly derived from the metal particle size effect, originating from the final state relaxation [27]. The surface modification of the Pt nanoclusters with C12H25SH caused a further increase in the Pt 4f7/2 electron binding energies of 0.3 eV, which is mainly caused by the formation of the Pt–S bond and the coordination of mercaptan groups on the surface Pt atoms. Although the measured Pt 4f electron binding energies of the Pt nanoclusters protected by C12H25NH2, PVP, PVA or PPh3 are very similar to each other, the charge distribution of these metal nanoclusters should be quite different since the intensities of electronic interaction between these ligands and Pt nanoclusters are different. Recently, the catalytic activities of these protected Pt nanoclusters for the hydrogenation of para-chloronitrobenzene (p-CNB) were measured [45]. The initial catalytic activities of the C12H25SH-, PPh3-, C18H37NH2- or PVP-modified Pt nanoclusters with the same sized Pt nanoclusters were 4.7, 4.1, 3.1 and 1.6 molhydrogen (molPt S)−1, respectively, and the selectivity for the byproduct aniline at a p-CNB conversion of about 40% followed the trend: Pt–PPh3 > Pt–C18H37NH2 > Pt–PVP > Pt–C12H25SH. WResearchers believe that the activity and selectivity of these protected Pt NCs are related to the different charge distribution of the metal NCs. The size effects of Pt nanoclusters in Pt/Fe2O3 catalysts for the CO oxidation at room-temperature were investigated by Zhang et al. [131][66]. The Pt/Fe2O3-a, Pt/Fe2O3-b and Pt/Fe2O3-c catalysts were prepared by depositing unprotected Pt nanoclusters with mean diameters of 1.1, 1.9 and 2.7 nm on the surface of Fe(OH)3 powders, respectively, followed by high-temperature calcination in a flow of 20% O2/Ar. Unprotected Pt nanoclusters colloids with different average particle sizes were prepared by changing the metal concentration or water content in the alkaline EG method [10,131][10][66]. The catalytic tests on the oxidation of CO to CO2 at a low temperature showed that the Pt/Fe2O3-b catalyst exhibited the highest activity among the tested catalysts, with a 42% conversion of CO at room temperature and a complete conversion of CO at around 60 ℃. For Pt/Fe2O3-a and Pt/Fe2O3-c, the CO conversion rates were 20% and 8% at room temperature, while the complete conversion of CO was realized at 70 and 90 ℃, respectively. The XPS and XANES measurement results indicate that the Pt nanocluster size affected the Pt species chemical states in the Pt/Fe2O3 catalysts. The Pt atoms in Pt/Fe2O3-a had been largely oxidized to Pt2+, while the majority of the Pt atoms in Pt/Fe2O3-b and Pt/Fe2O3-c were in the Pt0 oxidation state, with Pt/Fe2O3-c containing the most Pt atoms in a metallic state.
Figure 1. The charge density difference (isosurface unit = 4 × 10−3 e per Bohr3) and Bader charges of Pt40 NCs of (a) Pt40/C and (b) Pt40/MMC. The yellow and blue surface represent the charge increase and decrease, respectively. The Bader charges of the Pt atoms at the marked adsorption sites for OOH* and OH−* on Pt/C with an average value of −0.049 |e| are less than that on Pt/MMC (with an average value of −0.077 |e|). Reproduced with permission, Copyright 2022 Wiley-VCH GmbH [116][64].The high negative charge of the catalytic site weakens the adsorption of OH−*. On the other hand, the formation of a hydrogen bond between the intermediate OOH* and N atoms in MMC on some catalytic sites of Pt40/MMC increases the adsorption energy of OOH*. The obviously enhanced catalytic activity for ORR of Pt/MMC compared that of Pt/C is attributed to the decrease in OH−* adsorption energy and the increase in OOH* adsorption energy at some sites of Pt/MMC. The chemical bonds between the Pt nanoclusters and the MMC carriers explain the high durability of Pt/MMC. In 2004, wresearchers were the first to present that compared with the catalytic sites of the metal nanocluster themselves, the catalytic sites composed of metal nanocluster surface atoms and the adjacent surface oxygen vacancy of the metal oxide supports may exhibit much better catalytic properties [11], indicating a synergistic effect between the metal nanoclusters and adjacent surface oxygen vacancies. For a Ru/SnO2 catalyst assembled with unprotected Ru nanoclusters and tin oxide colloidal nanoparticles, with an average Ru particle size of 1.3 nm, the average catalytic activity (4.3 × 10−2 molo-CNB mol−1Ru s−1) for the selective hydrogenation of ortho-chloronitrobenzene (o-CNB) to ortho-chloroaniline (o-CAN) was much higher than that of the PVP-protected Ru nanoclusters prepared with the same unprotected Ru nanoclusters (6.9 × 10−3 molo-CNB mol−1Ru s−1). It was proposed that the oxygen vacancies or coordination-unsaturated Sn4+ or Sn2+ species at the support surface surrounding the Ru metal nanoclusters may activate the polar NO2 groups of o-CNB and coordinate with the NH2 groups of the produced o-CAN molecules, thereby significantly promoting the hydrogenation of o-CNB and depressing the dehalogenation of o-CAN. The selectivity for o-CAN of Ru/SnO2 reached 99.9%, which is much higher than that of the Ru/SiO2 prepared with the same unprotected Ru nanoclusters (95%) after complete o-CNB conversion, which is due to the hydrodechlorination rate being much lower for Ru/SnO2. Soon after, this concept was successfully applied to develop highly efficient catalysts for the selective hydrogenation of halonitrobenzenes to corresponding haloanilines by depositing unprotected Pt metal nanoclusters onto iron oxide supports [12,16,19][12][16][19]. It was found that during the activation of the catalysts, Pt nanoclusters could catalyze the partial reduction of iron oxide, thus forming oxygen vacancies surrounding the Pt nanoclusters. The partially reduced iron-oxide supported Pt nanocluster catalyst showed much high catalytic activity and selectivity for o-CAN than the Pt/C catalyst with the same size of Pt nanoclusters did, and the catalytic dehalogenation side reaction of was completely suppressed at 100% conversion of the substrates over the Pt/iron oxide catalysts. The interaction between the Pt nanoclusters and the surface oxygen vacancies resulted in an increase in the electron binding energy of Pt 4f7/2 in the partially reduced Pt/γ-Fe2O3 (Pt/γ-Fe2O3-PR), which is 0.5 eV higher than that of the same sized Pt nanoclusters protected by PVP, as measured using an in situ XPS spectrometer, indicating the electron transfer from Pt nanoclusters to iron cations in the catalyst [16]. The high selectivity for haloanilines in the hydrogenation of halonitrobenzenes was attributed to the electron-deficient state of the Pt nanoclusters supported on the partially reduced iron oxide supports, which may have weakened the extent of electron feedback from the Pt particles to the aromatic ring in o-CAN and suppressed the hydrodechlorination of the haloanilines. Pt/γ-Fe2O3, Pt/α-Fe2O3, Ir/γ-Fe2O3, Rh/γ-Fe2O3 and Ru/γ-Fe2O3 were prepared by depositing the corresponding unprotected platinum group metal (PGM) NCs onto the iron oxide supports and treating the solid products with H2 at 373 K. A universal rule was revealed for the first time through IR-CO probe and CO chemisorption measurements on these samples, namely, the PGM NCs supported on partially reduced iron oxides have an extremely weak affinity for CO [16,19][16][19]. This should be derived from the electron transfer from PGMs NCs to the surface oxygen vacancies of the iron oxides and weaken the degree of electron back-donation from the nanocluster surface atoms to CO. This discovery is very helpful for understanding the unique catalytic properties of these catalysts in many reactions. WResearchers succeeded in the preparation of unprotected PtCu alloy nanoclusters with solid solution structures and controllable Pt-to-Cu ratio (1:3-9:1) using a modified alkaline glycol method by introducing acetate ions into the reaction system [21,118][21][65]. MMC-supported alloy nanocluster catalysts (PtCu/MMC) with an average diameter of ca. 2 nm and different Pt/Cu ratios were prepared by assembling the PtCu alloy nanoclusters and MMC, which were used as catalysts for ORR. The alloy catalyst with a Pt/Cu ratio of 3:1 showed not only the highest MA (1.59A mg−1Pt @0.9 V), but it also showed the highest specific activity (SA) (3.98 mA cm−2Pt @0.9 V) among the tested catalysts. As the particle sizes of the alloy nanoclusters in the catalysts are almost the same, the effect of the Pt/Cu ratio on the activity should be mainly derived from the electronic properties of the nanoclusters. As revealed by the in situ XPS measurements, the electron transfer from Cu to Pt occurred in the alloy nanoclusters, which may be the reason why PtCu alloy catalysts exhibit enhanced catalytic activity for ORR compared to that of Pt/MMC. From a further comparison of the catalytic activation of Pt3Cu1/MMC, Pt3Cu1/C and Pt/C, it was found that the alloying effect increased the MA by 1.4 times, while the promoting effect of MMC increased the MA by 3.2 times. WResearchers prepared a PtRu/NCNHs composite using N-doped carbon nanohorns (NCNHs) and unprotected PtRu NCs with an average PtRu particle size of 1.9 nm, which was used as a catalyst for the electrochemical oxidation of methanol [20]. The MA of PtRu/NCNHs (850 mA mg−1PtRu @0.9 V) was 2.5 and 1.7 times larger than those of a commercial PtRu/C catalyst and a homemade PtRu/Vulcan carbon catalyst, respectively. The SA of PtRu/NCNHs (1.35 mA cm−2PtRu @0.9 V) was 1.8 times larger than that of PtRu/Vulcan carbon. The TEM, ICP and XPS results showed that the three catalysts had similar PtRu particle sizes and Pt/Ru atomic ratios. The Pt 4f and Ru 3p electron binding energies of PtRu/NCNHs had a negative shift of 0.2 eV compared with the corresponding values of PtRu/Vulcan, which was attributed to metal–support interactions, indicating the electron transfer from NCNHs to PtRu NCs in the PtRu/NCNHs catalyst. Based on the proposed principle described above, the improved catalytic activity for the oxidation of CH3OH and carbonaceous intermediates of PtRu/NCNHs compared to that of PtRu/Vulcan carbon might be derived from the unique structure of complex catalytic sites in the catalyst. In order to reveal the ligand effects, wresearchers investigated the effect of the organic ligands on the Pt 4f electron binding energies of small Pt nanoclusters [27]. The same sized Pt nanoclusters (dav = 1.3 nm, size distribution of between 0.8 and 2.8 nm) modified with C12H25NH2, C12H25SH, PPh3, polyvinylpyrrolidone (PVP) or polyvinyl alcohol (PVA) were prepared by the surface modification of the unprotected Pt nanoclusters. The 4f7/2 level electron binding energies of Pt in the prepared C12H25NH2-, PVP-, PVA-, PPh3- and C12H25SH-protected Pt nanoclusters increased by 0.5, 0.5, 0.5, 0.6 and 0.8 eV than that of bulk Pt, respectively, as measured by XPS. Since the weak interaction between the PVA and Pt nanoclusters cannot affect the core-level electron binding energy of the Pt core to an observable extent, the increment in the Pt 4f binding energy of the PVA-protected Pt nanocluster was mainly derived from the metal particle size effect, originating from the final state relaxation [27]. The surface modification of the Pt nanoclusters with C12H25SH caused a further increase in the Pt 4f7/2 electron binding energies of 0.3 eV, which is mainly caused by the formation of the Pt–S bond and the coordination of mercaptan groups on the surface Pt atoms. Although the measured Pt 4f electron binding energies of the Pt nanoclusters protected by C12H25NH2, PVP, PVA or PPh3 are very similar to each other, the charge distribution of these metal nanoclusters should be quite different since the intensities of electronic interaction between these ligands and Pt nanoclusters are different. Recently, the catalytic activities of these protected Pt nanoclusters for the hydrogenation of para-chloronitrobenzene (p-CNB) were measured [45]. The initial catalytic activities of the C12H25SH-, PPh3-, C18H37NH2- or PVP-modified Pt nanoclusters with the same sized Pt nanoclusters were 4.7, 4.1, 3.1 and 1.6 molhydrogen (molPt S)−1, respectively, and the selectivity for the byproduct aniline at a p-CNB conversion of about 40% followed the trend: Pt–PPh3 > Pt–C18H37NH2 > Pt–PVP > Pt–C12H25SH. WResearchers believe that the activity and selectivity of these protected Pt NCs are related to the different charge distribution of the metal NCs. The size effects of Pt nanoclusters in Pt/Fe2O3 catalysts for the CO oxidation at room-temperature were investigated by Zhang et al. [131][66]. The Pt/Fe2O3-a, Pt/Fe2O3-b and Pt/Fe2O3-c catalysts were prepared by depositing unprotected Pt nanoclusters with mean diameters of 1.1, 1.9 and 2.7 nm on the surface of Fe(OH)3 powders, respectively, followed by high-temperature calcination in a flow of 20% O2/Ar. Unprotected Pt nanoclusters colloids with different average particle sizes were prepared by changing the metal concentration or water content in the alkaline EG method [10,131][10][66]. The catalytic tests on the oxidation of CO to CO2 at a low temperature showed that the Pt/Fe2O3-b catalyst exhibited the highest activity among the tested catalysts, with a 42% conversion of CO at room temperature and a complete conversion of CO at around 60 ℃. For Pt/Fe2O3-a and Pt/Fe2O3-c, the CO conversion rates were 20% and 8% at room temperature, while the complete conversion of CO was realized at 70 and 90 ℃, respectively. The XPS and XANES measurement results indicate that the Pt nanocluster size affected the Pt species chemical states in the Pt/Fe2O3 catalysts. The Pt atoms in Pt/Fe2O3-a had been largely oxidized to Pt2+, while the majority of the Pt atoms in Pt/Fe2O3-b and Pt/Fe2O3-c were in the Pt0 oxidation state, with Pt/Fe2O3-c containing the most Pt atoms in a metallic state.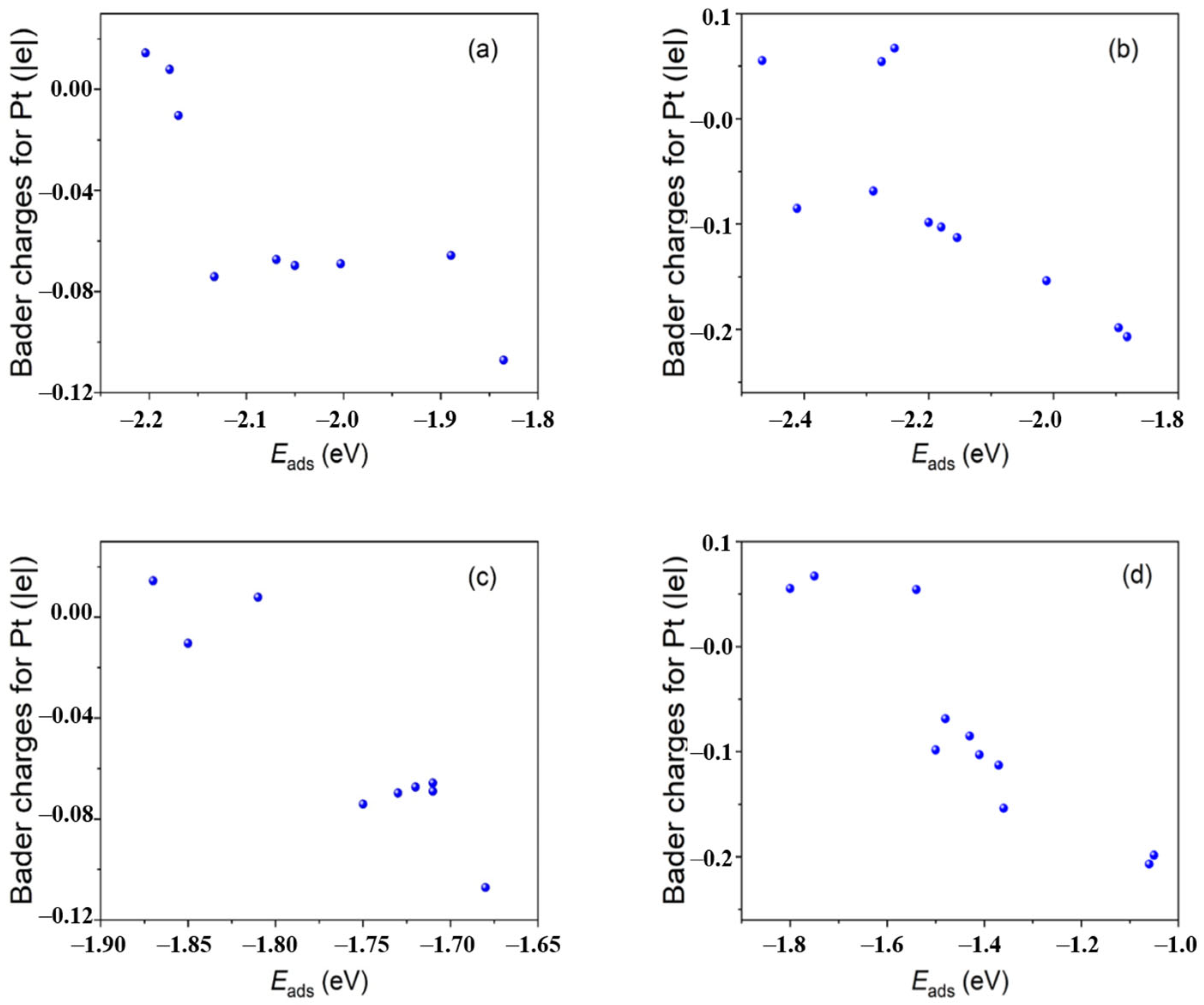 Figure 2. Plots of Bader charges of the representative adsorption site Pt atoms versus adsorption energies of OOH* on Pt40 NCs of Pt40/C (a) and Pt40/MMC (b); plots of Bader charges of the representative adsorption site Pt atoms versus adsorption energies of OH−* on Pt40 NCs of Pt40/C (c) and Pt40/MMC (d). Reproduced with permission, Copyright 2022 Wiley-VCH GmbH [116][64].
Figure 2. Plots of Bader charges of the representative adsorption site Pt atoms versus adsorption energies of OOH* on Pt40 NCs of Pt40/C (a) and Pt40/MMC (b); plots of Bader charges of the representative adsorption site Pt atoms versus adsorption energies of OH−* on Pt40 NCs of Pt40/C (c) and Pt40/MMC (d). Reproduced with permission, Copyright 2022 Wiley-VCH GmbH [116][64].2.1.2. Application in Fabrication of Smart Catalysts
The unprotected metal nanoclusters prepared based on the alkaline EG method have been widely applied in the fabrication of metal nanocluster-based catalysts with excellent catalytic properties [53,54,55,132,133,134,135,136,137,138,139,140,141][53][54][55][67][68][69][70][71][72][73][74][75][76]. Size- or shape-selective catalysts have important applications for the production of fine chemicals. Li and Yang et al. reported the first example of the encapsulation of unprotected Pt nanoclusters in zeolitic imidazolate frameworks (ZIFs) to prepare heterogeneous catalysts with high size selectivity for the substrates in the catalytic hydrogenation of alkenes [50]. In this preparation, 2-Methyl imidazole plays both the roles of the Pt NPs stabilizer and the conjunction linker of ZIF-8. The catalytic performance of the Pt@ZIF-8 catalysts prepared by this hetero-nucleation strategy was much better than that of the ZIF-8 encapsulated with PVP-protected Pt NPs. It would be expected that other polydentate ligands that are capable of capping the unprotected metal NPs could also be employed to fabricate various metal NPs@MOFs with the same strategy, providing an effective way to design and synthesize size- or shape-selective catalysts. Moreover, Li and Yang et al. reported an efficient approach for the encapsulation of the unprotected Pt nanoclusters into nanocages of cage-like mesoporous silicas (CMS) [142][77]. The prepared Pt/CMS catalysts exhibited high selectivity (>99%) for the corresponding CAN in the hydrogenation of CNB. The catalytic hydrogenation of carbon dioxide to produce multi-carbon compounds is of significance because it will make carbon dioxide an important carbon resource for the synthesis of sustainable energy sources or fine chemicals, thereby reducing the dependence on fossil fuels [143,144,145,146,147,148,149,150,151][78][79][80][81][82][83][84][85][86]. For most reported catalysts, the synthesis of multi-carbon compounds through CO2 hydrogenation usually needs to be carried out at high temperatures of 200–350 °C. The development of new catalytic systems to generate liquid fuels or highly valued fine chemicals by consuming CO2 under mild conditions would bring about many economic and environmental benefits. Recently, wresearchers reported a new method for the catalytic conversion of CO2 to multi-carbon compounds at low temperatures [53,152][53][87]. At 40–130 °C, in a catalyst of ferrous carbonate-supported Pt nanoclusters and Ru nanoclusters, the Pt and Ru nanoclusters can catalyze the hydrogenation of the carbonate ions to form high hydrocarbons and multi-carbon alcohols. The carbon atom number in the high hydrocarbons could be as high as 26. Coupling this process with the carbonation of the resulting metal species enables the catalytic conversion of CO2 to high hydrocarbons and multi-carbon alcohols [53]. The reaction equations are shown as follows: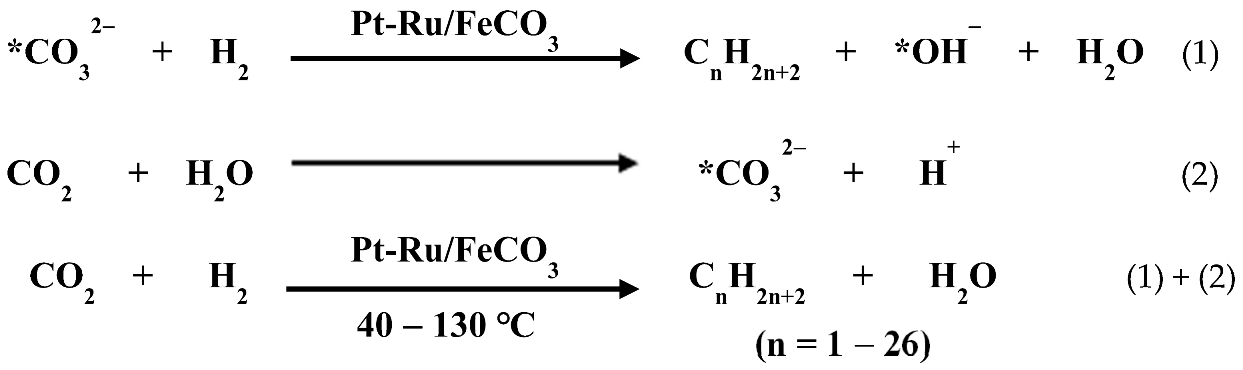 At 40 °C, the selectivities for C5–C26 hydrocarbons and C2+ compounds reached 49.6% and 77.7%, respectively, while at 130 °C, the selectivities for them were 26.1% and 45.8%, respectively, while for the conversion of carbon in the substrates (including FeCO3 and CO2) over 8 h, it was 5.1%. CO was not detected by gas chromatography (GC) in the products. The experimental results indicated that the simultaneous presence of platinum and ruthenium in the catalyst is beneficial to improve the selectivity of multi-carbon compounds. This encouraged uresearchers to improve the selectivity of multi-carbon compounds in the products by regulating the distribution of Pt and Ru elements in the catalyst. Pt nanocrystals anchoring small Ru clusters on carbon (Ru-co-Pt/C) [55] were prepared using unprotected Pt and Ru nanoclusters as starting materials through the Pt nanocluster catalyzing atomization of Ru nanoclusters and a surface growth process at 130 °C in a mixture of water and cyclohexane in a gaseous mixture of CO2 and H2 and characterized by HRTEM, EDX, EXAFS and XPS. Carbon-supported Pt or Ru nanoclusters were prepared by the immobilization of the Pt or Ru nanoclusters on a carbon support, and these were used as catalysts for comparison. At 130 °C, in a mixture of water and cyclohexane, the Ru/C catalyst exhibited a high activity for CO2 hydrogenation with a selectivity for methane of 94.3%, while the catalytic activity of the Pt/C catalyst was quite low under this condition. Ru-co-Pt/C could catalyze CO2 hydrogenation to produce hydrocarbons and alcohols with a selectivity of 90.1% for the C2+ compounds, far exceeding the previously reported values, which suggests that CO2 hydrogenation of Ru-co-Pt/C tends to produce multi-carbon compounds. The yield of organic hydrogenation products after 22 h of reaction reached 8.9% of Ru-co-Pt/C under this condition. Density functional theory (DFT) calculations were performed to study the origin of the significant increase in multi-carbon product selectivity in the CO2 hydrogenation products of the bimetallic catalyst compared to those of the Ru/C and Pt/C catalysts. The established model clusters used to simulate the three catalysts contain 50 metal atoms (Figure 3), and the bimetallic cluster has 3 Ru dimers and 2 individual Ru atoms anchored on the NC surface.
At 40 °C, the selectivities for C5–C26 hydrocarbons and C2+ compounds reached 49.6% and 77.7%, respectively, while at 130 °C, the selectivities for them were 26.1% and 45.8%, respectively, while for the conversion of carbon in the substrates (including FeCO3 and CO2) over 8 h, it was 5.1%. CO was not detected by gas chromatography (GC) in the products. The experimental results indicated that the simultaneous presence of platinum and ruthenium in the catalyst is beneficial to improve the selectivity of multi-carbon compounds. This encouraged uresearchers to improve the selectivity of multi-carbon compounds in the products by regulating the distribution of Pt and Ru elements in the catalyst. Pt nanocrystals anchoring small Ru clusters on carbon (Ru-co-Pt/C) [55] were prepared using unprotected Pt and Ru nanoclusters as starting materials through the Pt nanocluster catalyzing atomization of Ru nanoclusters and a surface growth process at 130 °C in a mixture of water and cyclohexane in a gaseous mixture of CO2 and H2 and characterized by HRTEM, EDX, EXAFS and XPS. Carbon-supported Pt or Ru nanoclusters were prepared by the immobilization of the Pt or Ru nanoclusters on a carbon support, and these were used as catalysts for comparison. At 130 °C, in a mixture of water and cyclohexane, the Ru/C catalyst exhibited a high activity for CO2 hydrogenation with a selectivity for methane of 94.3%, while the catalytic activity of the Pt/C catalyst was quite low under this condition. Ru-co-Pt/C could catalyze CO2 hydrogenation to produce hydrocarbons and alcohols with a selectivity of 90.1% for the C2+ compounds, far exceeding the previously reported values, which suggests that CO2 hydrogenation of Ru-co-Pt/C tends to produce multi-carbon compounds. The yield of organic hydrogenation products after 22 h of reaction reached 8.9% of Ru-co-Pt/C under this condition. Density functional theory (DFT) calculations were performed to study the origin of the significant increase in multi-carbon product selectivity in the CO2 hydrogenation products of the bimetallic catalyst compared to those of the Ru/C and Pt/C catalysts. The established model clusters used to simulate the three catalysts contain 50 metal atoms (Figure 3), and the bimetallic cluster has 3 Ru dimers and 2 individual Ru atoms anchored on the NC surface.
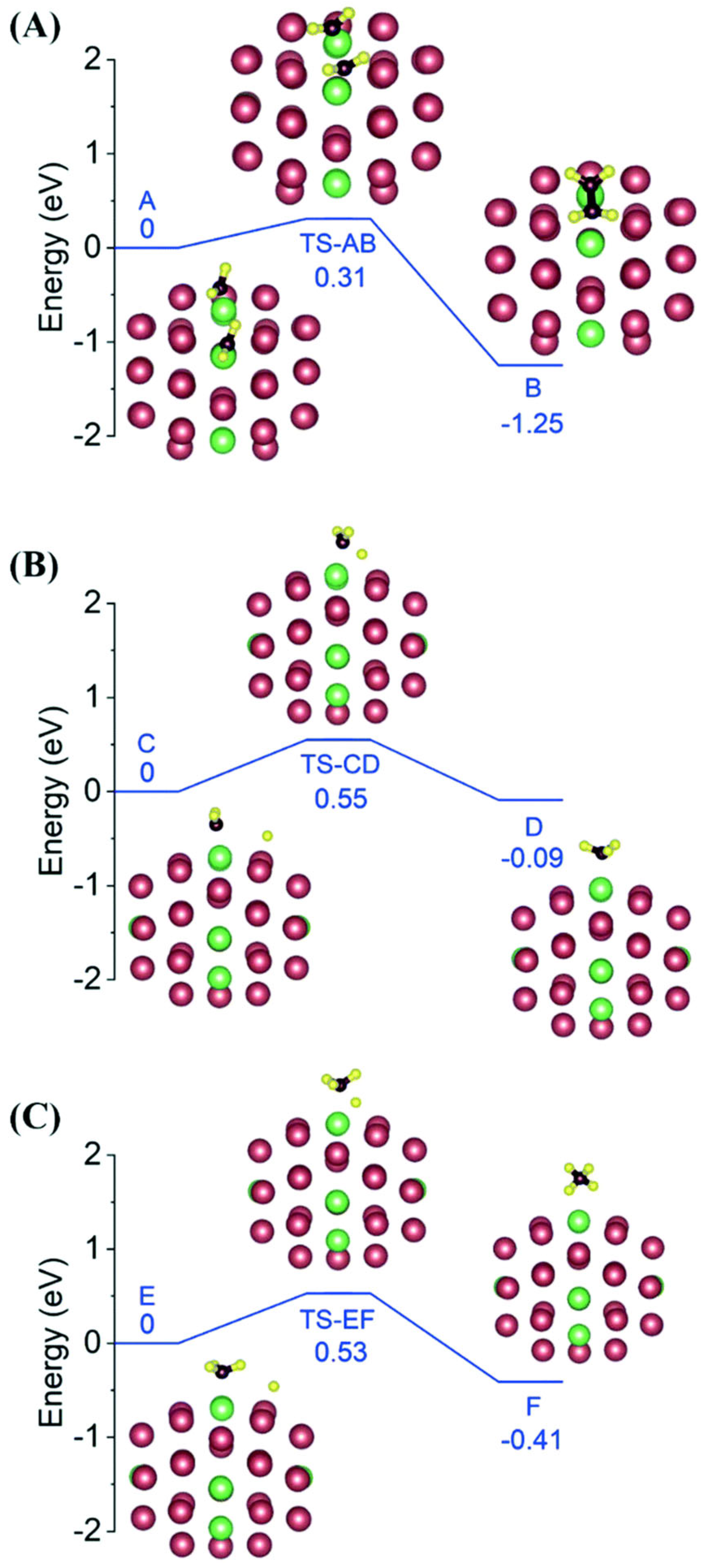 Figure 3. The optimized geometries of reactants, transition states and products for (A) CH2 + CH2 coupling, (B) CH2 hydrogenation and (C) CH3 hydrogenation on a Pt42-Ru8 bimetallic cluster, respectively. Reproduced with permission, Copyright 2022 The Royal Society of Chemistry [55].
Figure 3. The optimized geometries of reactants, transition states and products for (A) CH2 + CH2 coupling, (B) CH2 hydrogenation and (C) CH3 hydrogenation on a Pt42-Ru8 bimetallic cluster, respectively. Reproduced with permission, Copyright 2022 The Royal Society of Chemistry [55].
References
- Ferrando, R.; Jellinek, J.; Johnston, R.L. Nanoalloys: From theory to applications of alloy clusters and nanoparticles. Chem. Rev. 2008, 108, 845–910.
- Guo, S.J.; Wang, E.K. Noble metal nanomaterials: Controllable synthesis and application in fuel cells and analytical sensors. Nano Today 2011, 6, 240–264.
- Jin, R.C.; Zeng, C.J.; Zhou, M.; Chen, Y.X. Atomically Precise Colloidal Metal Nanoclusters and Nanoparticles: Fundamentals and Opportunities. Chem. Rev. 2016, 116, 10346–10413.
- Fievet, F.; Ammar-Merah, S.; Brayner, R.; Chau, F.; Giraud, M.; Mammeri, F.; Peron, J.; Piquemal, J.Y.; Sicard, L.; Viau, G. The polyol process: A unique method for easy access to metal nanoparticles with tailored sizes, shapes and compositions. Chem. Soc. Rev. 2018, 47, 5187–5233.
- Pan, M.F.; Yang, J.Y.; Liu, K.X.; Yin, Z.J.; Ma, T.Y.; Liu, S.M.; Xu, L.H.; Wang, S.O. Noble Metal Nanostructured Materials for Chemical and Biosensing Systems. Nanomaterials 2020, 10, 209.
- Lu, L.F.; Zheng, H.; Li, Y.X.; Zhou, Y.H.; Fang, B.Z. Ligand-free synthesis of noble metal nanocatalysts for electrocatalysis. Chem. Eng. J. 2023, 451, 138668.
- An, K.; Somorjai, G.A. Size and Shape Control of Metal Nanoparticles for Reaction Selectivity in Catalysis. ChemCatChem 2012, 4, 1512–1524.
- Schauermann, S.; Nilius, N.; Shaikhutdinov, S.; Freund, H.J. Nanoparticles for Heterogeneous Catalysis: New Mechanistic Insights. Acc. Chem. Res. 2013, 46, 1673–1681.
- Liu, L.C.; Corma, A. Metal Catalysts for Heterogeneous Catalysis: From Single Atoms to Nanoclusters and Nanoparticles. Chem. Rev. 2018, 118, 4981–5079.
- Wang, Y.; Ren, J.W.; Deng, K.; Gui, L.L.; Tang, Y.Q. Preparation of tractable platinum, rhodium, and ruthenium nanoclusters with small particle size in organic media. Chem. Mater. 2000, 12, 1622–1627.
- Zuo, B.J.; Wang, Y.; Wang, Q.L.; Zhang, J.L.; Wu, N.Z.; Peng, L.D.; Gui, L.L.; Wang, X.D.; Wang, R.M.; Yu, D.P. An efficient ruthenium catalyst for selective hydrogenation of ortho-chloronitrobenzene prepared via assembling ruthenium and tin oxide nanoparticles. J. Catal. 2004, 222, 493–498.
- Zhang, J.L.; Wang, Y.; Ji, H.; Wei, Y.G.; Wu, N.Z.; Zuo, B.J.; Wang, Q.L. Magnetic nanocomposite catalysts with high activity and selectivity for selective hydrogenation of ortho-chloronitrobenzene. J. Catal. 2005, 229, 114–118.
- Wang, Y.; Zhang, J.L.; Wang, X.D.; Ren, J.W.; Zuo, B.J.; Tang, Y.Q. Metal nanoclusters stabilized with simple ions and solvents—Promising building blocks for future catalysts. Top. Catal. 2005, 35, 35–41.
- Wang, X.D.; Liang, M.H.; Liu, H.Q.; Wang, Y. Selective hydrogenation of bromonitrobenzenes over Pt/γ-Fe2O3. J. Mol. Catal. A Chem. 2007, 273, 160–168.
- Wang, Y.; Wang, X.D. Metal Nanoclusters in Catalysis and Materials Science: The Issue of Size Control. In Solvent and Simple Ion-Stabilized Metal Nanoclusters: Chemical Synthesis and Application; Corain, B., Schmid, G., Toshima, N., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; p. 327.
- Liang, M.H.; Wang, X.D.; Liu, H.Q.; Liu, H.C.; Wang, Y. Excellent catalytic properties over nanocomposite catalysts for selective hydrogenation of halonitrobenzenes. J. Catal. 2008, 255, 335–342.
- Liu, Y.; Zheng, N.; Chao, W.; Liu, H.Q.; Wang, Y. A novel nanocomposite catalytic cathode for direct methanol fuel cells. Electrochim. Acta 2010, 55, 5617–5623.
- Zhang, L.W.; Zheng, N.; Gao, A.; Zhu, C.M.; Wang, Z.Y.; Wang, Y.; Shi, Z.J.; Liu, Y. A robust fuel cell cathode catalyst assembled with nitrogen-doped carbon nanohorn and platinum nanoclusters. J. Power Sources 2012, 220, 449–454.
- Xiao, C.; Liang, M.H.; Gao, A.; Xie, J.L.; Wang, Y.; Liu, H.C. Weak affinity for CO of platinum group metal nanoparticles supported on partially reduced iron oxides. J. Nanopart. Res. 2013, 15, 1822.
- Zhang, L.W.; Gao, A.; Liu, Y.; Wang, Y.; Ma, J.T. PtRu nanoparticles dispersed on nitrogen-doped carbon nanohorns as an efficient electrocatalyst for methanol oxidation reaction. Electrochim. Acta 2014, 132, 416–422.
- Liu, Y.; Chen, L.F.; Cheng, T.; Guo, H.Y.; Sun, B.; Wang, Y. Preparation and application in assembling high-performance fuel cell catalysts of colloidal PtCu alloy nanoclusters. J. Power Sources 2018, 395, 66–76.
- Yu, Y.L.; Huang, J.; Wang, Y. Catalytic Conversion of CO2 to Value-Added Products under Mild Conditions. ChemCatChem 2018, 10, 4863–4867.
- Cheng, Y.L.; Zhao, X.S.; Yu, Y.L.; Liu, Y.; Harada, M.; Ishihara, A.; Wang, Y. Indium oxide supported Pt-In alloy nanocluster catalysts with enhanced catalytic performance toward oxygen reduction reaction. J. Power Sources 2020, 446, 227332.
- Schrader, I.; Warneke, J.; Neumann, S.; Grotheer, S.; Swane, A.A.; Kirkensgaard, J.J.K.; Arenz, M.; Kunz, S. Surface Chemistry of "Unprotected" Nanoparticles: A Spectroscopic Investigation on Colloidal Particles. J. Phys. Chem. C 2015, 119, 17655–17661.
- Huang, B.; Ma, Y.T.; Xiong, Z.L.; Lu, W.D.; Ding, R.; Li, T.T.; Jiang, P.; Liang, M.H. Facile fabrication of Ir/CNT/rGO nanocomposites with enhanced electrocatalytic performance for the hydrogen evolution reaction. Sustain. Energy Fuels 2020, 4, 3288–3292.
- Ding, J.; Chan, K.Y.; Ren, J.W.; Xiao, F.S. Platinum and platinum-ruthenium nanoparticles supported on ordered mesoporous carbon and their electrocatalytic performance for fuel cell reactions. Electrochim. Acta 2005, 50, 3131–3141.
- Fu, X.Y.; Wang, Y.; Wu, N.Z.; Gui, L.L.; Tang, Y.Q. Surface modification of small platinum nanoclusters with alkylamine and alkylthiol: An XPS study on the influence of organic ligands on the Pt 4f binding energies of small platinum nanoclusters. J. Colloid Interface Sci. 2001, 243, 326–330.
- Du, X.Y.; Wang, Y.; Mu, Y.Y.; Gui, L.L.; Wang, P.; Tang, Y.Q. A new highly selective H2 sensor based on TiO2/PtO-Pt dual-layer films. Chem. Mater. 2002, 14, 3953–3957.
- Mu, Y.Y.; Du, X.Y.; Wang, Y.; Guo, H.Y.; Gui, L.L. Study on hydrogen sensitivities of TiO2/PtO-Pt and SnO2/PtO-Pt dual-layer films. Acta Chimica Sinica 2003, 61, 8–12.
- Mu, Y.Y.; Liang, H.P.; Hu, J.S.; Jiang, L.; Wan, L.J. Controllable Pt nanoparticle deposition on carbon nanotubes as an anode catalyst for direct methanol fuel cells. J. Phys. Chem. B 2005, 109, 22212–22216.
- Neumann, S.; Grotheer, S.; Tielke, J.; Schrader, I.; Quinson, J.; Zana, A.; Oezaslan, M.; Arenz, M.; Kunz, S. Nanoparticles in a box: A concept to isolate, store and re-use colloidal surfactant-free precious metal nanoparticles. J. Mater. Chem. A 2017, 5, 6140–6145.
- Lewis, L.N. Chemical catalysis by colloids and clusters. Chem. Rev. 1993, 93, 2693–2730.
- Schmid, G. Nanoclusters-Building blocks for future nanoelectronic devices? Adv. Eng. Mater. 2001, 3, 737–743.
- Sun, Y.G.; Xia, Y.N. Shape-controlled synthesis of gold and silver nanoparticles. Science 2002, 298, 2176–2179.
- Kuhn, J.N.; Tsung, C.-K.; Huang, W.; Somorjai, G.A. Effect of organic capping layers over monodisperse platinum nanoparticles upon activity for ethylene hydrogenation and carbon monoxide oxidation. J. Catal. 2009, 265, 209–215.
- Fenske, D.; Sonstroem, P.; Stoever, J.; Wang, X.D.; Borchert, H.; Al-Shamery, K. Colloidally Prepared Pt Nanoparticles for Heterogeneous Gas-Phase Catalysis: Influence of Ligand Shell and Catalyst Loading on CO Oxidation Activity. ChemCatChem 2010, 2, 198–205.
- Wang, X.D.; Stoever, J.; Zielasek, V.; Altmann, L.; Thiel, K.; Al-Shamery, K.; Baeumer, M.; Borchert, H.; Parisi, J.; Kolny-Olesiak, J. Colloidal Synthesis and Structural Control of PtSn Bimetallic Nanoparticles. Langmuir 2011, 27, 11052–11061.
- Sonstroem, P.; Arndt, D.; Wang, X.D.; Zielasek, V.; Baeumer, M. Ligand Capping of Colloidally Synthesized Nanoparticles-A Way to Tune Metal-Support Interactions in Heterogeneous Gas-Phase Catalysis. Angew. Chem. Int. Ed. 2011, 50, 3888–3891.
- Kunz, S.; Schreiber, P.; Ludwig, M.; Maturi, M.M.; Ackermann, O.; Tschurl, M.; Heiz, U. Rational design, characterization and catalytic application of metal clusters functionalized with hydrophilic, chiral ligands: A proof of principle study. Phys. Chem. Chem. Phys. 2013, 15, 19253–19261.
- Guo, Z.Y.; Xiao, C.X.; Maligal-Ganesh, R.V.; Zhou, L.; Goh, T.W.; Li, X.L.; Tesfagaber, D.; Thiel, A.; Huang, W.Y. Pt Nanoclusters Confined within Metal Organic Framework Cavities for Chemoselective Cinnamaldehyde Hydrogenation. ACS Catal. 2014, 4, 1340–1348.
- Speder, J.; Spanos, I.; Zana, A.; Kirkensgaard, J.J.K.; Mortensen, K.; Altmann, L.; Baeumer, M.; Arenz, M. From single crystal model catalysts to systematic studies of supported nanoparticles. Surf. Sci. 2015, 631, 278–284.
- Ning, X.M.; Yu, H.; Peng, F.; Wang, H.J. Pt nanoparticles interacting with graphitic nitrogen of N-doped carbon nanotubes: Effect of electronic properties on activity for aerobic oxidation of glycerol and electro-oxidation of CO. J. Catal. 2015, 325, 136–144.
- Altmann, L.; Wang, X.; Borchert, H.; Kolny-Olesiak, J.; Zielasek, V.; Parisi, J.; Kunz, S.; Baeumer, M. Influence of Sn content on the hydrogenation of crotonaldehyde catalysed by colloidally prepared PtSn nanoparticles. Phys. Chem. Chem. Phys. 2015, 17, 28186–28192.
- Darab, M.; Barnett, A.O.; Lindbergh, G.; Thomassen, M.S.; Sunde, S. The Influence of Catalyst Layer Thickness on the Performance and Degradation of PEM Fuel Cell Cathodes with Constant Catalyst Loading. Electrochim. Acta 2017, 232, 505–516.
- Yan, M.Y.; Wu, T.; Chen, L.F.; Yu, Y.L.; Liu, B.; Wang, Y.; Chen, W.X.; Liu, Y.; Lian, C.; Li, Y.D. Effect of Protective Agents upon the Catalytic Property of Platinum Nanocrystals. ChemCatChem 2018, 10, 2433–2441.
- Ilsemann, J.; Murshed, M.M.; Gesing, T.M.; Kopyscinski, J.; Baeumer, M. On the support dependency of the CO2 methanation—Decoupling size and support effects. Catal. Sci. Technol. 2021, 11, 4098–4114.
- Li, X.H.; You, X.; Ying, P.L.; Xiao, J.L.; Li, C. Some insights into the preparation of Pt/γ-Al2O3 catalysts for the enantioselective hydrogenation of a-ketoesters. Top. Catal. 2003, 25, 63–70.
- Lian, C.; Liu, H.Q.; Xiao, C.; Yang, W.; Zhang, K.; Liu, Y.; Wang, Y. Solvent-free selective hydrogenation of chloronitrobenzene to chloroaniline over a robust Pt/Fe3O4 catalyst. Chem. Commun. 2012, 48, 3124–3126.
- Liu, M.H.; Mo, X.X.; Liu, Y.Y.; Xiao, H.L.; Zhang, Y.; Jing, J.Y.; Colvin, V.L.; Yu, W.W. Selective hydrogenation of o-chloronitrobenzene using supported platinum nanoparticles without solvent. Appl. Catal. A Gen. 2012, 439, 192–196.
- Wang, P.; Zhao, J.; Li, X.B.; Yang, Y.; Yang, Q.H.; Li, C. Assembly of ZIF nanostructures around free Pt nanoparticles: Efficient size-selective catalysts for hydrogenation of alkenes under mild conditions. Chem. Commun. 2013, 49, 3330–3332.
- Jawale, D.V.; Gravel, E.; Boudet, C.; Shah, N.; Geertsen, V.; Li, H.; Namboothiri, I.N.N.; Doris, E. Selective conversion of nitroarenes using a carbon nanotube-ruthenium nanohybrid. Chem. Commun. 2015, 51, 1739–1742.
- Schrader, I.; Warneke, J.; Backenkoehler, J.; Kunz, S. Functionalization of Platinum Nanoparticles with L-Proline: Simultaneous Enhancements of Catalytic Activity and Selectivity. J. Am. Chem. Soc. 2015, 137, 905–912.
- Yu, Y.L.; Huang, J.; Wang, Y. Catalytic conversion of ferrous carbonate to higher hydrocarbons under mild conditions and its application in transformation of CO2 to liquid fuels. Sustain. Energy Fuels 2020, 4, 96–100.
- Liu, Y.; Guo, W.; Li, X.J.; Jiang, P.; Zhang, N.; Liang, M.H. Copper Single-Atom-Covered Pt Nanoparticles for Selective Hydrogenation of Phenylacetylene. ACS Appl. Nano Mater. 2021, 4, 5292–5300.
- Yu, Y.L.; Cai, Y.C.; Liang, M.H.; Tan, X.; Huang, J.; Jiang, H.; Harada, M.; Wang, Y. Highly selective synthesis of multicarbon compounds by carbon dioxide hydrogenation over Pt nanocrystals anchoring Ru clusters. Catal. Sci. Technol. 2022, 12, 3786–3792.
- Wang, Y.J.; Zhao, N.N.; Fang, B.Z.; Li, H.; Bi, X.T.T.; Wang, H.J. Carbon-Supported Pt-Based Alloy Electrocatalysts for the Oxygen Reduction Reaction in Polymer Electrolyte Membrane Fuel Cells: Particle Size, Shape, and Composition Manipulation and Their Impact to Activity. Chem. Rev. 2015, 115, 3433–3467.
- Shao, M.H.; Chang, Q.W.; Dodelet, J.P.; Chenitz, R. Recent Advances in Electrocatalysts for Oxygen Reduction Reaction. Chem. Rev. 2016, 116, 3594–3657.
- Sharma, G.; Kumar, A.; Sharma, S.; Naushad, M.; Dwivedi, R.P.; Alothman, Z.A.; Mola, G.T. Novel development of nanoparticles to bimetallic nanoparticles and their composites: A review. J. King Saud Univ. Sci. 2019, 31, 257–269.
- Quinson, J. Colloidal surfactant-free syntheses of precious metal nanoparticles for electrocatalysis. Curr. Opin. Electrochem. 2022, 34, 100977.
- Li, H.Q.; Sun, G.Q.; Cao, L.; Jiang, L.H.; Xin, Q. Comparison of different promotion effect of PtRu/C and PtSn/C electrocatalysts for ethanol electro-oxidation. Electrochim. Acta 2007, 52, 6622–6629.
- Speder, J.; Altmann, L.; Roefzaad, M.; Baeumer, M.; Kirkensgaard, J.J.K.; Mortensen, K.; Arenz, M. Pt based PEMFC catalysts prepared from colloidal particle suspensions—A toolbox for model studies. Phys. Chem. Chem. Phys. 2013, 15, 3602–3608.
- Quinson, J.; Inaba, M.; Neumann, S.; Swane, A.A.; Bucher, J.; Kunz, S.; Arenz, M. Investigating Particle Size Effects in Catalysis by Applying a Size-Controlled and Surfactant-Free Synthesis of Colloidal Nanoparticles in Alkaline Ethylene Glycol: Case Study of the Oxygen Reduction Reaction on Pt. ACS Catal. 2018, 8, 6627–6635.
- Martinez, E.Y.; Li, C.W. Surface functionalization of Pt nanoparticles with metal chlorides for bifunctional CO oxidation. Polyhedron 2019, 170, 239–244.
- Cheng, T.; Tan, X.; Chen, L.F.; Zhao, X.S.; Kotegawa, F.; Huang, J.; Liu, Y.; Jiang, H.; Harada, M.; Wang, Y. A Robust Electrocatalyst for Oxygen Reduction Reaction Assembled with Pt Nanoclusters and a Melem-Modified Carbon Support. Energy Technol. 2022, 10, 2200680.
- Chen, L.F. Study on the Structure and Properties of Novel Nanocomposite Catalysts for Fuel Cell. Ph.D. Thesis, Peking University, Beijing, China, 2019.
- An, N.; Li, S.; Duchesne, P.N.; Wu, P.; Zhang, W.L.; Jia, M.J.; Zhang, W.X. Size Effects of Platinum Colloid Particles on the Structure and CO Oxidation Properties of Supported Pt/Fe2O3 Catalysts. J. Phys. Chem. C 2013, 117, 21254–21262.
- Zheng, N.; Zhu, C.M.; Sun, B.; Shi, Z.J.; Liu, Y.; Wang, Y. Nanocomposite Cathode Catalyst with High Methanol Tolerance and Durability. Acta Phys.-Chim. Sin. 2012, 28, 2263–2268.
- Yu, W.J.; Lou, L.-L.; Yu, K.; Li, S.S.; Shi, Y.; Liu, S.X. Pt nanoparticles stabilized by thermosensitive polymer as effective and recyclable catalysts for the asymmetric hydrogenation of ethyl pyruvate. RSC Adv. 2016, 6, 52500–52508.
- Liu, J.; Yin, J.; Feng, B.; Li, F.; Wang, F. One-pot synthesis of unprotected PtPd nanoclusters with enhanced catalytic activity, durability, and methanol-tolerance for oxygen reduction reaction. Appl. Surf. Sci. 2019, 473, 318–325.
- Zhang, J.; Wang, M.; Gao, Z.R.; Qin, X.T.; Xu, Y.; Wang, Z.H.; Zhou, W.; Ma, D. Importance of Species Heterogeneity in Supported Metal Catalysts. J. Am. Chem. Soc. 2022, 144, 5108–5115.
- Jawale, D.V.; Kouatchou, J.A.T.; Fossard, F.; Miserque, F.; Geertsen, V.; Gravel, E.; Doris, E. Catalytic hydrothiolation of alkenes and alkynes using bimetallic RuRh nanoparticles on carbon nanotubes. Green Chem. 2022, 24, 1231–1237.
- Bizzotto, F.; Arenz, M.; Quinson, J. Surfactant-free Ir nanoparticles synthesized in ethanol: Catalysts for the oxygen evolution reaction. Materials Letters 2022, 308, 131209.
- Kawawaki, T.; Shimizu, N.; Funai, K.; Mitomi, Y.; Hossain, S.; Kikkawa, S.; Osborn, D.J.; Yamazoe, S.; Metha, G.F.; Negishi, Y. Simple and high-yield preparation of carbon-black-supported similar to 1 nm platinum nanoclusters and their oxygen reduction reactivity. Nanoscale 2021, 13, 14679–14687.
- Kim, J.; Kim, W.; Seo, Y.; Kim, J.-C.; Ryoo, R. n-Heptane hydroisomerization over Pt/MFI zeolite nanosheets: Effects of zeolite crystal thickness and platinum location. J. Catal. 2013, 301, 187–197.
- Tang, R.; Zhu, Z.J.; Li, C.R.; Xiao, M.Q.; Wu, Z.Y.; Zhang, D.K.; Zhang, C.C.; Xiao, Y.; Chu, M.Y.; Genest, A.; et al. Ru-Catalyzed Reverse Water Gas Shift Reaction with Near-Unity Selectivity and Superior Stability. ACS Mater. Lett. 2021, 3, 1652–1659.
- Pei, W.B.; Dai, L.Y.; Liu, Y.X.; Deng, J.G.; Jing, L.; Zhang, K.F.; Hou, Z.Q.; Han, Z.; Rastegarpanah, A.; Dai, H.X. PtRu nanoparticles partially embedded in the 3DOM Ce0.7Zr0.3O2 skeleton: Active and stable catalysts for toluene combustion. J. Catal. 2020, 385, 274–288.
- Li, X.B.; Liu, X.; Yang, Y.; Zhao, J.; Li, C.; Yang, Q.H. Entrapment of metal nanoparticles within nanocages of mesoporous silicas aided by co-surfactants. J. Mater. Chem. 2012, 22, 21045–21050.
- Zhao, K.; Nie, X.W.; Wang, H.Z.; Chen, S.; Quan, X.; Yu, H.T.; Choi, W.Y.; Zhang, G.H.; Kim, B.; Chen, J.G.G. Selective electroreduction of CO2 to acetone by single copper atoms anchored on N-doped porous carbon. Nat. Commun. 2020, 11, 2455.
- Xu, Y.F.; Li, X.Y.; Gao, J.H.; Wang, J.; Ma, G.Y.; Wen, X.D.; Yang, Y.; Li, Y.W.; Ding, M.Y. A hydrophobic catalyst increases olefins from syngas by suppressing C1 by-products. Science 2021, 371, 610–613.
- Li, Z.L.; Wang, J.J.; Qu, Y.Z.; Liu, H.L.; Tang, C.Z.; Miao, S.; Feng, Z.C.; An, H.Y.; Li, C. Highly Selective Conversion of Carbon Dioxide to Lower Olefins. ACS Catal. 2017, 7, 8544–8548.
- Wei, J.; Ge, Q.J.; Yao, R.W.; Wen, Z.Y.; Fang, C.Y.; Guo, L.S.; Xu, H.Y.; Sun, J. Directly converting CO2 into a gasoline fuel. Nat. Commun. 2017, 8, 15174.
- Gao, P.; Li, S.G.; Bu, X.N.; Dang, S.S.; Liu, Z.Y.; Wang, H.; Zhong, L.S.; Qiu, M.H.; Yang, C.G.; Cai, J.; et al. Direct conversion of CO2 into liquid fuels with high selectivity over a bifunctional catalyst. Nat. Chem. 2017, 9, 1019–1024.
- Zhou, C.; Shi, J.Q.; Zhou, W.; Cheng, K.; Zhang, Q.H.; Kang, J.C.; Wang, Y. Highly Active ZnO-ZrO2 Aerogels Integrated with H-ZSM-5 for Aromatics Synthesis from Carbon Dioxide. ACS Catal. 2020, 10, 302–310.
- Boreriboon, N.; Jiang, X.; Song, C.S.; Prasassarakich, P. Fe-based bimetallic catalysts supported on TiO2 for selective CO2 hydrogenation to hydrocarbons. J. CO2 Util 2018, 25, 330–337.
- Ni, Y.M.; Chen, Z.Y.; Fu, Y.; Liu, Y.; Zhu, W.L.; Liu, Z.M. Selective conversion of CO2 and H2 into aromatics. Nat. Commun. 2018, 9, 3457.
- Wang, Y.; Tan, L.; Tan, M.H.; Zhang, P.P.; Fang, Y.; Yoneyama, Y.; Yang, G.H.; Tsubaki, N. Rationally Designing Bifunctional Catalysts as an Efficient Strategy to Boost CO2 Hydrogenation Producing Value-Added Aromatics. ACS Catal. 2019, 9, 895–901.
- Huang, J.; Cai, Y.C.; Yu, Y.L.; Wang, Y.A. Conversion of CO2 to Multi-carbon Compounds over a CoCO3 Supported Ru-Pt Catalyst Under Mild Conditions. Chem. Res. Chin. Univ. 2022, 38, 223–228.
More
