Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Anna Barra Caracciolo and Version 2 by Rita Xu.
Hydrocarbons occur in fossil fuels such as crude oil and consist mainly of hydrogen and carbon. They are natural chemicals, crude oil refining results in commercial products with new physico-chemical properties, which can increase their complexity and toxicity, and hamper their degradation. The presence of biodiverse natural microbial communities is a prerequisite for an effective homeostatic response to the various hydrocarbons, that contaminate ecosystems.
- microbial communities
- regulation ecosystem services
- aliphatic hydrocarbons
- polycyclic aromatics hydrocarbons
1. Introduction
Economic activities are strongly dependent on the use of fossil fuels, which consist of hydrocarbon-containing material formed naturally in the Earth’s crust from dead plant and animal residues. Hydrocarbons provide energy for civil and industrial purposes, and for transport [1]. Owing to the growth of the human population, the worldwide demand for hydrocarbons is increasing, and petrochemical industries, accidental oil spills, disconnection between oil wells and combustion processes (e.g., industry emissions), abandoned refining sites, and vehicle combustion have been constantly polluting air, water, and soil [2][3][4][2,3,4]. The risk of accidental leaks in ecosystems has increased exponentially with the growing global demand for oil and it has been estimated that every year, ca. 1.3 million liters of oil reach natural environments [5]. Hydrocarbons are the organic contaminants most commonly found in ecosystems [1][6][1,6], and it is fundamental to evaluate their environmental fate and effects in different matrices.
Hydrocarbons are chemicals naturally occurring in crude oil and consisting mainly of hydrogen and carbon. Crude oil is a dark, viscous, and easily flammable complex liquid mixture of hydrocarbons (83–87%), comprising variable amounts of hydrogen (10–14%), oxygen (0.05–1.5%), sulfur (0.005–6.0%), nitrogen (0.1–0.2%), and metals (<1000 mg/L) such as nickel, iron, and copper. The specific composition depends on the oil field’s geological age, location, and depth. After crude oil refining, the resulting products have new physico-chemical properties, which increase their complexity and can hamper their biodegradation [7].
Microorganisms play key roles in natural ecosystem functioning, such as primary production, organic matter decomposition, nutrient cycling, and biodegradation of contaminants, including hydrocarbons, thus contributing in different ways to soil and water purification processes. The maintenance of these regulating ecosystem services is linked to bacterial diversity and metabolic versatility, which makes it potentially possible to biodegrade a huge variety of aliphatic and aromatic hydrocarbons.
2. Aliphatic Hydrocarbons and Polycyclic Aromatic Hydrocarbons
Hydrocarbons can be divided in accordance with their chemical structure into: aliphatic hydrocarbons or saturated hydrocarbons, aromatic hydrocarbons (monocyclic aromatic and polycyclic aromatic hydrocarbons); and heteroatomic compounds (saturated and aromatic ones), including resins and asphaltenes.
Aliphatic hydrocarbons (AH) (Figure 1A), also known as paraffins, are mainly present in deposits of natural gas and oil formed by plant and animal decomposition. AH have no double bonds (general formula CnH2n) and represent the highest percentage of the constituents of crude oil. The lack of functional groups makes them strongly apolar, so they have low water solubility and are poorly reactive at room temperature [8]. In accordance with their structure, they are classified as alkanes (which have a linear structure) and cycloalkanes (which have a condensed ring structure); they can be linear or branched [9].
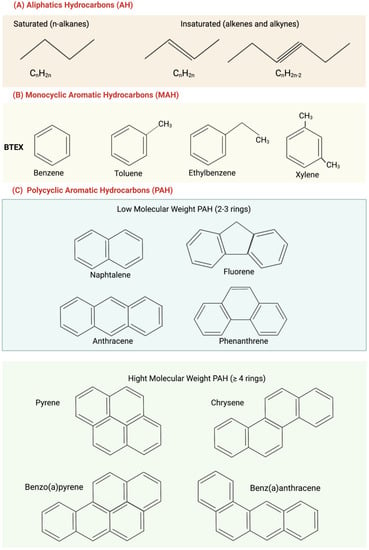
Figure 1. Structure of main hydrocarbons: (A) Aliphatic hydrocarbons (AH); (B) monocyclic aromatic hydrocarbons (MAH); (C) polycyclic aromatic hydrocarbons (PAH), subdivided into low molecular weight (in blue) and high molecular weight (in green).
Aromatic hydrocarbons have one or more benzene rings and are generally replaced with different aliphatic hydrocarbons. They are mainly divided into monocyclic aromatic hydrocarbons (MAH), such as benzene, toluene, ethylbenzene, and xylene, which together constitute BTEX (widely studied and making up 2–20% of oil) (Figure 1B) and polycyclic aromatic hydrocarbons (PAH), (Figure 1C).
PAHs are a group of lipophilic organic pollutants that derive from biological processes or are formed as products of incomplete combustion from natural (forest fires and shrubs) or anthropic (vehicle emissions, domestic heating, and cigarette smoke) sources. Owing to their ubiquitous presence and persistence in air, water, and soil, as well as their toxicity for both humans and biota, they are compounds of environmental concern [10][11][10,11].
PAHs with 2–3 benzene rings, such as naphthalene, fluorene, anthracene, and phenanthrene, are low-molecular-weight ones. PAHs with four or more benzene rings, such as pyrene, chrysene, benzo(a)pyrene, and benz(a)anthracene, are high molecular weight ones (Figure 1C). Low-molecular-weight PAHs are gases and tend to escape into the atmosphere, while those with a higher molecular weight are liquids or in a solid state at room temperature and often have a separate phase in water [8]. PAH solubility in aqueous solution decreases as the number of benzene rings increases, and this increases their environmental persistence. A high persistence increases the possibility of a compound making its toxicity felt.
It has recently been recognized that life quality is connected to that of the environment, as expressed by the new concept of ‘One Health’ proposed by the World Health Organization [12]. The One Health approach means that human health is connected to that of animals and the environment. For this reason, the ubiquitous occurrence (water, air, soil, and sediment) of hydrocarbons is a serious threat to both human and environmental health [13].
3. Environmental Fate and Toxic Effects
Hydrocarbons interact with both the abiotic and biotic components of ecosystems. Hydrocarbons can be divided into specific fractions, equivalent to the number of carbon atoms. These fractions can also be described for their physical, chemical, and toxicological characteristics. Fraction 1 (F1) contains hydrocarbons with a carbon number between 6 and 10 (C6–C10) and is classified as volatiles. Fraction 2 (F2) represents the semi-volatile hydrocarbons with at least 10 carbon atoms (C10–C16). Fraction 3 (F3) includes hydrocarbons with a carbon number between 16 and 34 (C16–C34) and are considered non-volatile. Fraction 4 (F4) refers to the hydrocarbon class with the lowest volatility and solubility, and more than 35 carbon atoms (>C35). Toxicity generally increases with the molecular weight [7][9][7,9]. For example, polycyclic aromatic hydrocarbons with a high molecular weight and boiling point have higher toxicities [7].
Due to their complex characteristics, the lightest and most volatile hydrocarbon fractions are released into the atmosphere, the amphipathic and hydrophilic fractions dissolve in water, and the lipophilic ones tend to bind to soil/sediment particles and organic matter [7].
The toxicity of a hydrocarbon also depends on its bioavailability, which in turn is influenced by its physical and chemical characteristics. Once a bioavailable chemical is absorbed by an organism, it interacts with cellular receptor sites and can cause lethal or sub-lethal effects. The effects depend on the concentration and duration (short or long) of exposure [7]. Lethality occurs from short-term exposure due to acute oil spills in aquatic environments in which hydrophobic compounds destroy the central nervous systems of organisms through their location in neuron cell membranes [7].
Sub-lethal toxic effects due to hydrocarbon exposure can cause lesions (internal or external), defects in early development, anoxia, and changes in molecular and behavioral functions related to nutrition and reproduction. Exposure to petroleum hydrocarbon products can cause acute effects such as changes in the nervous, ocular, and respiratory systems. Along with migraines and headaches, people living in oil-polluted areas also experience nausea, upper respiratory tract infections, and nose and eye irritations [5].
PAHs can potentially not only affect the nervous, immune, and excretory systems but also cause tumors and mutations [7][9][7,9]. Chronic exposure to PAHs can cause cancer in humans, and the cataractogenic properties of PAHs (i.e., alterations at the dermal and ocular levels) have been well documented [14]. Exposure to PAHs can occur through inhalation, ingestion (through contaminated food or drink), or dermally (direct contact). Once introduced into the body, these molecules rapidly diffuse due to their high fat solubility, which makes them able to cross cell membranes and deposit themselves in adipose tissue, the kidneys, and the liver. Exposure to these substances causes blood damage, and probable immunosuppression. Depending on the route of exposure, PAHs (particularly those characterized by 3–7 aromatic rings) cause lung, digestive, or skin cancers. The carcinogenic potential derives from the metabolism of these substances in the liver, which leads to a biotransformation into reactive intermediates capable of binding to DNA and RNA with consequent damage to genetic information. It has been empirically demonstrated that PAHs interact metabolically, resulting in synergistic and additive effects [15].
The toxicity of hydrocarbons affects humans, plants, animals, and microorganisms, with consequences for ecosystem biodiversity and functioning. Environmental contamination by hydrocarbons and derivatives has caused the local extinction of many species of plants and animals [5].
Hydrocarbons are freshwater, wastewater, and seawater contaminants. Because they contain a large number of hydrophobic components, which are adsorbed onto particulates and sediments in aquatic ecosystems, they can become bioavailable for benthic species. In fact, invertebrates, fish, eggs laid by fish, and filter feeders such as bivalves are in contact with these contaminants by filtration of suspended petroleum [7].
Hydrocarbons can hamper water and oxygen transfer through porous spaces in sediments and soil, influencing their permeability, moisture content, pH, nutrient availability (e.g., nitrate, phosphate, and sodium), and redox condition [3]. This is the case of higher molecular weight PAHs, which thanks to their low desorption from soil and low water solubility can form a surface layer that can prevent both bioaccessibility and vegetation development for several decades [16]. Damaging effects also occur on plant species directly exposed to hydrocarbons because hydrocarbons prevent light access and make plants unable to acquire nutrients and water, reducing primary and agricultural productivity [5][17][5,17].
Hydrocarbons can also be released into deeper waters from soil by percolation or atmospheric subsidence, bringing them into contact with underground ecosystems [18].
Hydrocarbons can also eliminate or inhibit microbial species, altering their corresponding ecosystem functions [17]. The high selective pressure exerted by hydrocarbons can reduce their diversity, producing rapid shifts in microbial populations [19]. The elimination or alteration of microbial species with key roles in biogeochemical cycles and primary production can also affect higher trophic levels of the food chain due to biomagnification [17]. Bacteria in soil, e.g., nitrifying bacteria, are susceptible to hydrocarbon exposure, which inhibits their enzyme ammonium monooxygenase, through competitive binding with low-weight hydrocarbons [7], with consequences for soil fertility.
Hydrocarbons act as a selective force, influencing the structure and functioning of natural microbial communities. Some microbial species are negatively affected by these toxic compounds [7]. However, others can respond to their presence with various mechanisms and remove them. Hydrocarbon biodegradation is possible if a high microbial metabolic diversity is present in a contaminated environment and the quantity of hydrocarbons is not so great as to prevent or inhibit the activity of the microbes [17].
