The oral cavity is a unique environment that consists of teeth surrounded by periodontal tissues, oral mucosae with minor salivary glands, and terminal parts of major salivary glands that open into the oral cavity. It is a first-line defense against most viral and bacterial pathogens. Fibrinolytic factors of the plasminogen (Plg)/plasmin (Pm) system, their soluble and membrane receptors, and fragments, such as suPAR (soluble urokinase plasminogen activator receptor) modulate physiological and pathological conditions, especially inflammation. Fibrinolysis, the removal of fibrin, is the primary function of fibrinolytic factors. Under physiological conditions, fibrinolytic factors are present in the oral cavity and secreted mostly with saliva. Under the inflammation plasminogen/plasmin system performs fibrinolytic and non-fibrinolytic functions: cytokines or proteases (MMPs) are activated, receptors such as suPAR are shed from the surface promoting cell migration, and modulation of the inflammatory response. Viruses, like SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2), exploit the fibrinolytic system to promote host cell infection.
- plasmin
- COVID-19
- suPAR
- oral cavity
1. Introduction
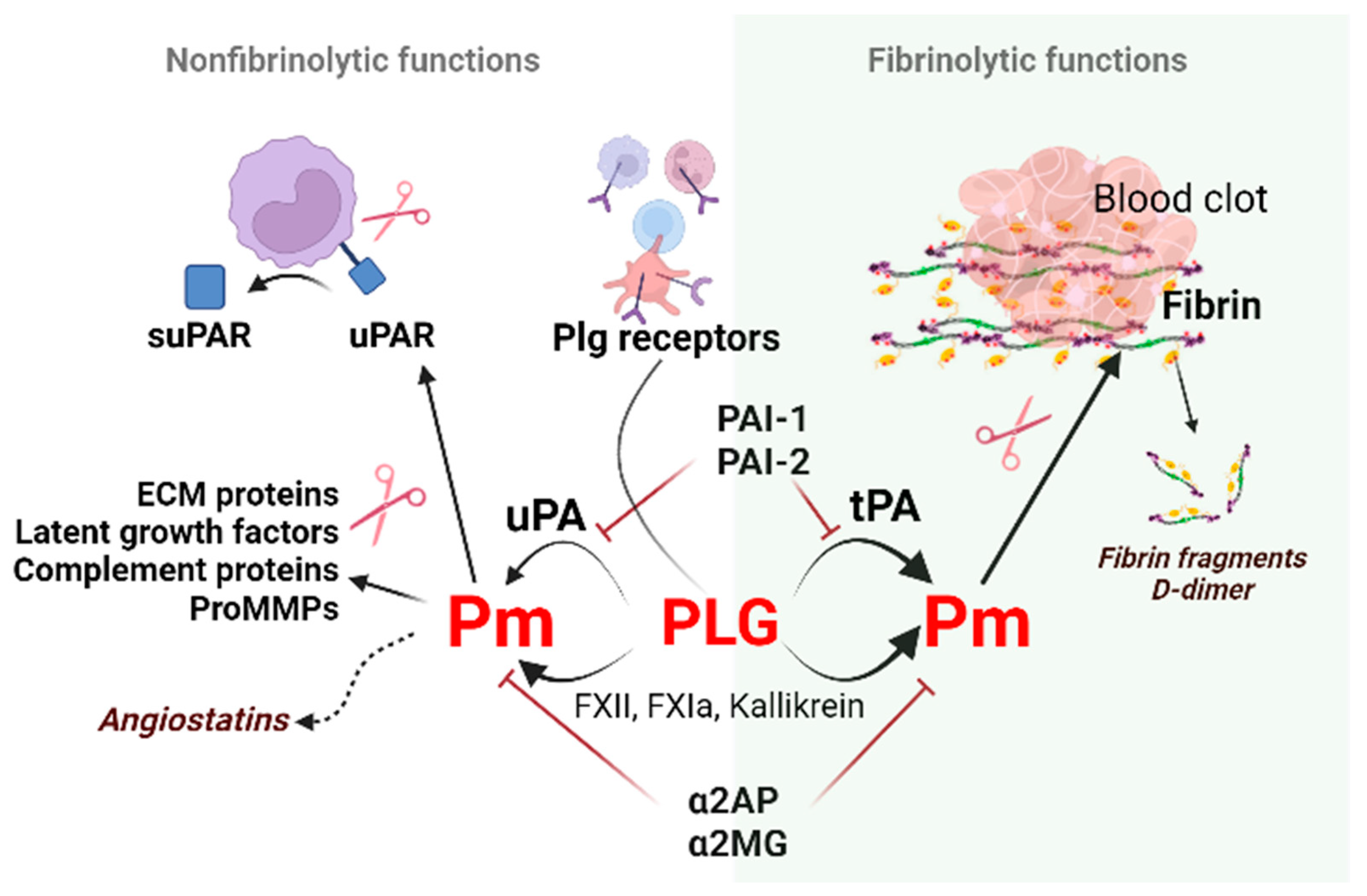
2. Regulators of Fibrinolysis
2.1. Plasminogen (Plg)
2.2. Plasminogen Activators (PAs)
2.3. Plasminogen Activator Inhibitors
3.3. Soluble uPAR, a New Biomarker of Inflammation
3.3. Soluble uPAR, a new biomarker of inflammation
uPAR (CD87; Plasminogen Activator, Urokinase Receptor [Plaur gene]) is a membrane-linked protein found in immunologically active cells (monocytes, neutrophils, activated T lymphocytes, macrophages), endothelial cells, keratinocytes, fibroblasts, smooth muscle cells, megakaryocytes, and certain tumor cells. Apart from uPA, uPAR can also interact with integrin or other partners, including vitronectin, high molecular weight kininogen, G protein-coupled receptor, tyrosine kinase receptors that can trigger plasmin generation and degradation of the extracellular matrix (ECM) along the leading edge of a migratory cell [62] and activate downstream signaling pathways. uPA via plasmin can increase cell proliferation through the proteolytic activation of growth factors and adhesion molecules, remodeling of tissues/ECM, regulate adhesion and invasion of normal and cancer cells [63].
Cleavage of uPAR at the GPI (glycosyl phosphatidylinositol) anchor by proteases like plasmin can shed the extracellular part of uPAR, releasing the soluble form of the receptor (suPAR) into the blood, mucosa, urine, and saliva [64].
3. Fibrinolytic fFactors dDuring iInflammation in the oral cOral Cavity
Lined with keratinized and non-keratinized stratified squamous epithelium, oral mucosa covers the oral cavity. It is moistened with excretes of the major parotid, submandibular, sublingual, and minor salivary glands within the oral cavity. Mechanical mucosal trauma occurs while eating, drinking, and talking (and even tobacco inhalation). Oral mucosa is a first-line defense that interacts with pathogens (e.g., bacteria, viruses, or fungi) and provides specific (immune) or non-specific protective response against pathogenic microorganisms such as pattern recognition receptors including C-type lectin receptors (Dectin-1, Dectin-2) or TLR1-1 (TLRs, toll-like receptors). Gradual desquamation of mucosal epithelium is a protective mechanism to eliminate adherent pathogenic microorganisms and to prevent their further invasion into underlying tissues [65]. A host organism reacts with the release of proinflammatory cytokines and proteases to fight oral microorganisms in the gingiva and periodontal ligament space.
Salivary glands provide local mucosal-specific and non-specific immunity. Proper qualitative and quantitative composition of saliva and salivation rate protect and maintain the integrity of the oral cavity. Salivary mucins avert plaque formation on teeth surfaces via bacteria binding. Salivary lysozyme, an enzyme that lyses bacteria cell walls prevent the overgrowth of oral microbiota. Several studies are available regarding the fibrinolytic properties of salivary glands and other fluids of the oral cavity [66] during steady state and stress/inflammation.
Salivary suPAR, tumor necrosis factor α (TNF α), and interleukins like (IL)-1β levels increased in healthy subjects exposed to psychological stress [76] and showed strong positive baseline and post-stress correlations. Elevated saliva suPAR levels were detected and proposed as a biomarker of gingivitis and periodontitis [67][68]. Aside from suPAR, Plg receptors such as glyceraldehyde-3-phosphate dehydrogenase, α-enolase, and annexin A2 are also found in the saliva [69][70][71][72], but their function in the oral cavity remains unclear. Excretory ducts and acinar cells highly expressed PAI-2. tPA was expressed in serous but not mucous acini [66]. Overall, several independent studies confirmed the existence of tPA in saliva, but the results of different investigators varied on the presence/dominance of PAI-1 or PAI-2 in salivary gland tissues. PAI-1 increases in saliva were associated with insulin resistance and inflammation and ascribed as a proinflammatory marker and valuable diagnostic marker to track periodontal therapy [73].
References
- Bharadwaj, A.G.; Holloway, R.W.; Miller, V.A.; Waisman, D.M. Plasmin and Plasminogen System in the Tumor Microenvironment: Implications for Cancer Diagnosis, Prognosis, and Therapy. Cancers 2021, 13, 1838.
- Heissig, B.; Salama, Y.; Osada, T.; Okumura, K.; Hattori, K. The Multifaceted Role of Plasminogen in Cancer. Int. J. Mol. Sci. 2021, 22, 2304.
- Heissig, B.; Ohki-Koizumi, M.; Tashiro, Y.; Gritli, I.; Sato-Kusubata, K.; Hattori, K. New functions of the fibrinolytic system in bone marrow cell-derived angiogenesis. Int. J. Hematol. 2012, 95, 131–137.
- Medcalf, R.L.; Keragala, C.B. The Fibrinolytic System: Mysteries and Opportunities. HemaSphere 2021, 5, e570.
- Myöhänen, H.; Vaheri, A. Regulation and interactions in the activation of cell-associated plasminogen. Cell. Mol. Life Sci. CMLS 2004, 61, 2840–2858.
- Rijken, D.C.; Lijnen, H.R. New insights into the molecular mechanisms of the fibrinolytic system. J. Thromb. Haemost. 2009, 7, 4–13.
- Urano, T.; Castellino, F.J.; Suzuki, Y. Regulation of plasminogen activation on cell surfaces and fibrin. J. Thromb. Haemost. 2018, 16, 1487–1497.
- Heissig, B.; Salama, Y.; Takahashi, S.; Osada, T.; Hattori, K. The multifaceted role of plasminogen in inflammation. Cell. Signal. 2020, 75, 109761.
- Baker, S.K.; Strickland, S. A critical role for plasminogen in inflammation. J. Exp. Med. 2020, 217, e20191865.
- Shen, Y.; Guo, Y.; Mikus, P.; Sulniute, R.; Wilczynska, M.; Ny, T.; Li, J. Plasminogen is a key proinflammatory regulator that accelerates the healing of acute and diabetic wounds. Blood 2012, 119, 5879–5887.
- Kessler, A.T.; Bhatt, A.A. Review of the Major and Minor Salivary Glands, Part 1: Anatomy, Infectious, and Inflammatory Processes. J. Clin. Imaging Sci. 2018, 8, 47.
- Rijken, D.C.; Sakharov, D.V. Basic Principles in Thrombolysis: Regulatory Role of Plasminogen. Thromb. Res. 2001, 103, S41–S49.
- Pryzdial, E.L.G.; Leatherdale, A.; Conway, E.M. Coagulation and complement: Key innate defense participants in a seamless web. Front. Immunol. 2022, 13, 918775.
- Twining, S.S.; Wilson, P.M.; Ngamkitidechakul, C. Extrahepatic synthesis of plasminogen in the human cornea is up-regulated by interleukins-1alpha and -1beta. Biochem. J. 1999, 339 Pt 3, 705–712.
- Castellino, F.J.; McCance, S.G. The Kringle Domains of Human Plasminogen. In Ciba Foundation Symposium 212—Plasminogen-Related Growth Factors; Novartis Foundation Symposia; John Wiley & Sons: Hoboken, NJ, USA, 2007; pp. 46–65.
- Rudd, P.M.; Woods, R.J.; Wormald, M.R.; Opdenakker, G.; Downing, A.K.; Campbell, I.D.; Dwek, R.A. The effects of variable glycosylation on the functional activities of ribonuclease, plasminogen and tissue plasminogen activator. Biochim. Biophys. Acta (BBA)—Protein Struct. Mol. Enzymol. 1995, 1248, 1–10.
- Zhang, L.; Gong, Y.; Grella, D.K.; Castellino, F.J.; Miles, L.A. Endogenous plasmin converts Glu-plasminogen to Lys-plasminogen on the monocytoid cell surface. J. Thromb. Haemost. 2003, 1, 1264–1270.
- Silverstein, R.L.; Friedlander, R.J., Jr.; Nicholas, R.L.; Nachman, R.L. Binding of Lys-plasminogen to monocytes/macrophages. J. Clin. Investig. 1988, 82, 1948–1955.
- Cao, Y.; Xue, L. Angiostatin. Semin. Thromb. Hemost. 2004, 30, 83–93.
- O’Reilly, M.S.; Holmgren, L.; Shing, Y.; Chen, C.; Rosenthal, R.A.; Moses, M.; Lane, W.S.; Cao, Y.; Sage, E.H.; Folkman, J. Angiostatin: A novel angiogenesis inhibitor that mediates the suppression of metastases by a lewis lung carcinoma. Cell 1994, 79, 315–328.
- Syed, S.P.; Martin, A.-M.; Haupt, H.M.; Arenas-Elliot, C.P.; Brooks, J.J. Angiostatin receptor annexin II in vascular tumors including angiosarcoma. Hum. Pathol. 2007, 38, 508–513.
- Yatsenko, T.A.; Rybachuk, V.M.; Yusova, O.I.; Kharchenko, S.M.; Grinenko, T.V. Effect of fibrin degradation products on fibrinolytic process. Ukr. Biochem. J. 2016, 88, 16–24.
- Schneider, M.; Nesheim, M. A Study of the Protection of Plasmin from Antiplasmin Inhibition within an Intact Fibrin Clot during the Course of Clot Lysis. J. Biol. Chem. 2004, 279, 13333–13339.
- Lamarre, J.; Vasudevan, J.; Gonias, S.L. Plasmin cleaves betaglycan and releases a 60 kDa transforming growth factor-β complex from the cell surface. Biochem. J. 1994, 302, 199–205.
- Sahni, A.; Francis, C.W. Plasmic degradation modulates activity of fibrinogen-bound fibroblast growth factor-2. J. Thromb. Haemost. 2003, 1, 1271–1277.
- Matsuoka, H.; Sisson, T.H.; Nishiuma, T.; Simon, R.H. Plasminogen-Mediated Activation and Release of Hepatocyte Growth Factor from Extracellular Matrix. Am. J. Respir. Cell Mol. Biol. 2006, 35, 705–713.
- Friedrich, C.; Neugebauer, L.; Zamora, M.; Robles, J.P.; Martínez de la Escalera, G.; Clapp, C.; Bertsch, T.; Triebel, J. Plasmin generates vasoinhibin-like peptides by cleaving prolactin and placental lactogen. Mol. Cell. Endocrinol. 2021, 538, 111471.
- Novak, J.F.; Hayes, J.D.; Nishimoto, S.K. Plasmin-Mediated Proteolysis of Osteocalcin. J. Bone Miner. Res. 1997, 12, 1035–1042.
- Wang, N.; Zhang, L.; Miles, L.; Hoover-Plow, J. Plasminogen regulates pro-opiomelanocortin processing. J. Thromb. Haemost. 2004, 2, 785–796.
- Ishii, T.; Fukano, K.; Shimada, K.; Kamikawa, A.; Okamatsu-Ogura, Y.; Terao, A.; Yoshida, T.; Saito, M.; Kimura, K. Proinsulin C-peptide activates α-enolase: Implications for C-peptide–cell membrane interaction. J. Biochem. 2012, 152, 53–62.
- Okaji, Y.; Tashiro, Y.; Gritli, I.; Nishida, C.; Sato, A.; Ueno, Y.; Del Canto Gonzalez, S.; Ohki-Koizumi, M.; Akiyama, H.; Nakauchi, H.; et al. Plasminogen deficiency attenuates postnatal erythropoiesis in male C57BL/6 mice through decreased activity of the LH-testosterone axis. Exp. Hematol. 2012, 40, 143–154.
- Magnussen, S.N.; Hadler-Olsen, E.; Costea, D.E.; Berg, E.; Jacobsen, C.C.; Mortensen, B.; Salo, T.; Martinez-Zubiaurre, I.; Winberg, J.O.; Uhlin-Hansen, L.; et al. Cleavage of the urokinase receptor (uPAR) on oral cancer cells: Regulation by transforming growth factor—beta1 (TGF-beta1) and potential effects on migration and invasion. BMC Cancer 2017, 17, 350.
- Monea, S.; Lehti, K.; Keski-Oja, J.; Mignatti, P. Plasmin activates pro-matrix metalloproteinase-2 with a membrane-type 1 matrix metalloproteinase-dependent mechanism. J. Cell. Physiol. 2002, 192, 160–170.
- Heissig, B.; Lund, L.R.; Akiyama, H.; Ohki, M.; Morita, Y.; Rømer, J.; Nakauchi, H.; Okumura, K.; Ogawa, H.; Werb, Z.; et al. The Plasminogen Fibrinolytic Pathway Is Required for Hematopoietic Regeneration. Cell Stem Cell 2008, 3, 120.
- Plow, E.F.; Doeuvre, L.; Das, R. So Many Plasminogen Receptors: Why? J. Biomed. Biotechnol. 2012, 2012, 141806.
- Miles, L.A.; Vago, J.P.; Sousa, L.P.; Parmer, R.J. Functions of the plasminogen receptor Plg-RKT. J. Thromb. Haemost. 2020, 18, 2468–2481.
- Bharadwaj, A.G.; Kempster, E.; Waisman, D.M. The ANXA2/S100A10 Complex—Regulation of the Oncogenic Plasminogen Receptor. Biomolecules 2021, 11, 1772.
- Bharadwaj, A.; Kempster, E.; Waisman, D.M. The Annexin A2/S100A10 Complex: The Mutualistic Symbiosis of Two Distinct Proteins. Biomolecules 2021, 11, 1849.
- Salama, Y.; Lin, S.Y.; Dhahri, D.; Hattori, K.; Heissig, B. The fibrinolytic factor tPA drives LRP1-mediated melanoma growth and metastasis. FASEB J. 2019, 33, 3465–3480.
- Ichinose, A.; Kisiel, W.; Fujikawa, K. Proteolytic activation of tissue plasminogen activator by plasma and tissue enzymes. FEBS Lett. 1984, 175, 412–418.
- Sappino, A.P.; Huarte, J.; Vassalli, J.D.; Belin, D. Sites of synthesis of urokinase and tissue-type plasminogen activators in the murine kidney. J. Clin. Investig. 1991, 87, 962–970.
- Padró, T.; van den Hoogen, C.M.; Emeis, J.J. Distribution of tissue-type plasminogen activator (activity and antigen) in rat tissues. Blood Coagul. Fibrinolysis 1990, 1, 601–608.
- Kristensen, P.; Larsson, L.-I.; Nielsen, L.S.; Grøndahl-Hansen, J.; Andreasen, P.A.; Danø, K. Human endothelial cells contain one type of plasminogen activator. FEBS Lett. 1984, 168, 33–37.
- Silva, M.M.C.G.; Thelwell, C.; Williams, S.C.; Longstaff, C. Regulation of fibrinolysis by C-terminal lysines operates through plasminogen and plasmin but not tissue-type plasminogen activator. J. Thromb. Haemost. 2012, 10, 2354–2360.
- Collen, D.; Lijnen, H.R. The Tissue-Type Plasminogen Activator Story. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 1151–1155.
- Cheng, X.-F.; Brohlin, M.; Pohl, G.; Bäck, O.; Wallén, P. Binding of tissue plasminogen activator to endothelial cells: The effect on functional properties. Localization of a ligand in the B-chain of tPA. Thromb. Res. 1995, 77, 149–164.
- Sashindranath, M.; Sales, E.; Daglas, M.; Freeman, R.; Samson, A.L.; Cops, E.J.; Beckham, S.; Galle, A.; McLean, C.; Morganti-Kossmann, C.; et al. The tissue-type plasminogen activator–plasminogen activator inhibitor 1 complex promotes neurovascular injury in brain trauma: Evidence from mice and humans. Brain 2012, 135, 3251–3264.
- Heissig, B.; Eiamboonsert, S.; Salama, Y.; Shimazu, H.; Dhahri, D.; Munakata, S.; Tashiro, Y.; Hattori, K. Cancer therapy targeting the fibrinolytic system. Adv. Drug Deliv. Rev. 2016, 99, 172–179.
- Leu, S.; Day, Y.-J.; Sun, C.-K.; Yip, H.-K. tPA-MMP-9 Axis Plays a Pivotal Role in Mobilization of Endothelial Progenitor Cells from Bone Marrow to Circulation and Ischemic Region for Angiogenesis. Stem Cells Int. 2016, 2016, 5417565.
- Seillier, C.; Hélie, P.; Petit, G.; Vivien, D.; Clemente, D.; Le Mauff, B.; Docagne, F.; Toutirais, O. Roles of the tissue-type plasminogen activator in immune response. Cell. Immunol. 2022, 371, 104451.
- Hu, K.; Lin, L.; Tan, X.; Yang, J.; Bu, G.; Mars, W.M.; Liu, Y. tPA Protects Renal Interstitial Fibroblasts and Myofibroblasts from Apoptosis. J. Am. Soc. Nephrol. 2008, 19, 503.
- Das, L.; Azmoon, P.; Banki, M.A.; Mantuano, E.; Gonias, S.L. Tissue-type plasminogen activator selectively inhibits multiple toll-like receptors in CSF-1-differentiated macrophages. PLoS ONE 2019, 14, e0224738.
- Husain, S.S. Single-chain urokinase-type plasminogen activator does not possess measurable intrinsic amidolytic or plasminogen activator activities. Biochem.-Us 1991, 30, 5797–5805.
- Mahmood, N.; Mihalcioiu, C.; Rabbani, S.A. Multifaceted Role of the Urokinase-Type Plasminogen Activator (uPA) and Its Receptor (uPAR): Diagnostic, Prognostic, and Therapeutic Applications. Front. Oncol. 2018, 8, 24.
- Bansal, V.; Roychoudhury, P.K. Production and purification of urokinase: A comprehensive review. Protein Expr. Purif. 2006, 45, 1–14.
- Yu, S.; Sui, Y.; Wang, J.; Li, Y.; Li, H.; Cao, Y.; Chen, L.; Jiang, L.; Yuan, C.; Huang, M. Crystal structure and cellular functions of uPAR dimer. Nat. Commun. 2022, 13, 1665.
- Sillen, M.; Declerck, P.J. Thrombin Activatable Fibrinolysis Inhibitor (TAFI): An Updated Narrative Review. Int. J. Mol. Sci. 2021, 22, 3670.
- Tjärnlund-Wolf, A.; Brogren, H.; Lo, E.H.; Wang, X. Plasminogen Activator Inhibitor-1 and Thrombotic Cerebrovascular Diseases. Stroke 2012, 43, 2833–2839.
- Kubala, M.H.; DeClerck, Y.A. The plasminogen activator inhibitor-1 paradox in cancer: A mechanistic understanding. Cancer Metastasis Rev. 2019, 38, 483–492.
- Yasar Yildiz, S.; Kuru, P.; Toksoy Oner, E.; Agirbasli, M. Functional Stability of Plasminogen Activator Inhibitor-1. Sci. World J. 2014, 2014, 858293.
- Sillen, M.; Miyata, T.; Vaughan, D.E.; Strelkov, S.V.; Declerck, P.J. Structural Insight into the Two-Step Mechanism of PAI-1 Inhibition by Small Molecule TM5484. Int. J. Mol. Sci. 2021, 22, 1482.
- Smith, H.W.; Marshall, C.J. Regulation of cell signalling by uPAR. Nat. Rev. Mol. Cell Biol. 2010, 11, 23–36.
- Mahmood, N.; Mihalcioiu, C.; Rabbani, S.A. Multifaceted Role of the Urokinase-Type Plasminogen Activator (uPA) and Its Receptor (uPAR): Diagnostic, Prognostic, and Therapeutic Applications. Front. Oncol. 2018, 8, 24.
- Huang, J.; Liu, G.; Zhang, Y.; Cui, Z.; Wang, F.; Liu, X.; Chu, R.; Zhao, M. Urinary soluble urokinase receptor levels are elevated and pathogenic in patients with primary focal segmental glomerulosclerosis. BMC Med. 2014, 12, 81.
- Skrypnyk, M.; Petrushanko, T.; Neporada, K.; Vynnyk, N.; Petrushanko, V.; Skrypnyk, R. Colonization resistance of oral mucosa in individuals with diverse body mass index. J. Stomatol. 2022, 75, 171–175.
- Virtanen, O.J.; Sirén, V.; Multanen, J.; Färkkilä, M.; Leivo, I.; Vaheri, A.; Koskiniemi, M. Plasminogen activators and their inhibitors in human saliva and salivary gland tissue. Eur. J. Oral Sci. 2006, 114, 22–26.
- Skottrup, P.D.; Dahlén, G.; Baelum, V.; Lopez, R. Soluble urokinase-type plasminogen activator receptor is associated with signs of periodontitis in adolescents. Eur. J. Oral Sci. 2018, 126, 292–299.
- Taşdemir, İ.; Erbak Yılmaz, H.; Narin, F.; Sağlam, M. Assessment of saliva and gingival crevicular fluid soluble urokinase plasminogen activator receptor (suPAR), galectin-1, and TNF-α levels in periodontal health and disease. J. Periodontal Res. 2020, 55, 622–630.
- Wen, J.; Nikitakis, N.G.; Chaisuparat, R.; Greenwell-Wild, T.; Gliozzi, M.; Jin, W.; Adli, A.; Moutsopoulos, N.; Wu, T.; Warburton, G.; et al. Secretory leukocyte protease inhibitor (SLPI) expression and tumor invasion in oral squamous cell carcinoma. Am. J. Pathol. 2011, 178, 2866–2878.
- Sejima, T.; Holtappels, G.; Bachert, C. The Expression of Fibrinolytic Components in Chronic Paranasal Sinus Disease. Am. J. Rhinol. Allergy 2011, 25, 1–6.
- Firinu, D.; Arba, M.; Vincenzoni, F.; Iavarone, F.; Costanzo, G.; Cabras, T.; Castagnola, M.; Messana, I.; Del Giacco, S.R.; Sanna, M.T. Proteomic Analysis of the Acid-Insoluble Fraction of Whole Saliva from Patients Affected by Different Forms of Non-histaminergic Angioedema. J. Clin. Immunol. 2020, 40, 840–850.
- Wang, J.; Li, Y.; Pan, L.; Li, J.; Yu, Y.; Liu, B.; Zubair, M.; Wei, Y.; Pillay, B.; Olaniran, A.O.; et al. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) moonlights as an adhesin in Mycoplasma hyorhinis adhesion to epithelial cells as well as a plasminogen receptor mediating extracellular matrix degradation. Vet. Res. 2021, 52, 80.
- Guru, S.R.; Aghanashini, S. Impact of scaling and root planing on salivary and serum plasminogen activator inhibitor-1 expression in patients with periodontitis with and without type 2 diabetes mellitus. J. Periodontol. 2023, 94, 20–30.
