Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Suhaib Bandh and Version 2 by Jessie Wu.
Blue carbon was established as a metaphor to highlight that, apart from terrestrial ecosystems, coastal ecosystems also contribute significantly to carbon sequestration. Apart from being recognized as a helpful carbon sink, blue carbon ecosystems provide various other services, including shelter for different migratory birds, fishes, and crabs. It is also vital in minimizing net carbon emissions.
- blue carbon
- climate change
- biofuels
1. Spatiotemporal Distribution of Blue Carbon Ecosystems
Coastal vegetated habitats including salt marshes, seagrasses, and mangroves have long provided humans with advantages. More recently, notwithstanding data limitations, their importance as carbon reserves has been recognized in climate change mitigation [1][2][1,8]. As illustrated in Figure 1, spatiotemporal distribution and the importance of blue carbon ecosystems in controlling climate change can be seen [3][9].
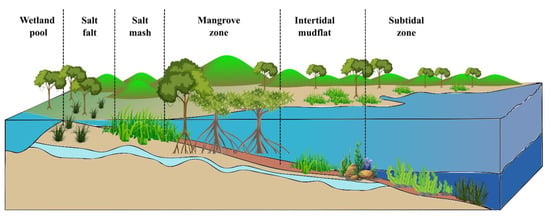
Figure 1.
Spatiotemporal distribution and the importance of blue carbon ecosystems.
Coastal zone environments, including seagrass beds, rocky reefs and corals, intertidal marshes, sandy beaches, kelp forests, and mangrove forests [4][10], help combat climate change by effectively storing and sequestering CO2, known as “coastal Blue Carbon” [5][11]. Salt marshes, seagrasses, and mangroves, for example, often form a spatially connected continuum of intertidal ecosystems. Unvegetated mudflats and sandbars are ecosystems that contain and sequester vast quantities of organic carbon [6][7][12,13]. Blue carbon soil is anaerobic, mainly in contrast to the terrestrial ground, which causes carbon stored in these soils to decay at a slow rate, and thus the carbon accumulates for hundreds to thousands of years [8][9][14,15]. The coastal wetland vegetation acts as a buffer zone between land and oceans, capable of storing surplus water during the rainy season and preventing floods [10][16]. They also help to protect coastlines and are considered to be more cost-effective than complicated structures such as seawalls and levees, as they are cheaper to manage and will be able to keep up with rising sea levels [11][12][17,18]. They also exhibit high burial rates leading to the seafloor’s rise, acting as a barrier against rising sea levels and wave actions linked with climate change [13][19]. They serve as a motivator for ecosystem-based adaptation to protect humans, infrastructure, and property from the negative impacts of climate change [14][20].
Mangroves occur in tropical and sub-tropical regions [15][21]. They are found in 118 countries worldwide, with 15 countries accounting for 75% of their overall coverage. West Africa is home to nearly a quarter of the world’s mangroves, containing almost 0.854 billion metric tons of carbon in below-ground and above-ground biomass [16][22]. Similarly, Indonesia alone accounts for 23% [17][23]. But a considerable loss of 0.16–0.39% per year has been recorded in the mangroves since 2000 [18][24]. Mangroves have an excellent ability to store carbon in the root system and act as carbon-rich forests in the tropics; hence their management and conservation need to be prioritized [19][25]. Geological evidence indicates the adaptation of mangroves to earlier climate and sea-level change [20][21][26,27]. They play a crucial role in promoting sedimentation in sensitive coastal regions, hence withstanding climate-induced impacts such as rising sea levels [22][28]. Their wide variety of aerial root structures such as pneumatophores, prop roots, plank roots, and knee roots help prevent soil erosion and differ in their efficacy to reserve sediments [23][29]. Moreover, mangroves speed up land development through a rise in sedimentation, lower wave exposure, and peat formation, consequently mitigating exposure to tropical storm surges and sea-level rise [24][30].
Seagrasses are another blue carbon ecosystem found mainly in shallow coastal margins across zero latitudes. Seagrasses use photosynthesis to take in carbon dioxide and assimilate it into their biomass. The above-ground/water vegetation traps suspended particulate matter (sedimentation) that later adds to the sedimentary storage component [25][31]. They have colossal mitigation potential for neutralizing CO2 emissions, which leads to improvements in carbon estimates stored in seagrass sediments and incorporate seagrass ecosystems [26][27][32,33]. The total global area under seagrass ranges from 300,000–600,000 km2 [28][34]. However, there has been a sharp decrease in recent decades, with a sevenfold decline reported from 1990 to 2009 [29][35]. Globally, seagrasses are declining by 2–5% each year as 30,000 Km2 of seagrass have been destroyed in recent decades [30][36]. Every year, organic carbon oxidation in degraded seagrass meadows potentially releases 0.03–0.33 petagrams of carbon dioxide back into the atmosphere [31][37]. Seagrasses cover 4.8 million hectares in West Africa, holding an estimated 673 million tons of carbon [16][22]. In coastal waters, the restoration of seagrasses has led to increased sequestration of blue carbon [32][38]. However, they have a poor carbon storage capacity compared to mangroves.
Unlike seagrasses and mangroves, salt marshes differ in having low methane emissions [33][34][39,40]. Salt marshes cover 1.2 million hectares in West Africa, holding 303 million metric tons of CO2 [16][22]. In recent studies, an area of 45,000 Km2 has been reported for salt marshes [35][41]. Apart from carbon sinks, they are prodigious inorganic carbon sources of coastal oceans [36][42]. Tidal marshes, mapped only in 43 countries of the world, represent 14% of the global coastal area [37][43]. The minimum yearly global loss rate of tidal marshes is 1–2% [38][44]. Although the blue carbon ecosystems have proved their ability as ideal carbon sinks, both natural and artificial threats destroy these ecosystems. Due to the rise in sea level, marshes sink to stress and shrink with time [39][45]. Further, marine accidents, such as massive oil spills, are also responsible for the damage to these ecosystems [40][41][42][46,47,48]. Hence, to avail the maximum benefits of these ecosystems, proper policymaking and guiding mechanisms should be established to preserve and manage these blue carbon ecosystems.
Other coastal ecosystems such as barrier islands, dunes, and beaches made of sand, play a pivotal role in dispersing wave energy, besides having vital sediment reserves that aid in preserving coastlines, and to a certain extent, in adapting to rising sea levels [43][44][49,50]. It is debatable if coral reefs are the sinks or sources of atmospheric CO2 [45][51]. However, they make remarkable structures, ranging from deep oceans to their surfaces and parallel to coastlines in many places extending up to several kilometres, in such a way that they form a significant part of the coastal defense. The mass flow of energy from overlying waters into the coral systems significantly reduces wave activity—a vital function of reef roughness [46][47][52,53]. However, as per Pendleton et al. [31][37], enormous reserves of carbon sequestered in the past are affected by the transformation of these coastal ecosystems, as blue carbon present in the sediments is released into the atmosphere when these ecosystems are degraded [31][37]. As a result, the value of blue carbon habitats in sequestering organic carbon has boosted conservation efforts as a means to reduce climate change and offset CO2 emissions [48][54]. Furthermore, their contribution to strengthening coastal resilience to weather disasters and changing climate has led to their participation in many countries’ nationwide defined commitments (NDCs) for climate change adaptation and mitigation [49][50][55,56].
2. Blue Carbon as a Potential Source for Biofuel Production
The sustainability of the first-generation bio-based fuels (1G) was also called into doubt since their utilization threatened the traditional food supply, particularly in developing nations [51][52][197,198]. The second-generation biofuels (2G/cellulosic biofuels) which were derived from cellulosic energy crops including municipal solid wastes, lignocellulosic residues, or agro-industrial wastes, provided an alternative option because of their plentiful availability [53][54][55][56][57][58][199,200,201,202,203,204]. However, this type of fuel also coped with a lot of failures due to higher investment expenses and technical problems in down streaming. Furthermore, the generation of 1G/2G biofuels necessitated additional crop cultivation acreage, and hence they could not be viewed as a viable alternative to fossil fuels because the yield gained might not fulfil the global energy demand. In addition, the third-generation biofuels (3G/advanced biofuels) were made from aquatic biomass such as algae [59][60][205,206]. Algae gained a lot of interest among third-generation biofuels because of their low lignin concentration and high productivity, which reduced the consumption of energy throughout fuel generation [61][62][207,208]. Moreover, blue carbon sources were biomasses that were morphologically and systematically more similar to plants on the ground than seaweeds [63][209]. Hence, exploiting blue carbon sources appeared to be an appealing solution for renewable energy generation, avoiding the significant drawbacks related to 1G and 2G bio-derived fuels. Indeed, the biofuel production pathway from blue carbon sources could be illustrated in Figure 2.
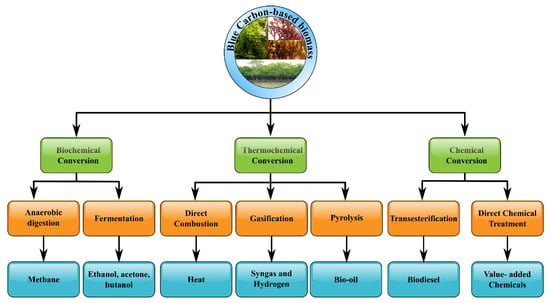
Figure 2.
Suggested technologies for biofuel production from blue carbon sources-based biomass.
It could be seen from Figure 2 that transesterification, direct combustion, gasification, or pyrolysis were all methods for producing biofuel from dry blue carbon-based biomass [64][65][66][67][210,211,212,213]. Meanwhile, energy generation techniques from wet blue carbon-based biomass included enzyme hydrolysis, hydrothermal treatments, anaerobic digestion, and fermentation to biobutanol/bioethanol/biohydrogen [68][69][70][71][214,215,216,217]. It was noted that utilizing blue carbon-based biomass for biofuel generation was in the early stages of research and development. Besides, a lot of non-glucose-derived sugars such as cell wall polysaccharides and mannitol were accumulated in seaweed, but not so many glucose-originated polysaccharides [72][218]. As a result, industrial bioethanol synthesis from blue carbon-based biomass necessitated the fermentation of not only non-glucose but also glucose-based sugars [73][219]. For chemical compositions, the blue carbon sources and terrestrial plants differed substantially in general. For example, seaweeds have high water content (90% fresh wt), protein content (from 7 to 15% dry wt), carbohydrate content (25–50% dry wt), as well as low concentration of lipid (between 1 and 5% dry wt) in comparison with terrestrial biomass [74][220].
As reported, the lipid content in the blue carbon sources was low; however, their carbohydrate content was high, permitting them to be employed as a feedstock for the generation of different fermentative bio-base fuels [75][221]. Though fermentation facilities using macroalgae were known as relatively expensive to operate and build, they were dependable and provided large yields [76][222]. This is partly because of the high content of water (from 70 to 90%), the protein concentration of around 10%, and the presence of various amounts of carbohydrates [77][223]. Furthermore, because there was a small amount of lignin and hemicellulose in macroalgae cells, the enzymatic and chemical pretreatment stages in the production of biofuel were removed [78][224]. More significantly, the carbohydrate concentration of macroalgae varied greatly depending on strains, species, and cultivars. In addition, because the potential growth speed and carbohydrate concentration of the green macroalgae Ulva lactuca were high, it was thought of as a promising aquatic energy crop [79][225]. Regarding brown macroalgae Laminaria spp., there could be up to 55% carbohydrates in it with dry weight, principally free sugars, cellulose, hemicellulose as well as the energy storage molecules mannitol and laminarin [80][226]. Indeed, biohydrogen generation from blue carbon received a lot of interest because of carbohydrate-rich blue carbon. In a study by Yukesh et al. [81][227], they improved the generation of biohydrogen from seagrass using new ozone-linked rotor-stator homogenization. In particular, rotor-stator homogenization required 510 kJ/kg TS of specific energy to accomplish 10.45% seagrass lysis while ozone-linked rotor-stator homogenization obtained 23.7% seagrass lysis with less energy (only 212.4 kJ/kg TS) input. It was noted that the ozone-coupled rotor-stator homogenization sample’s biohydrogen generation capability was evaluated and compared with using biohydrogenesis.
The generation of biogas was considered a long-time technology. Interestingly, there were multiple operational biogas systems, ranging from large-scale to small-scale and they were supplied by a variety of feedstocks such as animal wastes, agricultural products, certain residential rubbish, and sewage sludge [82][83][228,229]. Additionally, because macroalgae contained more water than terrestrial biomass (ranging between 80 and 85%), they were more suited for microbial conversion instead of thermochemical conversion [84][85][230,231]. Indeed, producing biogas from macroalgae was more technically feasible than generating biogas from other fuels because all organic components in macroalgae (such as protein, carbohydrates, and so on) could be transformed into biogas via anaerobic digestion [86][87][232,233]. Besides, a low lignocellulose concentration of macroalgae facilitated biodegradation more than that of their relative microalgae to create considerable amounts of biogas [88][89][234,235]. However, microalgae could be cultivated using pollutant water and CO2 [90][91][236,237] and could be used to synthesize many types of biofuels such as bioethanol, biodiesel, and bio-oil [92][93][94][95][238,239,240,241].
Many works successfully established the practical usefulness of seaweed as a feedstock for the anaerobic digestion process. For example, the generation of biogas from marine wrack might reduce GHG emissions while also bringing economic benefits to local island people. Apart from that, Marquez et al. [96][242] discovered biogas generation by employing three different microbial seeds including marine sediment, marine wrack-related microflora, and manure of cow. Accordingly, the authors discovered that the average biogas generated was 1223 mL from marine wrack-related microflora, 2172 mL from marine sediment, and 551 mL from the manure of cow. Although the methane potential at 396.9 mL CH4/g volatile solid was calculated using marine wrack proximate values in comparison with other feedstock, this parameter was low when the greatest methane yield of 94.33 mL CH4/g volatile solid was considered. Interestingly, among the microbial seeds tested, sediment in the marine platform was found to be the most effective source of microorganisms in terms of using seawater and marine wrack biomass to produce biogas. Nonetheless, sand deposition in salinity and digesters might cause trouble in the long-term anaerobic digestion process [97][98][243,244]. As observed, several factors, including growing method, species type, harvesting time, and seaweed production per hectare all made a great contribution to the anaerobic digestion process. It was noted that the balance of material and energy, harvesting biomass cost, carbon balance, as well as expenses of creating biogas from seaweed were not evaluated [99][100][245,246]. As reported, methane yields in biogas produced from the anaerobic digestion process of blue carbon sources could be changed with biochemical composition and they were linked to ash concentration and the degree of sugars stored [88][234]. Therefore, to increase methane yields, Banu et al. [101][247] used disperser-tenside (polysorbate 80) disintegration so as to improve the biomethanation ability of seagrass (namely Syringodium isoetifolium). Indeed, dispersion-assisted tenside disintegration had a more significant influence on bio-acidification as well as biomethanation assays in terms of methane production (0.256 g/g COD) and volatile fatty acid content (1100 mg/L) when compared to dispersion disintegration, which was 0.198 g/g COD; 800 mg/L. As a result, S. isoetifolium was seen as a potential substrate for achieving third-generation biofuel targets in the foreseeable future.
Apart from that, marine algae, which contained a high concentration of hydrolyzable carbohydrates, cellulose, glucan, and galactan, might serve as a possible feedstock to produce liquid biofuels [102][248]. As reported, two popular liquid transportation biofuels are synthetic biodiesel, bioethanol, and biobutanol using marine macroalgae feedstock. In comparison with edible as well as lignocellulosic biomass sources, marine macroalgae biomass was gaining popularity as a renewable feedstock to produce bioethanol [88][103][234,249]. As mentioned above, macroalgae possessed a high carbohydrate concentration and low lignin [104][250], making them appropriate for use as a substrate in the fermentation process to generate bioethanol after hydrolysis. The current techniques for bioethanol synthesis from seaweed were separate hydrolysis and fermentation, and simultaneous saccharification and fermentation, as illustrated in Figure 3 [74][105][106][220,251,252]. As for separate hydrolysis and fermentation, seaweed biomass was hydrolyzed before being fermented in discrete units using yeast or bacteria [72][107][218,253]. Regarding simultaneous saccharification and fermentation, however, both fermentation and hydrolysis occurred concurrently in a single stage [108][109][254,255].
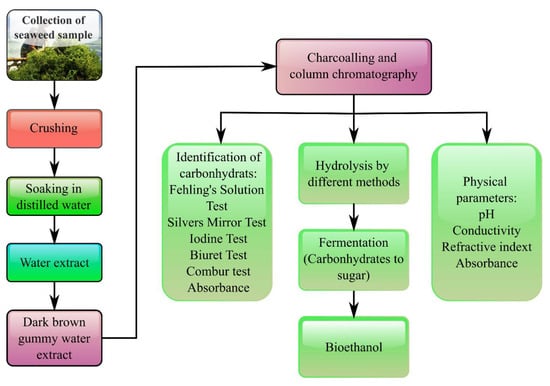
Figure 3.
Scheme of bioethanol production from seaweed via hydrolysis and fermentation process.
Even though experiments on bioethanol generation from macroalgae were scarce, it was not hard to determine that using marine macroalgae waste for bio-derived fuel feedstock could lead to less rivalry for biofuels among food [75][110][221,256]. According to several investigations, the findings of using seagrass biowaste for bioethanol production appeared to be promising in terms of making this a reality [111][112][113][257,258,259]. In an investigation by Mahmoud et al. [114][260], they employed seven samples of beach-cast seagrasses (associated with Z. marina, S. filiforme, Z. noltii, P. australis, T. testudinum, and P. oceanic) gathered from maritime environments worldwide with carbohydrate concentration ranging between 73% and 81% (w/dry weight of biomass). With no pretreatment, enzymatic hydrolysis with a single step was designed to effectively extract the monomeric sugars present in biomass originating from seagrass. In shake flasks, P. oceanica hydrolysate was observed to produce higher lipid yields (at 6.8 g/L) in comparison with the synthetic minimum medium (just 5.1 g /L). Additionally, it was then used as the only fermentation medium for oleaginous yeast T. oleaginous under the technical scale with the use of a fed-batch bioreactor, yielding 224.5 g /L lipids (0.35 g /L.h). Furthermore, the proportion of sugar/lipid conversion (w/w) was seen to be 0.41. According to cumulative statistics, roughly 4 million tons of microbial oils might be created by harvesting just half of the beach-cast seagrass in the world. Besides, Ravikumar et al. [111][257] presented their research on manufacturing bioethanol from seagrass biowastes with the use of Saccharomyces cerevisiae. The greatest bioethanol generation (0.047 mL/g) was observed in fresh seagrass leaves under acid pretreatment. As a result, fresh seagrass leaves might be one of the appropriate substrates for bioethanol synthesis. Furthermore, an investigation by Uchida et al. [115][261] studied the seagrass seeds (Zostera marina) bioethanol fermentation. On a dry weight basis, there were 83.5% carbs in the seeds, which included 48.1% crude starch. This parameter was equivalent to that of cereals such as corn and wheat flour. As reported, the saccharification of seeds went smoothly with no heating pretreatment, which showed that the starch present in seagrass seeds possessed a molecular form being ready to be digested by glucoamylase. Besides, the authors proposed that it might be possible to develop alcoholic drinks and foods from seagrass seeds, resulting in the creation of a unique marine fermentation sector in the future. The treatment of Laminaria japonica, Gelidium amansii, Ulva fasciata, Ulva lactuca, and Sargassum fulvellum biomass with acid and hydrolytic enzymes resulted in hydrolyzates with distinct proportions of mannose, glucose, mannitol, galactose, and other sugars [116][262]. As reported, Laminaria japonica hydrolyzate produced 0.4 g bioethanol for each gram of carbohydrate in case hydrolytic enzymes were utilized [117][263]. In another study, Adams et al. [118][264] investigated the generation of ethanol through laminarin polysaccharide yeast fermentation from the brown macroalga Saccharina latissimi using a variety of pretreatments. Meanwhile, in an experiment by Wi et al. [102][248], fermentation pretreatments were researched for a red microalgae species (namely Ceylon moss) with a high carbohydrate concentration (normally 23% galactose and 20% glucose). Accordingly, they proved that pretreatment approaches could be utilized to broaden the range of macroalga species appropriate for bioethanol generation. Moreover, Ge et al. [119][265] investigated the utilization of floating residual wastes from the industry of alginate from Laminaria japonica (a brown alga) to generate bioethanol after they were pretreated with diluted sulphuric acid as well as experienced enzymatic hydrolysis. Likewise, Horn et al. [120][266] showed the ability of fermented extracts of Laminaria hyporbea to synthesize ethanol with the employment of Pichia angophorea (yeast), while El-Sayed et al. [121][267] assessed the utilization of reducing sugars from U. lactuca to produce bioethanol via Saccharomyces cerevisiae.
In the case of biobutanol, there existed just a few studies that researched the manufacture of biobutanol from macroalgae. In other words, macroalgae, especially brown algae, and their potential for biochemical transformation to butanol and other solvents by Clostridium spp. via acetone-butanol fermentation were not studied. However, the brown macroalgae biomass’s acetone butanol fermentation feasibility via C. acetobutylicum was proved, and the results showed that the butanol content in the hydrolysate reached around 0.26 g butanol/g sugar while 0.29 g butanol/g sugar was obtained in the pilot investigation [122][123][268,269]. In addition, HMF was regarded as among the chemical platforms that have the most potential for the conversion of industrially important bio-originated chemical compounds. According to several researchers, a greater starch concentration was accumulated in seagrass seeds [124][125][270,271]. Moreover, several studies showed that raw biomass sources rich in non-structural carbohydrates, such as sucrose, fructose, starch, and glucose were employed as biomaterials for HMF generation [126][127][272,273]. Furthermore, by utilizing beach-cast seagrasses without feedstock expenses, seagrass feedstocks might contribute to sustainably and cost-effectively manufacturing HMF, which showed that seagrass biomasses were considered the most attractive source of bio-based feedstock to produce HMF sustainably.
Macroalgae were used to produce biogas and bioethanol instead of biodiesel since they lacked triglycerides. Typically, macroalgae were transformed into bio-derived oils such as free fatty acids and lipids, and more importantly, the lipids were separated to generate bio-based diesel. Even though free fatty acids were a precursor to biodiesel, the excessive quantity of free fatty acids in the oil might stymie the intended transformation. In an experiment, Tamilarasan [128][274] esterified the free fatty acids in Enteromorpha compressa algal oil from 6.3% to 0.34%, and subsequently, two stages for biodiesel synthesis were developed. More notably, Xu [129][275] recently tried to use macroalgae as a carbon source for oleaginous yeast aiming to create bio-based diesel, and the maximal lipid concentration was observed to reach 48.30%. In contrast, the by-product-free fatty acids accompanying mannitol could be utilized to cultivate the oleaginous yeast. Also, several innovative approaches, such as ultrasonic irradiation, were employed to support transesterification through the formation of fine emulsions between alcohol and oil, and the rate of reaction was enhanced due to cavitation [128][274]. Furthermore, biodiesel output from wet biomass achieved was nearly 10 times lower compared to that obtained from dry biomass, suggesting that water had a detrimental influence on transesterification experiments, and hence the dehydration process was required to attain high efficiency [130][276]. Moreover, Saengsawang et al. [131][277] investigated whether Rhizoclonium sp. oil could be employed as a biodiesel alternative to optimize the reaction conditions required for the process of chemical transesterification. The biodiesel weight of 0.174 ± 0.034 g along with 82.2% of the whole FAME was produced during the transesterification procedure from macroalgae oil. Besides, this research indicated that biodiesel produced from Rhizoclonium might be utilized as an alternative fuel, and more research would make it appropriate for large-scale manufacturing.
Thermochemical techniques are also considered potential solutions for converting biomass sources into biofuels [132][133][134][278,279,280]. Indeed, pyrolysis was the most used technique for extracting bio-oil [84][230, [135]281]. Pyrolytic cracking could quickly transform dried seaweed biomass into bio-originated oil and solid residue. Furthermore, investigations on the behaviors of pyrolysis and product properties of some macroalgae, such as brown algae, green algae, and red algae [92][136][238,282], revealed that the macroalgae’s pyrolysis process to produce biofuels and that of terrestrial plants were alike [137][138][283,284], even though the macroalgae had higher activation energy than that of terrestrial biomass [139][285]. Importantly, pyrolysis of macroalgae operating under 500 °C was shown to be a favorable temperature for maximizing bio-oil output [108][140][254,286]. Liquefaction was seen as a process where biomass experienced complex thermochemical reactions in a solvent solution, resulting in mostly liquid products. Remarkably, hydrothermal liquefaction mostly neglected macroalgae in the role of a feedstock for bio-originated oil since microalgae were assumed to have a greater lipid concentration intrinsically [141][142][287,288]. Elliott et al. [143][289] reported on the hydrothermal liquefaction of Macrocystis sp. with the employment of a batch reactor that was fed with 10% kelp dry mass in water. According to the oil product’s solvent separation, an oil yield of 19.2 wt% was observed. Utilizing Na2CO3 as a catalyst, Zhou et al. [144][290] investigated the hydrothermal liquefaction of the green marine macroalgae named Enteromorpha prolifera and got a maximal bio-oil output of 23.0% dw as well as an energy density of 29.89 MJ/kg. In another study, Neveux et al. [145][291] used hydrothermal liquefaction in a batch reactor to convert six types of freshwater and marine green macroalgae into bio-crude. The findings showed that the high ash concentration of macroalgae caused poorer bio-oil yields when compared to the results achieved from hydrothermal liquefaction of a variety of microalgae (in the range of 26–57% dw) [146][292]. Although the gasification of biomass on a wide scale was successfully demonstrated, it was still comparatively costly in contrast to fossil-fuel energy [147][148][293,294]. Indeed, gasification was able to generate hydrogen and syngas at a competitive price in the market. Actually, several nations had very few pilot gasification factories, more widespread industrial penetration appeared to be dependent on integration into the chain of biofuel from seaweed [149][295]. Table 1 compared and showed the benefits and drawbacks of several biofuel generation methods from blue carbon sources.
Table 1. Advantages and disadvantages of various processing techniques for converting blue carbon to biofuels [74][150][151][152][153][154].
Advantages and disadvantages of various processing techniques for converting blue carbon to biofuels [220,296,297,298,299,300].
| Processing Techniques | Target Products | Benefits | Drawbacks |
|---|---|---|---|
| Anaerobic digestion | Biogas | Finishing technology without drying process | High inhibition and salt |
| Fermentation | Bioethanol/biobutanol | High content of carbohydrate | Low efficiency in forming various mixed sugars |
| Transesterification | Biodiesel | No required the dewatering process | Low yield |
| Pyrolysis/Gasification/Liquefaction | Bio-oil, syngas, hydrogen, bio-char | Fast rate without required chemicals | High energy consumption |
| Processing Techniques | Target Products | Benefits | Drawbacks |
|---|---|---|---|
| Anaerobic digestion | Biogas | Finishing technology without drying process | High inhibition and salt |
| Fermentation | Bioethanol/biobutanol | High content of carbohydrate | Low efficiency in forming various mixed sugars |
| Transesterification | Biodiesel | No required the dewatering process | Low yield |
| Pyrolysis/Gasification/Liquefaction | Bio-oil, syngas, hydrogen, bio-char | Fast rate without required chemicals | High energy consumption |
