Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Sirius Huang and Version 1 by lai hongbin.
Extracellular vesicles (EVs) are lipid bound particles derived from their original cells, which play critical roles in intercellular communication through their cargoes, including protein, lipids, and nucleic acids. Status of EVs in pulp/periodontal tissue regeneration and the potential mechanisms are discussed.
- extracellular vesicle
- pulp regeneration
- periodontal regeneration
1. Introduction
Extracellular vesicles (EVs) are encapsulated particles secreted by all types of parent cells. In addition, EVs can be found in various biological fluids, such as blood, urine, and saliva [1,2][1][2]. They were firstly observed in plasma 50 years ago [3], but were regarded as cellular waste and thought insignificant for a long time. However, research in the past decade has markedly increased our knowledge about EVs in terms of their classification, characteristics, and functions. With respect to the guidelines proposed by the International Society of Extracellular Vesicles (ISEV) in 2018, EVs can be characterized by their size, origin, protein composition, and functions [4]. EVs are all enclosed by a lipid bilayer and carry complex contents such as proteins, lipids, and nucleic acids [5,6][5][6]. They participate in both physiological and pathological processes and exert similar functions to their origin cells through intercellular communication and material transmission [5,7][5][7]. The application of EVs as disease biomarkers, therapeutic targets, novel drug agents, and acellular therapeutics has attracted considerable attention [6].
Dental pulp is a vital, highly vascularized, and innervated tissue that provides several functions for teeth, such as response to bacterial insult and injury. The presence or absence of dental pulp can greatly affect the prognosis of a treated tooth [8]. However, dental pulp is susceptible to trauma and infection caused by dental caries, periodontitis, retrograde infection, or iatrogenic causes, which eventually leads to irreversible pulp injury or necrosis [9]. Endodontic therapy has been considered the primary choice for clinical treatment; however, this approach increases the fracture rate of treated teeth and the failure rate of endodontic therapy ranges from 19.1% to 25.3% [10]. Therefore, dental pulp regeneration has marked advantages in maintaining the function of teeth after pulp disease. Although cell-based therapies have been reported to be able to regenerate three-dimensional pulp tissue with blood vessels and sensory nerves [11], the application of cell-based therapies has been limited because of concerns regarding immune rejection, safety, or medical ethics [12]. Thus, there is an urgent need for alternative cell-free approaches.
Periodontitis is a highly prevalent, multifactorial, chronic inflammatory disease of the periodontium characterized by the irreversible destruction of tooth-supporting structures, including the gingiva, periodontal ligament (PDL), cementum, and alveolar bone [13]. Over 30% of adults worldwide are subjected to periodontitis, which is the main cause of adult tooth loss. However, regular treatment strategies have limited efficacy in regenerating damaged periodontal tissues [14]. Stopping disease progression and maintaining therapeutic achievements are the major goals of conventional periodontal treatments; however, complete and functional periodontal regeneration remains a clinical challenge [15].
2. EVs in Pulp Regeneration
EVs play a critical role in multiple tissue regeneration, including that of dental pulp [17][16]. Regenerative endodontics comprises several aspects, including pulp revascularization, pulpal tissue regeneration, dentin formation, and neurological recovery, which involves the migration, proliferation, and differentiation of vascular endothelial cells and dental stem cells, such as dental pulp stem cells (DPSCs), periodontal ligament stem cells (PDLSCs), gingival mesenchymal stem cells (GMSCs), dental follicle stem cells (DFSCs), stem cells from human exfoliated deciduous teeth (SHED), and stem cells from apical papilla (SCAP). The potential functions of EVs, mostly derived from dental stem cells, in endodontic regeneration will be discussed below and summarized in Table 1 and Figure 1.
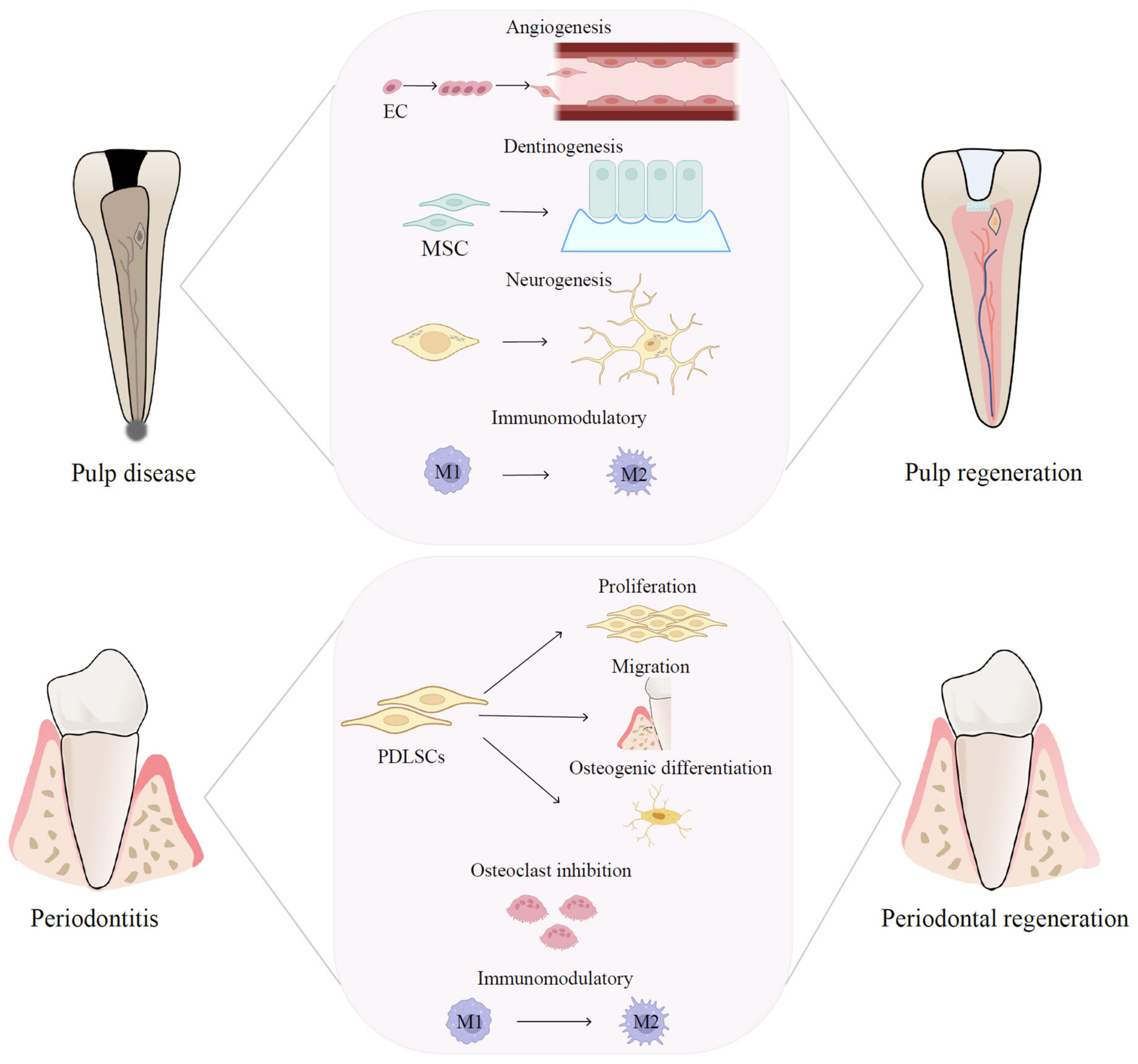
Figure 1. Therapeutic mechanisms of EVs in pulp and periodontal regeneration. EV-based therapies are promising therapeutic approaches for pulp and periodontal regeneration and have the potential to regulate the immune microenvironment, boost angiogenesis, facilitate neural regeneration, and promote MSC proliferation and differentiation.
2.1. Pulp Vascularization
Pulp vascularization is fundamental for functional pulp regeneration because sprouting angiogenesis is the predominant process during pulp regeneration and therapeutic processes [87][17]. The key point for successful angiogenesis during pulp regeneration is to provide an adequate blood supply. EVs derived from various stem cells have been reported to promote angiogenesis by increasing endothelial cell viability, proliferation, migration, tube formation, and ameliorating damaged endothelial cells (ECs) [88][18]. Interestingly, EVs from dental stem cells exhibited a high potential for pulp vascularization [16][19]. Xian et al. revealed that dental pulp cell-derived EVs are capable of facilitating human umbilical vein endothelial cell (HUVEC) proliferation, tube formation, and proangiogenic factor expression, including fibroblast growth factor 2 (FGF-2), vascular endothelial growth factor A (VEGFA), kinase insert domain receptor (KDR), and matrix metalloproteinase 9 (MMP-9), indicating vital roles in angiogenesis. Moreover, p38 MAPK signaling inhibition enhanced EV-induced tube formation, suggesting that p38 MAPK signaling participates in EV-mediated angiogenesis [89][20]. Using a tooth fragment model in immunocompromised mice, Wu et al. showed that SHED aggregate-derived EVs (SA-EVs) effectively improved pulp tissue regeneration and angiogenesis in vivo via promoting SHED endothelial differentiation and enhancing the angiogenesis of HUVECs. Mechanistically, SA-EV-transferred-miR-26a improved angiogenesis both in SHED and HUVECs by regulating the TGFβ/SMAD2/3 pathway, which contributes to pulp regeneration [90][21].
Apoptosis is an autonomously regulated programmed cell death and used to be considered a passive phenomenon, whereas recent studies suggest that apoptosis has an important role in modulating tissue homeostasis and regeneration [44,91,92][22][23][24]. Cells undergo apoptosis after implantation in an ischemic-hypoxic environment; however, the roles of EVs released by apoptotic cells are largely unknown. Recently, Li et al. demonstrated that ApoVs released by SHED can be internalized by endothelial cells to augment their angiogenic capacities, including their proliferation, migration, differentiation, and secretion. ApoV-shuttled mitochondrial Tu translation elongation factor could modulate the activation of endogenous ECs through the transcription factor EB-autophagy pathway. In a beagle model, endogenous ECs were recruited by ApoVs to promote the formation of blood vessel-rich dental-pulp-like tissue [92][24]. The experimental data revealed the significance of apoptosis in tissue regeneration and demonstrated the potential of using ApoVs to promote angiogenesis in pulp regeneration.
Notably, EVs derived from cells in an inflammatory state possess higher proangiogenic potential. EVs secreted by DPSCs isolated from periodontally diseased teeth exerted enhanced potential to promote the angiogenesis of ECs in vitro and were better able to accelerate cutaneous wound healing and promote vascularization in vivo, compared with those from periodontally healthy teeth [93][25]. Similarly, the number of EVs derived from PDLSCs was enhanced by inflammation, while EVs could promote the angiogenesis of HUVECs by mediating VEGFA transfer via miR-17-5p [94][26]. Furthermore, EVs originating from lipopolysaccharide (LPS)-preconditioned DPSCs had a more significant effect in modulating BMMSC proliferation, migration, angiogenesis, and differentiation compared with EVs isolated from normal cells. The results demonstrated that EVs released by DPSCs in a mild inflammatory microenvironment are capable of facilitating the regeneration of dental pulp through functional healing instead of scar healing, which has potential applications in regenerative endodontics [95][27]. The above findings confirmed a generally accepted conclusion that submitting cells to suitable environmental stress factors, such as radiation, oxidative stress, hypoxia, or inflammation, could increase the pro-angiogenic potential of their EVs. The underlying angiogenesis potential of EVs stimulated by stress factors needs to be explored for dental pulp regeneration.
2.2. Nerve Regeneration and Neural Repair
Neuralization of the damaged tissue is critical for the regeneration of functional dental pulp because nerves perform sensory functions in the pulp and are responsible for responding to external stimuli [96][28]. The ideal regenerative endodontics also involve nerve regeneration and neural recovery. Dental stem cells, originating from cranial neural crests, are suitable for the induction of neural differentiation during pulp regeneration procedures. Previous studies have shown that transplantation of DPSCs can promote neurite extension and neuron growth during pulp regeneration, but little is known about the neurogenesis capability of EVs for pulp regeneration [11,97][11][29]. EVs have been reported to improve neural recovery and induce neurogenesis in some neural diseases [98][30]. Schwann cells (SCs) play a vital role in the support, maintenance, and regeneration of nerve fibers in dental pulp [99][31]. Recently, Wang et al. revealed that EVs derived from SCs (SC-EVs) enhanced neurite outgrowth and neuron migration of rat dorsal root ganglia explants after coculture, suggesting that SC-EVs are able to promote neural regeneration and might facilitate the regeneration of functional nerve fibers in dental pulp tissue [98][30]. To investigate the role of EVs from the dental pulp tissue (DPT-EVs), Chen et al. built an in vivo “cell homing” model by filling the root canal of swine teeth with a mixture of treated dental matrix and DPT-EV-laden scaffolds. After 8 weeks of subcutaneous implantation into immunodeficient nude mice, the results showed that the DPT-EVs promoted the neurogenetic differentiation of SCAP by expressing neurogenetic markers MBP101 and neurofilament protein (NF200), indicating the potential of DPT-EVs in nerve regeneration [96][28].
Recent studies suggested the positive effects of EVs on neurogenesis, indicating that EVs are a potential biomimetic tool during pulp regeneration. However, the underlying mechanism and role of EVs during pulp nerve regeneration and neural recovery remains to be clarified.
2.3. Dentin-Pulp Complex Regeneration
An ideal outcome of dental pulp regeneration also requires recruited or implanted MSCs’ multipotency to form the dentin-pulp complex, which is the frontier of reparative dentin formation and the foundation of ideal pulp regeneration [100][32]. On this basis, the biological function of dental pulp is subsequently reconstructed. EVs have been proven to improve cell differentiation and can be used as a powerful tool for pulp regeneration [101][33].
Endocytosis of dental pulp cell-derived EVs by DPSCs and BMMSCs induced the odontogenic differentiation of both cells by triggering the increased expression of DSPP (encoding dentin sialophosphoprotein). When tested in vivo, EVs also triggered the regeneration of dental pulp-like tissue. Interestingly, EVs derived from cells under odontogenic conditions are more potent in inducing lineage-specific differentiation of DPSCs, suggesting that the source and state of EVs are critical for their therapeutic potential [102][34]. Hu et al. further investigated the underlying mechanism via microRNA sequencing and pathway analysis. They found that 28 microRNAs in EVs extracted from DPSCs cultured in odontogenic medium were significantly changed compared with those in regular medium. In addition, conditioned EV treatment or transfection of miR-27a-5p, which was upregulated in EVs under odontogenic induction, both enhanced TGFβ signaling and promoted odontogenic differentiation of DPSCs. Further experimental data verified that the EV-encapsulated miR-27a-5p promoted odontogenic differentiation through the TGFβ1/SMADs signaling pathway by downregulating latent TGF-β-binding protein 1 (LTBP1) [103][35]. Recently, EVs derived from SCAPs were observed to be taken up by BMMSCs and markedly improved their specific dentinogenesis in root fragments transplanted into nude mice [104][36]. A previous study reported that EVs derived from a Hertwig’s epithelial root sheath (HERS) cell line could promote the odontogenic differentiation of DPSCs and enhance dentin-pulp complex formation [105][37]. All these findings indicated that the use of EVs from dental stem cells could constitute a potential therapeutic approach for dentine-pulp complex regeneration in regenerative endodontic procedures.
2.4. Immunomodulatory Properties in Pulp Regeneration
The continuous production of inflammatory cytokines in pulp would maintain inflammatory reactions, eventually resulting in dental pulp necrosis. Therefore, suitable inflammation conditions are necessary for pulp regeneration. EVs inherit the function of their parent cells and have lower immunogenicity; therefore, they play important roles in immune regulation and tissue regeneration [106][38]. For example, EVs derived from human GMSCs (GMSC-EVs) could inhibit the inflammatory response of PDLSCs by regulating the expression of NF-κB signaling and Wnt5a, which restored the regenerative potential of PDLSCs and promoted periodontal tissue regeneration in patients with periodontitis [107][39].
Macrophages are the most abundant immune cells in pulp, acting as the critical regulators of inflammation-related diseases, such as pulpitis. They are crucial for inflammatory pulp regeneration because their interactions with pulpal inflammation can create a regulatory microenvironment for the odontogenesis of stem cells [108][40]. Zheng et al. clarified that microRNA-enriched EVs derived from DPSCs (DPSC-EVs) possess odonto-immunomodulatory properties by switching macrophages from the M1 to the M2 phenotype to enhance the odontogenesis of DPSCs. Mechanistically, miR-125a-3p was significantly upregulated in DPSC-EVs, which was proven to mediate macrophage phenotype switching via inhibition of NF-κΒ and Toll like receptor (TLR) signaling. Moreover, DPSC-EVs and the encapsulated miR-125a-3p both enhanced bone morphogenetic protein 2 (BMP2) release in macrophages, promoting the odontogenesis of DPSCs through BMP2 pathway activation [109][41]. The immune microenvironment is critical during inflammatory dental pulp regeneration, especially T cell-based immune functions. A previous study showed that SCAP-EVs could enhance Treg conversion and effectively alleviate inflammation in the dental pulp of rats, indicating that SCAP-EVs can modulate the local immune microenvironment to support tissue regeneration [110][42]. Recent studies have shown that EVs can alleviate inflammation to promote pulp regeneration in vitro and in vivo (Table 1); however, the detailed mechanism of how EVs regulate the immune balance during pulp regeneration requires further investigation.
Table 1.
Therapeutic effects of EVs in dental pulp regeneration.
| Origin of EVs | Types of EVs | Study Models | Key Functions/Potential Molecular Mechanism | References |
|---|---|---|---|---|
| Dental pulp stem cells (DPSCs) | sEVs | In vitro and in vivo (teeth root slices implanted subcutaneously into nude mice dorsum) | Induced lineage-specific differentiation of stem cells/Triggered the P38 MAPK pathway | Huang et al., 2016 [102][34] |
| Dental pulp cells | sEVs | In vitro | Promoted angiogenesis/Enhanced tubular morphogenesis via p38 MAPK signaling inhibition | Xian et al., 2018 [89][20] |
| DPSCs | sEVs | In vitro and in vivo (dental pulp capping in SD rat teeth) | Enhanced odontogenesis by switching macrophages toward pro-healing M2 cells/Promoted odontogenesis in DPSCs through BMP2 pathway activation |
Zheng et al., 2020 [109][41] |
| Hertwig’s epithelial root sheath (HERS) cells | sEVs-like vesicles | In vitro and in vivo (transplantation in renal capsule of rat; teeth root slices transplanted subcutaneously into nude mice dorsum) | Triggered regeneration of dental pulp-dentin-like tissue comprised of hard (reparative dentin-like tissue) and soft (blood vessels and neurons) tissue/Endocytosis of sEVs triggered the activation of P38 MAPK pathway | Zhang et al., 2020 [105][37] |
| DPSCs | EVs | In vitro | Promoted angiogenesis in an injectable hydrogel in vitro | Zhang et al., 2020 [111][43] |
| Stem cells from apical papilla (SCAPs) | sEVs | In vitro and in vivo (teeth root fragments implanted subcutaneously into nude mice dorsum) | Endocytosed by bone marrow-derived mesenchymal stem cells (BMMSCs) and significantly improved their specific dentinogenesis | Zhuang et al., 2020 [104][36] |
| Dental pulp cells | sEVs | In vitro | Induced the recruitment and proliferation of human mesenchymal stem cells | Ivica et al., 2020 [112][44] |
| Stem cells from human exfoliated deciduous teeth (SHED) | sEVs | In vitro and in vivo (teeth root fragments implanted subcutaneously into mice dorsum) |
Shuttled miR-26a to promote angiogenesis via TGF- β/SMAD2/3 signaling | Wu et al., 2021 |
| [ | ||||
| 92 | ||||
| ] | ||||
| [ | ||||
| 24 | ||||
| ] | ||||
3. EVs in Periodontal Regeneration
The American Academy of Periodontology defined periodontal regeneration as the formation of a new cementum, alveolar bone, and a functional periodontal ligament over a previously diseased root surface [118][50]. Tissue engineering and cell-based therapies have been considered novel alternatives to overcome the limitations of existing therapies, because of the reported functions of stem cells in promoting and regulating tissue regeneration [15,119][15][51]. MSCs participate in the regeneration of defective or damaged periodontal tissues because of their high proliferation, multipotency, paracrine effects, and immune regulation [120][52]. As an important part of the stem cell secretome, EVs have been demonstrated to participate in periodontal tissue repair and regeneration.
In 2019, Chew et al. investigated the therapeutic effects of MSC-EV-loaded collagen sponges to regenerate surgically created periodontal intrabony defects in an immunocompetent rat model. The data showed that EV-treated rats repaired the defects more efficiently with the regeneration of periodontal tissues, including newly-formed bone and PDL, possibly via increasing PDLSCs migration and proliferation [121][53]. PDLSCs, residing in the perivascular space of the periodontium possess MSC potentials and are a therapeutic target for periodontal regeneration [122][54]. A later study reported that EVs secreted from healthy PDLSCs (h-PDLSC-EVs) promoted the osteogenic differentiation of PDLSCs derived from periodontitis tissue. h-PDLSC-EV treatment accelerated bone formation in the defect of alveolar bone in rat models of periodontitis. Mechanistically, h-PDLSC-EVs inhibited the over-activation of canonical Wnt signaling to recover the osteogenesis of inflammatory PDLSCs [123][55]. Recently, EVs isolated from DFSCs (DFSC-EVs) have also been demonstrated to improve periodontal tissue regeneration by promoting the proliferation, migration, and osteogenic differentiation of PDLSCs. The effect of DFSC-EVs might be partially induced by the activation of the p38 MAPK signaling pathway [124][56]. BMMSCs are crucial for desired bone regeneration. EVs derived from SHED (SHED-EVs) have been reported to directly promote osteogenesis of BMMSCs and suppress adipogenesis, thereby enhancing bone formation in a mouse model of periodontitis [125][57]. SHED-EVs also present a potential to mobilize naïve BMMSCs, suggesting their relevance in assisting bone regeneration [126][58]. Local administration of EVs to treat periodontitis has limitations, such as the short half-lives of EVs and their rapid diffusion away from the delivery site. To overcome these drawbacks, “dual delivery” microparticles have been designed, which not only facilitate the microenvironment-sensitive release of EVs by metalloproteinases at the affected site but also are loaded with antibiotics to suppress bacterial biofilm growth. The results showed that the one-time administration of immunomodulatory GMSC-EV-decorated microparticles led to a significant improvement in the regeneration of the damaged periodontal tissues [127][59].
Periodontitis is mainly caused by a host immune-inflammatory response to bacterial insult; therefore, it is critical to suppress the inflammatory immune microenvironments that mediate periodontal tissue damage for high-quality healing [14]. EVs derived from dental stem cells can modulate the local immune microenvironment to support pulp regeneration, which also exhibit their immunoregulation effects in periodontal regeneration. Zarubova et al. have shown that GMSC-EVs can reduce the secretion of pro-inflammatory cytokines by immune cells, inhibit T-cell activation, and induce the formation of Tregs in vitro. In a rat model of periodontal disease, GMSC-EVs led to a significant improvement in the regeneration of the damaged periodontal tissue [127][59]. DPSC-EVs can alleviate periodontitis-induced epithelial lesions and reduce alveolar bone loss in experimental mice by converting macrophages from a pro-inflammatory phenotype (M1) to an anti-inflammatory phenotype (M2). It was further clarified that miR-1246 within DPSC-EVs accounted for the therapeutic effect [128][60]. Research suggested that macrophages are also critically involved in the periodontal regeneration mediated by EVs, similar to their roles in pulp regeneration. Other research demonstrated that BMMSC-EVs suppressed the development of periodontitis and immune damage of periodontal tissue, partly attributed to the regulation of macrophage polarization, TGF-β1 expression, and osteoclast function [129][61]. In accordance with the response to an inflammatory environment in pulp regeneration, studies also reported that preconditioned EVs possess enhanced therapeutic potential in periodontal bone defects. GMSCs under TNF-α stimulation not only secreted increased amounts of EVs but also regulated inflammation by inducing M2 macrophage polarization. Moreover, EVs derived from TNF-α-preconditioned GMSCs greatly enhanced their therapeutic efficiency in reducing periodontal bone resorption and the number of osteoclasts in a mouse model of periodontitis, which was mediated by the upregulated miR-1260b within EVs targeting the Wnt5a-mediated receptor activator of nuclear factor kappa B ligand (RANKL) pathway and inhibiting its osteoclastogenic activity [130][62]. Similarly, Kang et al. reported that the TNF-α-preconditioned MSC-EVs possess enhanced immunomodulatory properties by suppressing M1 macrophages and increasing M2 macrophages in vitro, and reducing inflammation in vivo in a rat calvarial defect model. An analysis of EV miRNA composition revealed significant changes in anti-inflammatory miRNAs in the preconditioned MSC-EVs [131][63]. Taken together, these studies indicated the specific function of TNF-α-preconditioned EV miRNAs in the immunomodulatory control of periodontal and bone regeneration. Paradoxically, LPS-preconditioned EVs were beneficial to repair lost alveolar bone in the early stage of treatment and to maintaining the level of alveolar bone in the late stage of treatment in experimental periodontitis rats; however, the therapeutic potential was similar to the unstimulated EVs [132][64]. Further investigations are required to explain the discrepancy.
The above studies showed that EV-based therapies, especially dental EVs, are promising therapeutic approaches and have the potential to enhance the success of periodontal regeneration, as summarized in Table 2 and Figure 1. However, the clinical application of EVs is still challenging and further clinical investigations are needed to testify to the effects of EVs on patients.
Table 2.
Therapeutic effects of EVs in periodontal regeneration.
| Origin of EVs | Types of EVs | Study Models | Key Functions/Potential Molecular Mechanism | References | |||
|---|---|---|---|---|---|---|---|
| Adipose-derived stem cells | sEVs | In vitro and in vivo (ligature-induced periodontitis in rat) |
Induced highly organized structures formation that was comparable to normal healthy periodontal tissue | Mohammed et al., 2018 [133][65] |
|||
| Mesenchymal stem cells (MSCs) | sEVs | In vitro and in vivo (periodontal intrabony defect in rat) | Increased periodontal ligament (PDL) cell migration and proliferation through CD73-mediated adenosine receptor activation of pro-survival AKT and ERK signaling | Chew et al., 2019 [121][53] | |||
| Dental follicle cells (DFCs) | sEVs | In vitro and in vivo (ligature-induced periodontitis in rat) |
Inhibited osteoclast formation and promoted periodontal regeneration via OPG/RANK/RANKL signaling pathway | Shi et al., 2020 [132][64] | |||
| DPSCs | sEVs | In vitro and in vivo (ligature-induced periodontitis in mouse) |
Modulated macrophages from a pro-inflammatory phenotype to an anti-inflammatory phenotype in the periodontium of mice with periodontitis, which could be associated with miR-1246 | Shen et al., 2020 [128][60] | |||
| SHED | sEVs | In vitro and in vivo (ligature-induced periodontitis +Porphyromonas gingivalis inoculation in mouse) | Directly promoted BMSCs osteogenesis, differentiation, and bone formation | Wei et al., 2020 [125][57] |
|||
| BMMSCs | sEVs | In vitro and in vivo (ligature-induced periodontitis in rat) |
Promoted the regeneration of periodontal tissues/Regulated the inflammatory immune response via the OPG–RANKL–RANK signaling pathway | Liu et al., 2021 [129][61] | |||
| [ | |||||||
| 90 | ] | [ | 21 | ] | |||
| DPSCs | sEVs | In vitro | Lipopolysaccharide (LPS)-stimulated sEVs displayed a better ability on regulating SCs migration and odontogenic differentiation than normal sEVs | Li et al., 2021 [113][45] |
|||
| DPSCs | sEVs | In vitro | LPS stimulated sEVs (LPS-sEVs) showed better proangiogenic potential of HUVECs compared with control sEVs/The expression of miR-146a-5p, miR-92b-5p, miR-218-5p, miR-23b-5p, miR-2110, miR-27a-5p, and miR-200b-3p was increased in the LPS-sEVs/The expression of miR-223-3p, miR-1246, and miR-494-3p was decreased in the LPS-sEVs | Huang et al., 2021 [114][46] | |||
| Dental pulp cells | sEVs | In vitro and in vivo (dental pulp capping in minipig teeth) | sEVs-treated dentin matrix promoted the formation of continuous reparative dentin | Wen et al., 2021 [115][47] | |||
| Dental pulp tissue and cells | sEVs | In vitro and in vivo (swine teeth implanted subcutaneously into mice dorsum) | Recruited SCAPs to regenerate connective tissue similar to natural dental pulp | Chen et al., 2022 [96][28] | |||
| Platelet | sEVs | ||||||
| Gingival mesenchymal stem cells (GMSCs) | sEVs | In vitro and in vivo (ligature-induced periodontitis in mouse) |
Induced the resolution of inflammation and prevented bone loss in the periodontal tissue/Inhibited osteoclastogenesis via the Wnt5a-mediated RANKL pathway | Nakao et al., 2021 [130][62] | |||
| Periodontal ligament stem cells (PDLSCs) | sEVs | In vitro and in vivo (surgically created periodontal defect and ligature-induced periodontitis +LPS inoculation in rat) |
Accelerated bone formation in the defect of alveolar bone in rat models of periodontitis/Recovered the osteogenic differentiation capacity of inflammatory PDLSCs via suppressing the over-activation of canonical Wnt signaling | Lei et al., 2022 [123][55] | |||
| Dental follicle stem cells (DFSCs) | sEVs | In vitro and in vivo (surgically created periodontal defect in rat) | Promoted periodontal tissue regeneration/Enhanced the proliferation, migration, and osteogenic differentiation of PDLSCs through the p38 MAPK signaling pathway | Ma et al., 2022 [124][56] | |||
| DFCs | MVs | In vitro and in vivo (surgically created periodontal defect in rat) | Strengthened alveolar bone regeneration through activating PLC/PKC/MAPK pathways | Yi et al., 2022 [134][66] | In vitro | 5% thrombin-activated platelet-derived sEVs had a high potential to induce dental pulp regeneration | Bagio et al., 2022 [116][48] |
| Dental pulp cells | EVs | In vitro and in vivo (subcutaneous transplantation in nude mice) | Regulated cellular NFIC level in SCAPs to promote the proliferation, migration of SCAPs, and dentinogenesis | Yang et al., 2022 [117][49] |
|||
| SHED | ApoVs | In vitro and in vivo (teeth fragments implanted subcutaneously into nude mice dorsum; orthotopic model of beagle dog) | Recruited endogenous endothelial cells (ECs) and facilitated the formation of dental-pulp-like tissue rich in blood vessels/ApoVs-carried mitochondrial Tu translation elongation factor modulated the angiogenic activation of ECs through the transcription factor EB-autophagy pathway | Li et al., 2022 |
References
- Doyle, L.M.; Wang, M.Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727.
- Yap, T.; Koo, K.; Cheng, L.; Vella, L.J.; Hill, A.F.; Reynolds, E.; Nastri, A.; Cirillo, N.; Seers, C.; McCullough, M. Predicting the Presence of Oral Squamous Cell Carcinoma Using Commonly Dysregulated Microrna in Oral Swirls. Cancer Prev. Res. 2018, 11, 491–502.
- Wolf, P. The Nature and Significance of Platelet Products in Human Plasma. Br. J. Haematol. 1967, 13, 269–288.
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal Information for Studies of Extracellular Vesicles 2018 (Misev2018): A Position Statement of the International Society for Extracellular Vesicles and Update of the Misev2014 Guidelines. J. Extracell. Vesicles 2018, 7, 1535750.
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding Light on the Cell Biology of Extracellular Vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228.
- Cheng, L.; Hill, A.F. Therapeutically Harnessing Extracellular Vesicles. Nat. Rev. Drug Discov. 2022, 21, 379–399.
- Kalluri, R.; LeBleu, V.S. The Biology, Function, and Biomedical Applications of Exosomes. Science 2020, 367, eaau6977.
- Itoh, Y.; Sasaki, J.I.; Hashimoto, M.; Katata, C.; Hayashi, M.; Imazato, S. Pulp Regeneration by 3-Dimensional Dental Pulp Stem Cell Constructs. J. Dent. Res. 2018, 97, 1137–1143.
- Ricucci, D.; Siqueira, J.F.; Abdelsayed, R.A.; Lio, S.G.; Rôças, I.N. Bacterial Invasion of Pulp Blood Vessels in Teeth with Symptomatic Irreversible Pulpitis. J. Endod. 2021, 47, 1854–1864.
- He, L.; Kim, S.G.; Gong, Q.; Zhong, J.; Wang, S.; Zhou, X.; Ye, L.; Ling, J.; Mao, J.J. Regenerative Endodontics for Adult Patients. J. Endod. 2017, 43, S57–S64.
- Xuan, K.; Li, B.; Guo, H.; Sun, W.; Kou, X.; He, X.; Zhang, Y.; Sun, J.; Liu, A.; Liao, L.; et al. Deciduous Autologous Tooth Stem Cells Regenerate Dental Pulp after Implantation into Injured Teeth. Sci. Transl. Med. 2018, 10, eaaf3227.
- Matsuzaka, Y.; Yashiro, R. Therapeutic Strategy of Mesenchymal-Stem-Cell-Derived Extracellular Vesicles as Regenerative Medicine. Int. J. Mol. Sci. 2022, 23, 6480.
- Hajishengallis, G. Periodontitis: From Microbial Immune Subversion to Systemic Inflammation. Nat. Rev. Immunol. 2015, 15, 30–44.
- Kinane, D.F.; Stathopoulou, P.G.; Papapanou, P.N. Periodontal Diseases. Nat. Rev. Dis. Prim. 2017, 3, 17038.
- Nuñez, J.; Vignoletti, F.; Caffesse, R.G.; Sanz, M. Cellular Therapy in Periodontal Regeneration. Periodontol 2000 2019, 79, 107–116.
- He, F.; Li, L.; Fan, R.; Wang, X.; Chen, X.; Xu, Y. Extracellular Vesicles: An Emerging Regenerative Treatment for Oral Disease. Front. Cell Dev. Biol. 2021, 9, 669011.
- Rombouts, C.; Giraud, T.; Jeanneau, C.; About, I. Pulp Vascularization During Tooth Development, Regeneration, and Therapy. J. Dent. Res. 2017, 96, 137–144.
- Yu, S.; Chen, H.; Gao, B. Potential Therapeutic Effects of Exosomes in Regenerative Endodontics. Arch. Oral Biol. 2020, 120, 104946.
- Li, Y.; Duan, X.; Chen, Y.; Liu, B.; Chen, G. Dental Stem Cell-Derived Extracellular Vesicles as Promising Therapeutic Agents in the Treatment of Diseases. Int. J. Oral Sci. 2022, 14, 2.
- Xian, X.; Gong, Q.; Li, C.; Guo, B.; Jiang, H. Exosomes with Highly Angiogenic Potential for Possible Use in Pulp Regeneration. J. Endod. 2018, 44, 751–758.
- Wu, M.; Liu, X.; Li, Z.; Huang, X.; Guo, H.; Guo, X.; Yang, X.; Li, B.; Xuan, K.; Jin, Y. Shed Aggregate Exosomes Shuttled Mir-26a Promote Angiogenesis in Pulp Regeneration Via Tgf-Beta/Smad2/3 Signalling. Cell Prolif. 2021, 54, e13074.
- Liu, D.; Kou, X.; Chen, C.; Liu, S.; Liu, Y.; Yu, W.; Yu, T.; Yang, R.; Wang, R.; Zhou, Y.; et al. Circulating Apoptotic Bodies Maintain Mesenchymal Stem Cell Homeostasis and Ameliorate Osteopenia Via Transferring Multiple Cellular Factors. Cell Res. 2018, 28, 918–933.
- György, B.; Szabó, T.G.; Pásztói, M.; Pál, Z.; Misják, P.; Aradi, B.; László, V.; Pállinger, É.; Pap, E.; Kittel, Á.; et al. Membrane Vesicles, Current State-of-the-Art: Emerging Role of Extracellular Vesicles. Cell. Mol. Life Sci. 2011, 68, 2667–2688.
- Li, Z.; Wu, M.; Liu, S.; Liu, X.; Huan, Y.; Ye, Q.; Yang, X.; Guo, H.; Liu, A.; Huang, X.; et al. Apoptotic Vesicles Activate Autophagy in Recipient Cells to Induce Angiogenesis and Dental Pulp Regeneration. Mol. Ther. 2022, 30, 3193–3208.
- Zhou, H.; Li, X.; Yin, Y.; He, X.-T.; An, Y.; Tian, B.-M.; Hong, Y.-L.; Wu, L.-A.; Chen, F.-M. The Proangiogenic Effects of Extracellular Vesicles Secreted by Dental Pulp Stem Cells Derived from Periodontally Compromised Teeth. Stem Cell Res. Ther. 2020, 11, 110.
- Zhang, Z.; Shuai, Y.; Zhou, F.; Yin, J.; Hu, J.; Guo, S.; Wang, Y.; Liu, W. Pdlscs Regulate Angiogenesis of Periodontal Ligaments Via Vegf Transferred by Exosomes in Periodontitis. Int. J. Med. Sci. 2020, 17, 558–567.
- Chen, W.-J.; Xie, J.; Lin, X.; Ou, M.-H.; Zhou, J.; Wei, X.-L. The Role of Small Extracellular Vesicles Derived from Lipopolysaccharide-Preconditioned Human Dental Pulp Stem Cells in Dental Pulp Regeneration. J. Endod. 2021, 47, 961–969.
- Chen, Y.; Ma, Y.; Yang, X.; Chen, J.; Yang, B.; Tian, W. The Application of Pulp Tissue Derived-Exosomes in Pulp Regeneration: A Novel Cell-Homing Approach. Int. J. Nanomed. 2022, 17, 465–476.
- Sui, B.; Chen, C.; Kou, X.; Li, B.; Xuan, K.; Shi, S.; Jin, Y. Pulp Stem Cell-Mediated Functional Pulp Regeneration. J. Dent. Res. 2019, 98, 27–35.
- Wang, D.; Lyu, Y.; Yang, Y.; Zhang, S.; Chen, G.; Pan, J.; Tian, W. Schwann Cell-Derived Evs Facilitate Dental Pulp Regeneration through Endogenous Stem Cell Recruitment Via Sdf-1/Cxcr4 Axis. Acta Biomater. 2022, 140, 610–624.
- Couve, E.; Schmachtenberg, O. Schwann Cell Responses and Plasticity in Different Dental Pulp Scenarios. Front. Cell. Neurosci. 2018, 12, 299.
- Xie, Z.; Shen, Z.; Zhan, P.; Yang, J.; Huang, Q.; Huang, S.; Chen, L.; Lin, Z. Functional Dental Pulp Regeneration: Basic Research and Clinical Translation. Int. J. Mol. Sci. 2021, 22, 8991.
- Ivica, A.; Zehnder, M.; Weber, F.E. Therapeutic Potential of Mesenchymal Stem Cell-Derived Extracellular Vesicles in Regenerative Endodontics. Eur. Cells Mater. 2021, 41, 233–244.
- Huang, C.-C.; Narayanan, R.; Alapati, S.; Ravindran, S. Exosomes as Biomimetic Tools for Stem Cell Differentiation: Applications in Dental Pulp Tissue Regeneration. Biomaterials 2016, 111, 103–115.
- Hu, X.; Zhong, Y.; Kong, Y.; Chen, Y.; Feng, J.; Zheng, J. Lineage-Specific Exosomes Promote the Odontogenic Differentiation of Human Dental Pulp Stem Cells (Dpscs) through Tgfβ1/Smads Signaling Pathway Via Transfer of Micrornas. Stem Cell Res. Ther. 2019, 10, 170.
- Zhuang, X.; Ji, L.; Jiang, H.; Liu, Y.; Liu, X.; Bi, J.; Zhao, W.; Ding, Z.; Chen, X. Exosomes Derived from Stem Cells from the Apical Papilla Promote Dentine-Pulp Complex Regeneration by Inducing Specific Dentinogenesis. Stem Cells Int. 2020, 2020, 5816723.
- Zhang, S.; Yang, Y.; Jia, S.; Chen, H.; Duan, Y.; Li, X.; Wang, S.; Wang, T.; Lyu, Y.; Chen, G.; et al. Exosome-Like Vesicles Derived from Hertwig’s Epithelial Root Sheath Cells Promote the Regeneration of Dentin-Pulp Tissue. Theranostics 2020, 10, 5914–5931.
- Kou, M.; Huang, L.; Yang, J.; Chiang, Z.; Chen, S.; Liu, J.; Guo, L.; Zhang, X.; Zhou, X.; Xu, X.; et al. Mesenchymal Stem Cell-Derived Extracellular Vesicles for Immunomodulation and Regeneration: A Next Generation Therapeutic Tool? Cell Death Dis. 2022, 13, 580.
- Sun, J.; Wang, Z.; Liu, P.; Hu, Y.; Li, T.; Yang, J.; Gao, P.; Xu, Q. Exosomes Derived from Human Gingival Mesenchymal Stem Cells Attenuate the Inflammatory Response in Periodontal Ligament Stem Cells. Front. Chem. 2022, 10, 863364.
- Park, H.C.; Quan, H.; Zhu, T.; Kim, Y.; Kim, B.; Yang, H.-C. The Effects of M1 and M2 Macrophages on Odontogenic Differentiation of Human Dental Pulp Cells. J. Endod. 2017, 43, 596–601.
- Zheng, J.; Kong, Y.; Hu, X.; Li, Z.; Li, Y.; Zhong, Y.; Wei, X.; Ling, J. Microrna-Enriched Small Extracellular Vesicles Possess Odonto-Immunomodulatory Properties for Modulating the Immune Response of Macrophages and Promoting Odontogenesis. Stem Cell Res. Ther. 2020, 11, 517.
- Yu, S.; Chen, X.; Liu, Y.; Zhuang, X.Y.; Wang, A.C.; Liu, X.M.; Zhu, S. Exosomes Derived from Stem Cells from the Apical Papilla Alleviate Inflammation in Rat Pulpitis by Upregulating Regulatory T Cells. Int. Endod. J. 2022, 55, 517–530.
- Zhang, S.; Thiebes, A.L.; Kreimendahl, F.; Rüetten, S.; Buhl, E.M.; Wolf, M.; Jockenhoevel, S.; Apel, C. Extracellular Vesicles-Loaded Fibrin Gel Supports Rapid Neovascularization for Dental Pulp Regeneration. Int. J. Mol. Sci. 2020, 21, 4226.
- Ivica, A.; Ghayor, C.; Zehnder, M.; Valdec, S.; Weber, F.E. Pulp-Derived Exosomes in a Fibrin-Based Regenerative Root Filling Material. J. Clin. Med. 2020, 9, 491.
- Li, J.; Ju, Y.; Liu, S.; Fu, Y.; Zhao, S. Exosomes Derived from Lipopolysaccharide-Preconditioned Human Dental Pulp Stem Cells Regulate Schwann Cell Migration and Differentiation. Connect. Tissue Res. 2021, 62, 277–286.
- Huang, X.; Qiu, W.; Pan, Y.; Li, J.; Chen, Z.; Zhang, K.; Luo, Y.; Wu, B.; Xu, W. Exosomes from Lps-Stimulated Hdpscs Activated the Angiogenic Potential of Huvecs in Vitro. Stem Cells Int. 2021, 2021, 6685307.
- Wen, B.; Huang, Y.; Qiu, T.; Huo, F.; Xie, L.; Liao, L.; Tian, W.; Guo, W. Reparative Dentin Formation by Dentin Matrix Proteins and Small Extracellular Vesicles. J. Endod. 2021, 47, 253–262.
- Bagio, D.A.; Julianto, I.; Margono, A.; Suprastiwi, E. Analysis of Thrombin-Activated Platelet-Derived Exosome (T-Apde) Potential for Dental Pulp Regeneration: In-Vitro Study. Eur. J. Dent. 2022.
- Yang, S.; Liu, Q.; Chen, S.; Zhang, F.; Li, Y.; Fan, W.; Mai, L.; He, H.; Huang, F. Extracellular Vesicles Delivering Nuclear Factor I/C for Hard Tissue Engineering: Treatment of Apical Periodontitis and Dentin Regeneration. J. Tissue Eng. 2022, 13, 20417314221084095.
- Reynolds, M.A.; Kao, R.T.; Camargo, P.M.; Caton, J.G.; Clem, D.S.; Fiorellini, J.P.; Geisinger, M.L.; Mills, M.P.; Nares, S.; Nevins, M.L. Periodontal Regeneration—Intrabony Defects: A Consensus Report from the Aap Regeneration Workshop. J. Periodontol. 2015, 86, S105–S107.
- Tassi, S.A.; Sergio, N.Z.; Misawa, M.Y.O.; Villar, C.C. Efficacy of Stem Cells on Periodontal Regeneration: Systematic Review of Pre-Clinical Studies. J. Periodontal Res. 2017, 52, 793–812.
- Chu, C.; Wei, S.; Wang, Y.; Wang, Y.; Man, Y.; Qu, Y. Extracellular Vesicle and Mesenchymal Stem Cells in Bone Regeneration: Recent Progress and Perspectives. J. Biomed. Mater. Res. Part A 2019, 107, 243–250.
- Chew, J.R.J.; Chuah, S.J.; Teo, K.Y.W.; Zhang, S.; Lai, R.C.; Fu, J.H.; Lim, L.P.; Lim, S.K.; Toh, W.S. Mesenchymal Stem Cell Exosomes Enhance Periodontal Ligament Cell Functions and Promote Periodontal Regeneration. Acta Biomater. 2019, 89, 252–264.
- Trubiani, O.; Pizzicannella, J.; Caputi, S.; Marchisio, M.; Mazzon, E.; Paganelli, R.; Paganelli, A.; Diomede, F. Periodontal Ligament Stem Cells: Current Knowledge and Future Perspectives. Stem Cells Dev. 2019, 28, 995–1003.
- Lei, F.; Li, M.; Lin, T.; Zhou, H.; Wang, F.; Su, X. Treatment of Inflammatory Bone Loss in Periodontitis by Stem Cell-Derived Exosomes. Acta Biomater. 2022, 141, 333–343.
- Ma, L.; Rao, N.; Jiang, H.; Dai, Y.; Yang, S.; Yang, H.; Hu, J. Small Extracellular Vesicles from Dental Follicle Stem Cells Provide Biochemical Cues for Periodontal Tissue Regeneration. Stem Cell Res. Ther. 2022, 13, 92.
- Wei, J.; Song, Y.; Du, Z.; Yu, F.; Zhang, Y.; Jiang, N.; Ge, X. Exosomes Derived from Human Exfoliated Deciduous Teeth Ameliorate Adult Bone Loss in Mice through Promoting Osteogenesis. J. Mol. Histol. 2020, 51, 455–466.
- Luo, L.; Avery, S.J.; Waddington, R.J. Exploring a Chemotactic Role for Evs from Progenitor Cell Populations of Human Exfoliated Deciduous Teeth for Promoting Migration of Naïve Bmscs in Bone Repair Process. Stem Cells Int. 2021, 2021, 6681771.
- Zarubova, J.; Hasani-Sadrabadi, M.M.; Dashtimoghadam, E.; Zhang, X.; Ansari, S.; Li, S.; Moshaverinia, A. Engineered Delivery of Dental Stem-Cell-Derived Extracellular Vesicles for Periodontal Tissue Regeneration. Adv. Healthc. Mater. 2022, 11, e2102593.
- Shen, Z.; Kuang, S.; Zhang, Y.; Yang, M.; Qin, W.; Shi, X.; Lin, Z. Chitosan Hydrogel Incorporated with Dental Pulp Stem Cell-Derived Exosomes Alleviates Periodontitis in Mice Via a Macrophage-Dependent Mechanism. Bioact. Mater. 2020, 5, 1113–1126.
- Liu, L.; Guo, S.; Shi, M.W.; Liu, Q.; Huo, F.; Wu, Y.; Tian, W. Bone Marrow Mesenchymal Stem Cell-Derived Small Extracellular Vesicles Promote Periodontal Regeneration. Tissue Eng. Part A 2021, 27, 962–976.
- Nakao, Y.; Fukuda, T.; Zhang, Q.; Sanui, T.; Shinjo, T.; Kou, X.; Chen, C.; Liu, D.; Watanabe, Y.; Hayashi, C.; et al. Exosomes from Tnf-Alpha-Treated Human Gingiva-Derived Mscs Enhance M2 Macrophage Polarization and Inhibit Periodontal Bone Loss. Acta Biomater. 2021, 122, 306–324.
- Kang, M.; Huang, C.-C.; Gajendrareddy, P.; Lu, Y.; Shirazi, S.; Ravindran, S.; Cooper, L.F. Extracellular Vesicles from Tnfα Preconditioned Mscs: Effects on Immunomodulation and Bone Regeneration. Front. Immunol. 2022, 13, 878194.
- Shi, W.; Guo, S.; Liu, L.; Liu, Q.; Huo, F.; Ding, Y.; Tian, W. Small Extracellular Vesicles from Lipopolysaccharide-Preconditioned Dental Follicle Cells Promote Periodontal Regeneration in an Inflammatory Microenvironment. ACS Biomater. Sci. Eng. 2020, 6, 5797–5810.
- Mohammed, E.; Khalil, E.; Sabry, D. Effect of Adipose-Derived Stem Cells and Their Exo as Adjunctive Therapy to Nonsurgical Periodontal Treatment: A Histologic and Histomorphometric Study in Rats. Biomolecules 2018, 8, 167.
- Yi, G.; Zhang, S.; Ma, Y.; Yang, X.; Huo, F.; Chen, Y.; Yang, B.; Tian, W. Matrix Vesicles from Dental Follicle Cells Improve Alveolar Bone Regeneration Via Activation of the Plc/Pkc/Mapk Pathway. Stem Cell Res. Ther. 2022, 13, 41.
More
