- autophagy
- cancer
- autophagy inhibitors
- autophagy activators
- autophagic cell death
- Cancer
- oncology
1. Introduction
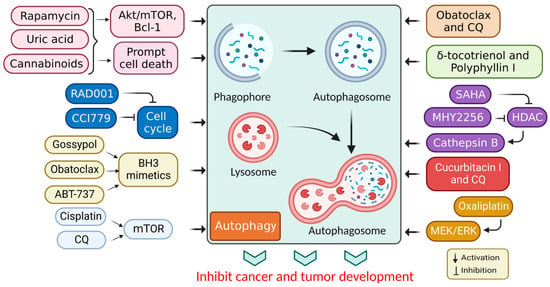
1. Targeted Drug for Autophagy Activation in Cancer Therapy
| Drug | Model | Molecular Mechanism | Reference |
|---|---|---|---|
| Azithromycin | Non-small-cell lung cancer (NSCLC) cells | Lysosomal membrane permeabilization for apoptosis induction | [32] |
| Penfluridol | Renal cell carcinoma | Induction of autophagy-mediated apoptosis and stemness inhibition | [33] |
| Canagliflozin | Acute kidney injury (AKI) | Cisplatin-mediated AKI by activating AMPK and autophagy | [34] |
| Oleanolic acid (3β-hydroxyolean-12-en-28-oic acid) | Different cancer model | Inducing tumor cell apoptosis via activating autophagy | [35] |
| Epigallocatechin-gallate | Human aortic epithelial cells | Autophagic flux stimulation | [36] |
| Korean red ginseng | Colon carcinoma cells (HCT-116 and SNU-1033) | Mitochondrial reactive oxygen species (ROS)-mediated autophagy and apoptosis. | [37] |
| Betulinic acid | Human malignant melanoma cell lines | Inhibits tumor growth and induces autophagy | [38] |
| Curcumin | Human thyroid cancer cells | Mitogen-activated protein kinase (MAPK) activation and mTOR inhibition with autophagy induction | [39] |
| Ursolic acid | Human breast cancer cells (MCF-7/MDA-MB-231) | Class III PI3K(VPS34)/Beclin-1 pathway autophagy induction | [40] |
| Obatoclax | Atypical teratoid/rhabdoid tumors | Dual mammalian target of rapamycin complex 1/2 (mTORC1/2) inhibition | [41] |
| Chloroquine | Triple-negative breast cancer (TNBC) | PI3K/Akt inhibitors and induces autophagy | [42] |
| Rapamycin | Kaposi’s sarcoma | PI3K/Akt/mTOR activation autophagy | [43] |
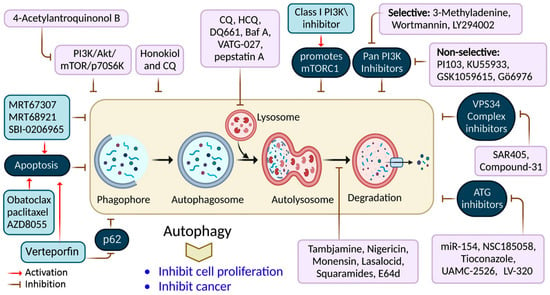
2. Targeted Drug for the Inhibition of Autophagy in Cancer Therapy
Abu Saim Mohammad Saikat is an enthusiastic and innovative individual with extensive experience in scientific research, leadership, team management, event planning, and social networking. He exhibits a high level of dedication, gets fully engaged, and has a clear vision of his goals. He does not get distracted, uses his energy entirely to manifest his dreams, and fully uses his resources.
He was born in January 2000 in Rajshahi in a small village near the northern border, where he grew up in a skimpy Bengali family with a strong interest in assisting his local communities from an early age. As an inquisitive child raised in a Southeast Asian household, he never had an uncomplicated time with it. He was introduced to a family that instilled an optimistic perspective on life despite the challenges he may face. He began his primary school education at the age of six in a school where he was consistently at the top of the class regarding excellence and academic performance and where continuity was harbored. He was admitted as a B.Sc. (Hons.) student in the Department of Biochemistry and Molecular Biology under the Life Science Faculty at Bangabandhu Sheikh Mujibur Rahman Science and Technology University, Gopalganj, Bangladesh, where he was furnished with several possibilities to participate in multiple academic and community activities. He is interested in, but not limited to, Cancer Biology, Immunology, Molecular Biology, Biochemistry, Genomics, Proteomics, and Bioinformatics.
| Drug | Model | Molecular Mechanism | Reference |
|---|---|---|---|
| Nigrosporins B | Human cervical cancer cells (Ca Ski) | PI3K/AKT/mTOR-mediated autophagy inhibition | [62] |
| 3-methyladenine (3-MA) | Gastric cancer cells | PI3K/AKT/FOXO3a inhibition | [63] |
| 4-acetylantroquinonol B | Human pancreatic cancer cells (MiaPaCa-2 and GEM-resistant MiaPaCa-2) | Upregulation of reactive oxygen species (ROS) promoted apoptosis via autophagy inhibition | [64] |
| LY294002 | Laryngeal squamous cell carcinoma | Inhibition of autophagy via the PI3K/mTOR pathway | [65] |
| MRT67307 | Several cancer models and lung cancer cells (A549) | ULK1 inhibition and autophagy inhibition | [66] |
| Honokiol | Human medulloblastoma | ERK/p38-MAPK-mediated autophagic inhibition | [67] |
| Obatoclax | Different cancer cells | Mitochondrial Ca2+ overload and autophagy inhibition | [68] |
| MHY2245 | Human colorectal cancer cells (HCT116) | Cell cycle arrest and apoptosis | [69] |
| Hydroxychloroquine | Human pancreatic cancer cells (PANC-1, MiaPaCa-2, and BxPC-3) | Promotes Bcl-xL inhibition-induced apoptosis with autophagy inhibition | [70] |
| Wortmannin | Pancreatic cancer cells (PANC-1, BxPC-3, and Capan-2) | Nutrient-starvation conditions via autophagy inhibition | [71] |
| Bafilomycin A1 | Thyroid cancer cells (MDA-T32, MDA-T68, FTC-133, and 8505c) | Autophagy inhibition reduced cell migration and invasion | [72] |
| Tioconazole | Human colorectal cancer cells (HCT116) | Autophagy inhibition | [73] |
2. Notable Contributions
His first journal article was on lactoferrin, its potential functions, pharmacological insights, and therapeutic promises [1]. He is enthusiastic about cancer biology research, and he contributed to cancer biology research. Cancer is a global menace to humankind and the most significant cause of mortality across the globe. Chemotherapeutic drugs are essential for treating and preventing cancer and are an integral part of cancer treatment. However, chemotherapy drugs cause oxidative stress and other adverse consequences on the human body [2].
He also worked on CCN proteins and their potential roles in breast cancer [3]. CCNs are a particular matricellular protein that serves many functions in multicellular eukaryotes as key signaling molecules. This protein family consists of six distinct members that are exclusive to vertebrates. CCN protein organization is multi-modular and comprises four different modules. CCN Proteins perform their principal functional activity via interacting with several integrin7 receptors. Among other biological interactions, the CCN family has been connected to cell adhesion, chemotaxis, migration, mitogenesis, cell longevity, angiogenesis, differentiation, cancer, chondrogenesis, as well as wound repair. Breast cancer is the most often diagnosed cancer globally, and CCN-regulated breast cancer ranks first. Recent research has demonstrated that CCN1 and CCN3 have a dual nature of tumor inhibition and suppression. Also mentioned are pharmacological advancements in the treatment of breast cancer by targeting CCN proteins [3].
Moreover, he was involved in research on the anticancer effects of acteoside, a phenylethanoid glycoside, and its mechanistic insights and therapeutic status for cancer treatment [4]. Several natural chemicals have been demonstrated to be effective in the treatment of various forms of cancer. Chemotherapeutic drugs are utilized in the prevention and treatment of cancer, among other applications. Acteoside is a phenylethanoid glycoside initially isolated from Verbascum sinuatum and has shown numerous effects[4].
Likewise, he also worked on computational studies on natural compounds that inhibit CDK9-induced cancer [5]. Given the role of cyclin-dependent kinases (CDKs) in regulating cell growth, gene transcription, and other vital biological processes, CDK blockers have been developed to treat various illnesses caused by CDK abnormalities. Moreover, CDK9 plays a crucial function in transcription by regulating anti-apoptotic genes with a short half-life, which are essential for the survival of cancer cells. Consequently, blocking CDK9 with inhibitors has emerged as a possible cancer therapy [5].
His recent work was on autophagy and cancer as the drug target in cancer treatment [6]. Recent data reveals that autophagy is a regulated catabolic framework that recycles nutrients from damaged organelles and other cellular components via lysosomal degradation. This process has been linked to the development of different pathologic illnesses, such as cancer and neurological disorders; however, current research suggests that autophagy has a dual function in cancer, operating as either a cytoprotective or cytotoxic mechanism. Numerous preclinical and clinical studies have demonstrated that suppressing autophagy increases the efficacy of anticancer drugs in various cancers [6].
