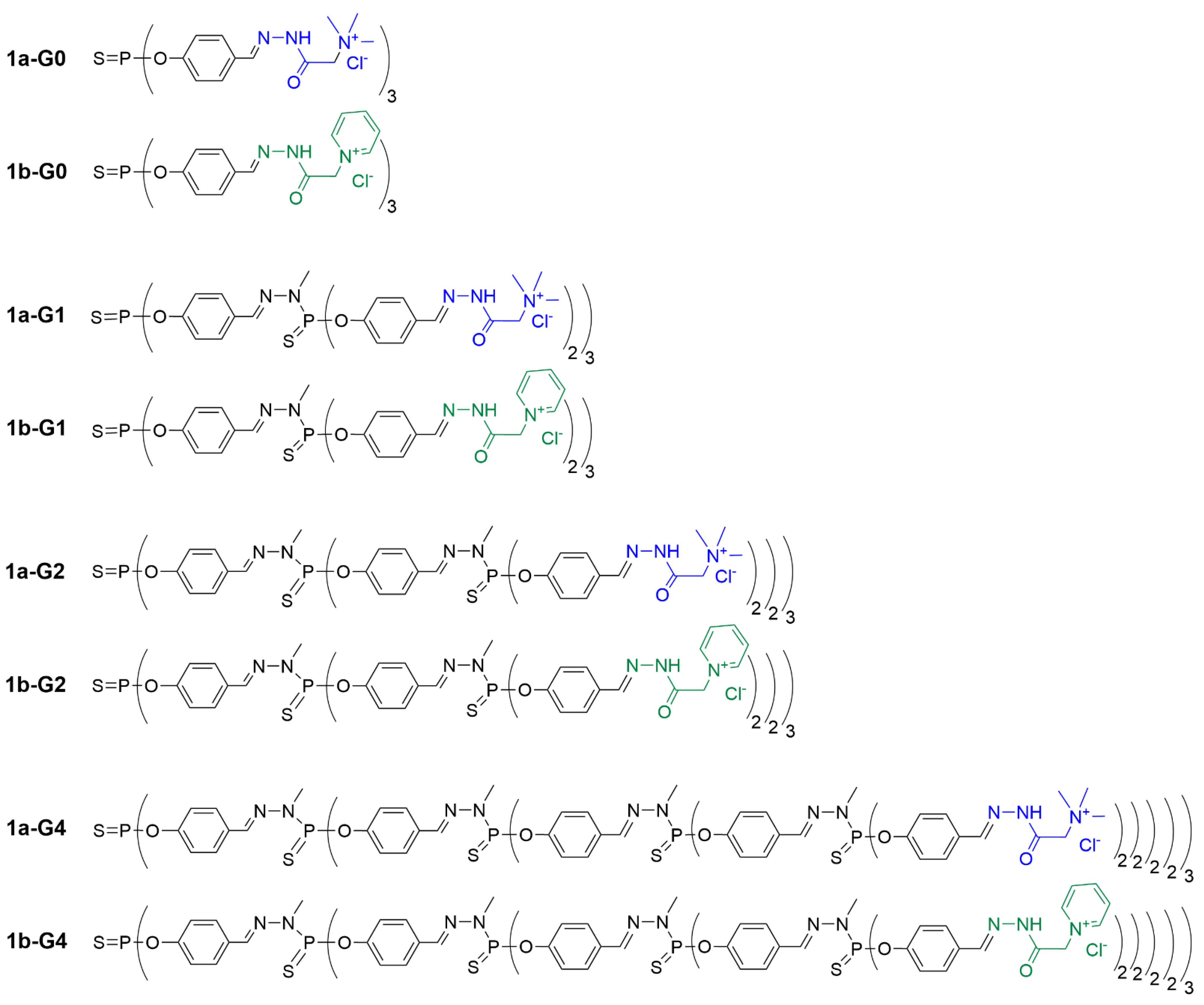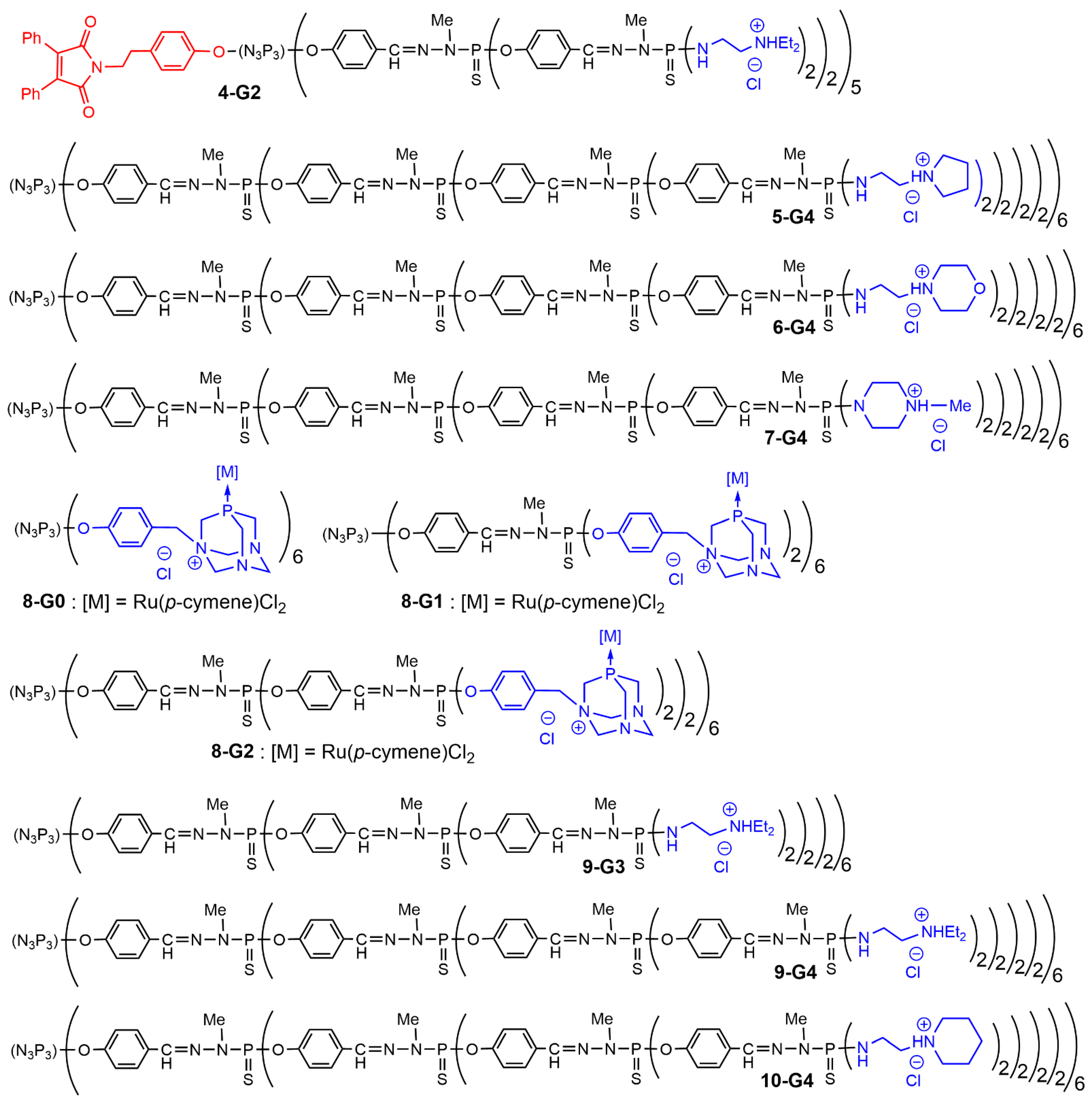1. Phosphorus Dendrimers as Building Blocks for Physical Hydrogels
The first examples of hydrogel-forming dendrimers concerned polycationic trimethylammonium acetohydrazone-terminated or pyridinium acetohydrazone-terminated phosphorus dendrimers of generations 0, 1, 2, and 4. They were synthesized by the grafting of the corresponding hydrazides (so-called Girard T and P reagents, respectively) to the aldehyde-terminated dendrimers, affording dendrimers
1a-Gn (Girard T) and
1b-Gn (Girard P) (n = 0, 1, 2 and 4), based on the trifunctional thiophosphate core
[1]. The hydrazone bond stabilized with the aromatic fragment on one side and the carbonyl group on the other side appeared to be quite stable in water and did not require reduction. The full structure of the second-generation dendrimers
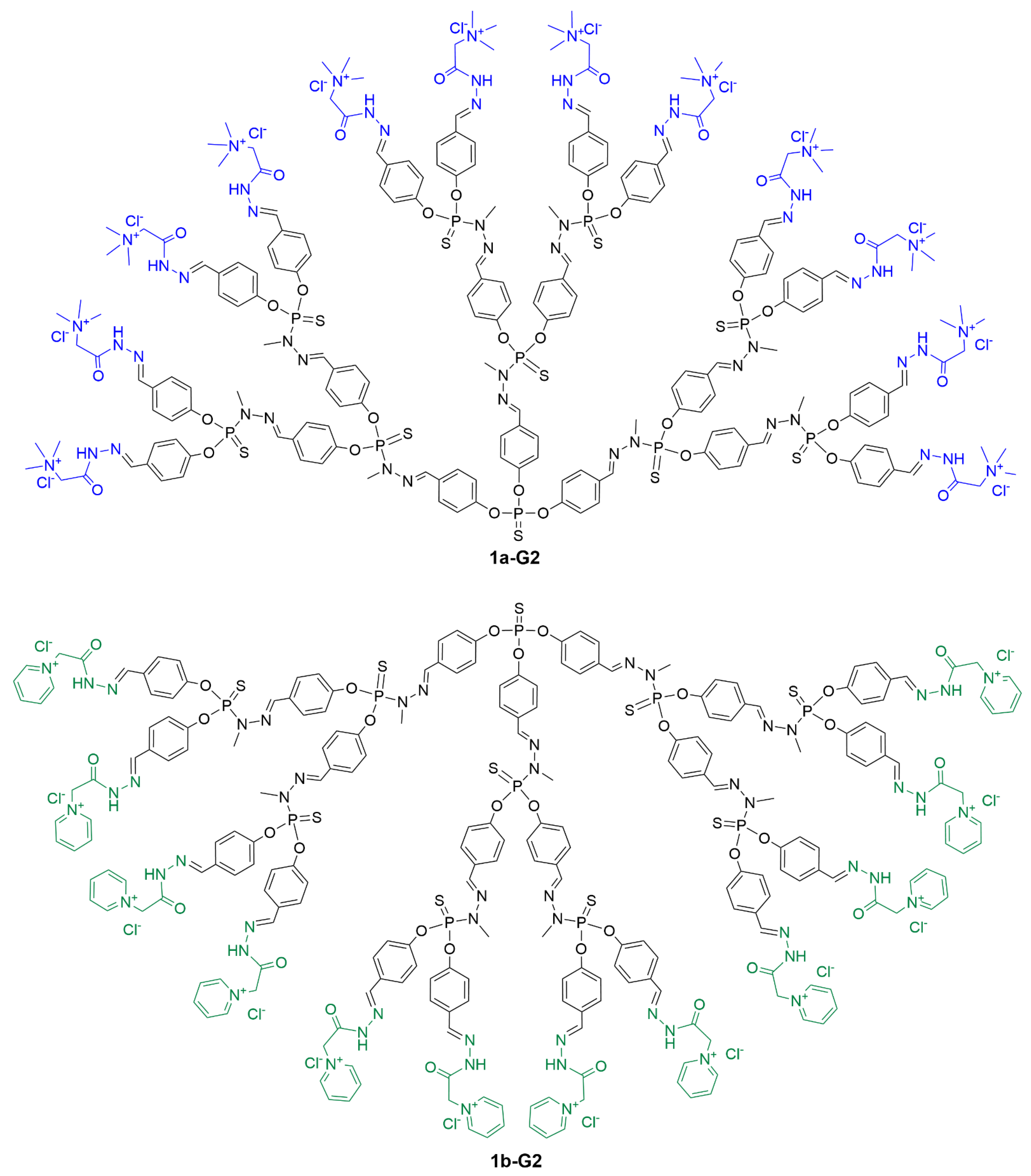
1a-G2 and
1b-G2 is shown in
Figure 1, whereas the linear structure of all dendrimers of this series is shown in
Figure 2.
Figure 1. Full chemical structure of dendrimers 1a-G2 (functionalized with the Girard T reagent) and 1b-G2 (functionalized with the Girard P reagent).
Figure 2. Linear representation of the chemical structure of phosphorus dendrimers built from a trifunctional core and functionalized with the Girard T reagent (series a, in blue) or the Girard P reagent (series b, in green) as hydrophilic terminal functions.
Polycationic acetohydrazone-modified dendrimers (1.8% in weight) formed stable physical hydrogels in water solutions when kept at 60 °C for several days. The formation of gels was driven by supramolecular interactions (hydrogen bonds, π−π aromatic stacking, and hydrophobic effects) of functional groups on the dendrimers’ surface and in distal fragments of dendrimer branches. The gelation speed depended on the dendrimer generation (higher-generation dendrimers gelated faster), the nature of the functional groups on the surface (pyridinium acetohydrazone-terminated dendrimers gelated faster than trimethylammonium ones) and the nature of the counter-anion (acetates gelated easier than chlorides, as shown by anion exchange with 1b-G1). Furthermore, the gelation was greatly accelerated in the presence of hydrophilic additives such as metal salts (Ni(Ac)2, Y(Ac)3, Er(Ac)3), organic acids (D,L-lactic acid, ascorbic acid, L-tartric acid, citric acid), dithioerythritol, or EDTA (sodium salt of ethylenediaminetetraacetate). The hydrogels contained a considerable amount of water (7500–70,000 water molecules per dendrimer molecule, depending on the generation, from G1 to G4, respectively) and were able to encapsulate up to 30% of Ni(Ac)2 [1].
Since these dendrimer hydrogels are physical gels, the gelation could be reversed by the addition of acetonitrile; gels dissociated and could be recovered upon the removal of acetonitrile. The gelation has been shown to be neither thermo-reversible nor pH-dependent. Interestingly, the three-dimensional hydrogel could be turned into an opaque aerogel by the freeze-drying-assisted removal of water, affording ramified fiber-like constructions, which look similar to green cabbage leaves.
Along with the formation of bulk hydrogels in the presence of additives, the directed gelation of these dendrimers was achieved using different modes of stimulated dendrimer gelation
[[2]]. The formation of hydrogels has been found to be forced by the addition of a flocculating salt. The values of the sol-gel transition temperature Tg strongly depended on the dendrimer generation as well as concentrations of the dendrimer and the flocculating salt. The best gelation was achieved using the
1a-G4 dendrimer and NaI. The formation occurred at room temperature (Tg ~17 ˚C), which facilitated further handling.
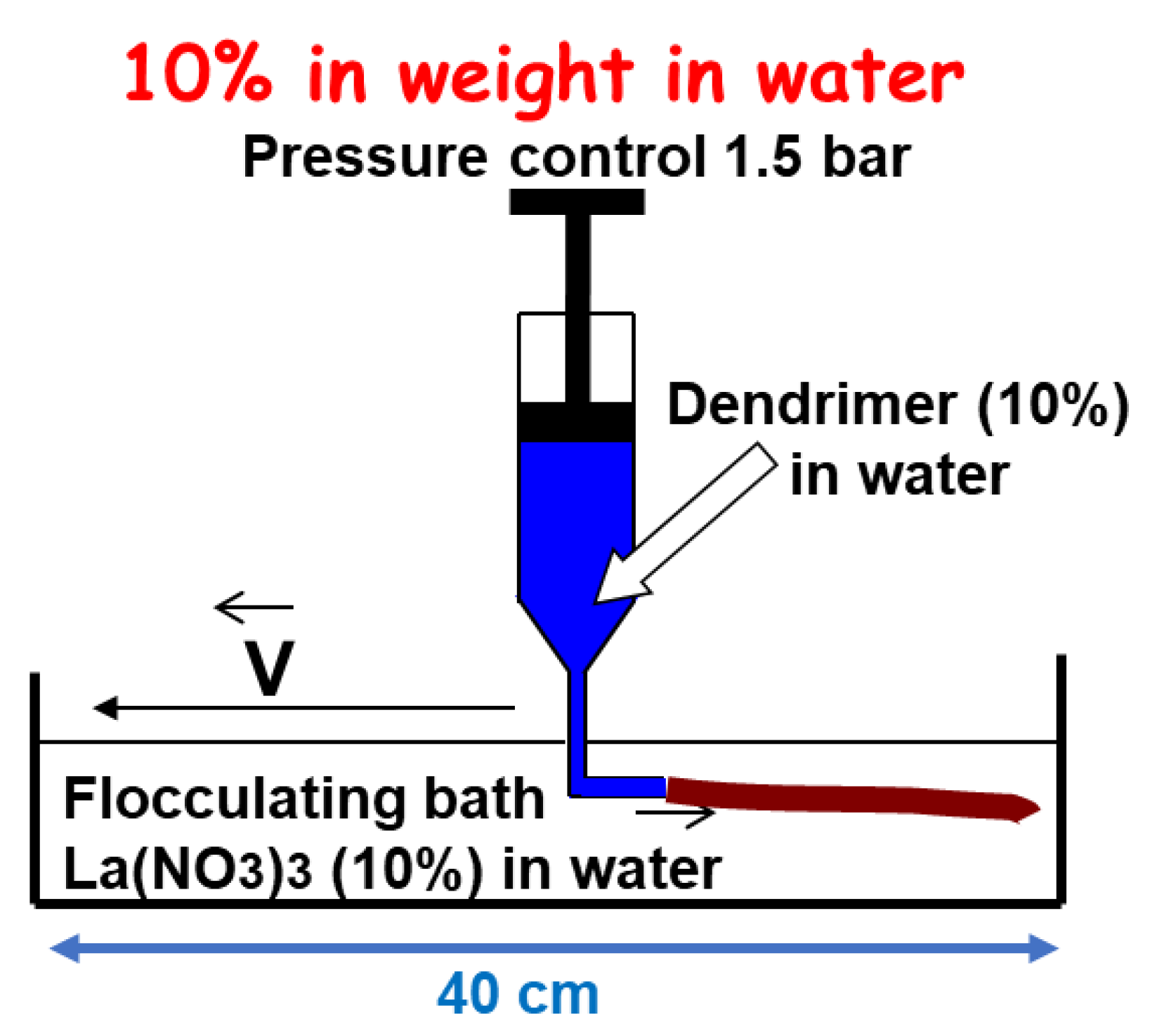
Based on these data, dendrimer hydrogel fibers have been produced. The 1a-G4 dendrimer solution (10% in weight) was purged through a syringe into a flocculating bath containing salt (10% La(NO3)3). Upon purging, stable hydrogel fibers were formed instantly (Figure 3), and after 40 min of incubation, they could be taken from the flocculating bath and remained stable and resistant to air. Denaturation of the macro-fiber with NaOH revealed that they were composed of thinner fibers, similar to long hair. The dendrimer fibers exhibited an elastic behavior, contrary to polymer fibers, which displayed a plastic behavior (irreversible elongation). The rupture occurred above 9 GPa for the dendrimer fibers. The Young’s modulus values were in the range of 0.5–3 GPa. It should be noted that such mechanical behavior is quite surprising for physical gels assembled exclusively by means of weak interactions [2].
Figure 3. Schematization of the method used for producing dendrimer fibers with dendrimer 1a-G4.
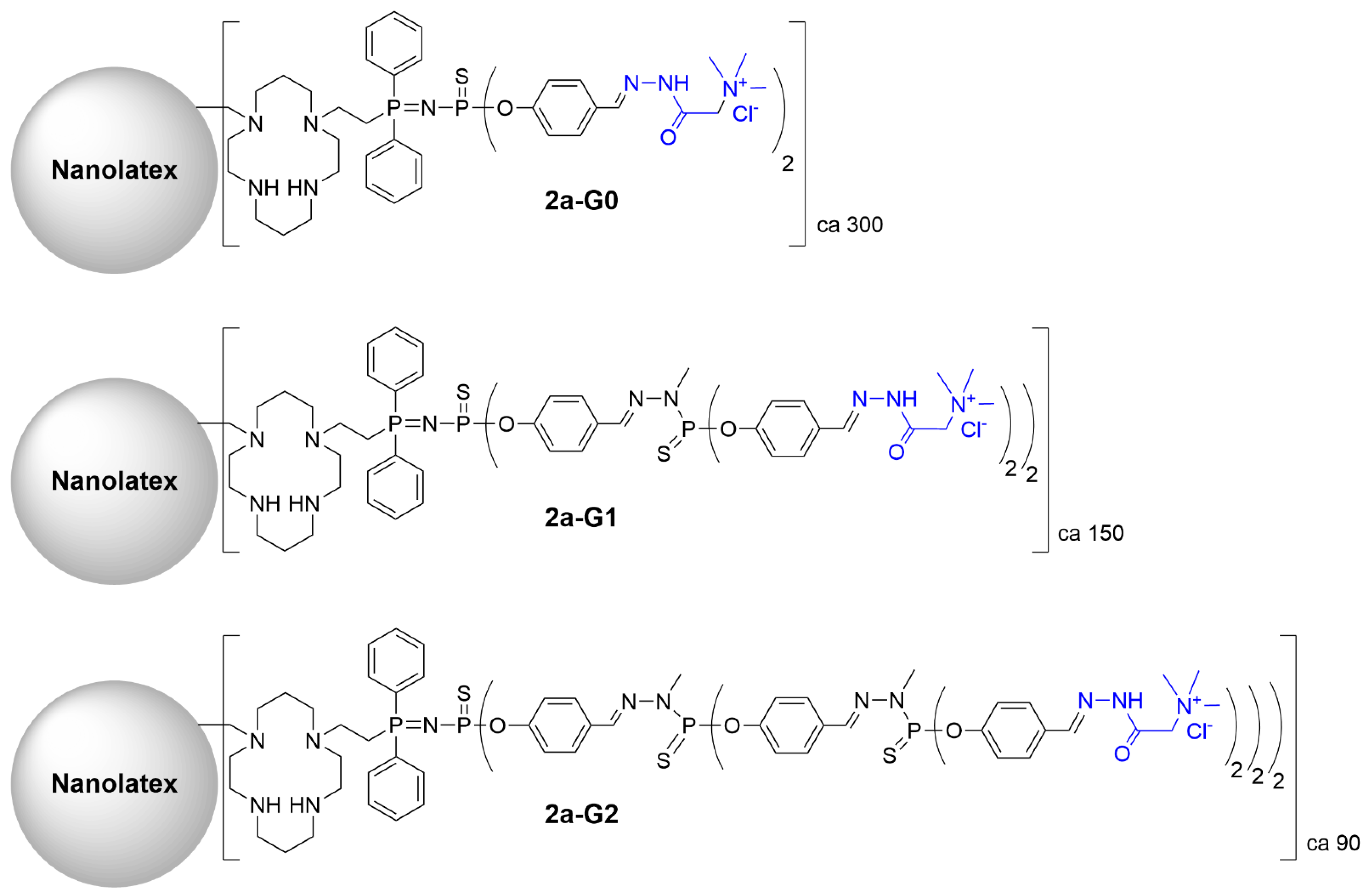
A series of cyclam-modified latex nanoparticles coated with phosphorus dendrons of generations 0, 1, and 2, bearing 2, 4 or 8 trimethylammonium acetohydrazone groups, respectively, was synthesized (
Figure 4)
[3]. The latex nanoparticles could accommodate on their surface about 300 molecules of the generation 0 dendron (
2a-G0), 150 of the generation 1 dendron (
2a-G1), and 90 of the generation 2 dendron (
2a-G2). Exposure to multiple charged groups greatly increased the colloidal stability of the nanoparticles in water. The gelation occurred at room temperature for 1 week. The nanolatex samples could be freeze-dried and then re-dispersed in water into individual particles. Furthermore, nanoparticles decorated with dendrons imitated dendrimers of high generations (G10, for instance), and this greatly facilitated their gelation potential. Interestingly, coating with cationic dendrons did not prevent the successful complexation of Cu
2+ ions by cyclam moieties on the surface of nanolatexes to form copper-containing hydrogels.
Figure 4. Nanolatex functionalized with phosphorus dendrons (generations 0 to 2) bearing the Girard T reagent as terminal functions. Cyclam moieties were suitable for the complexation of Cu2+ ions.
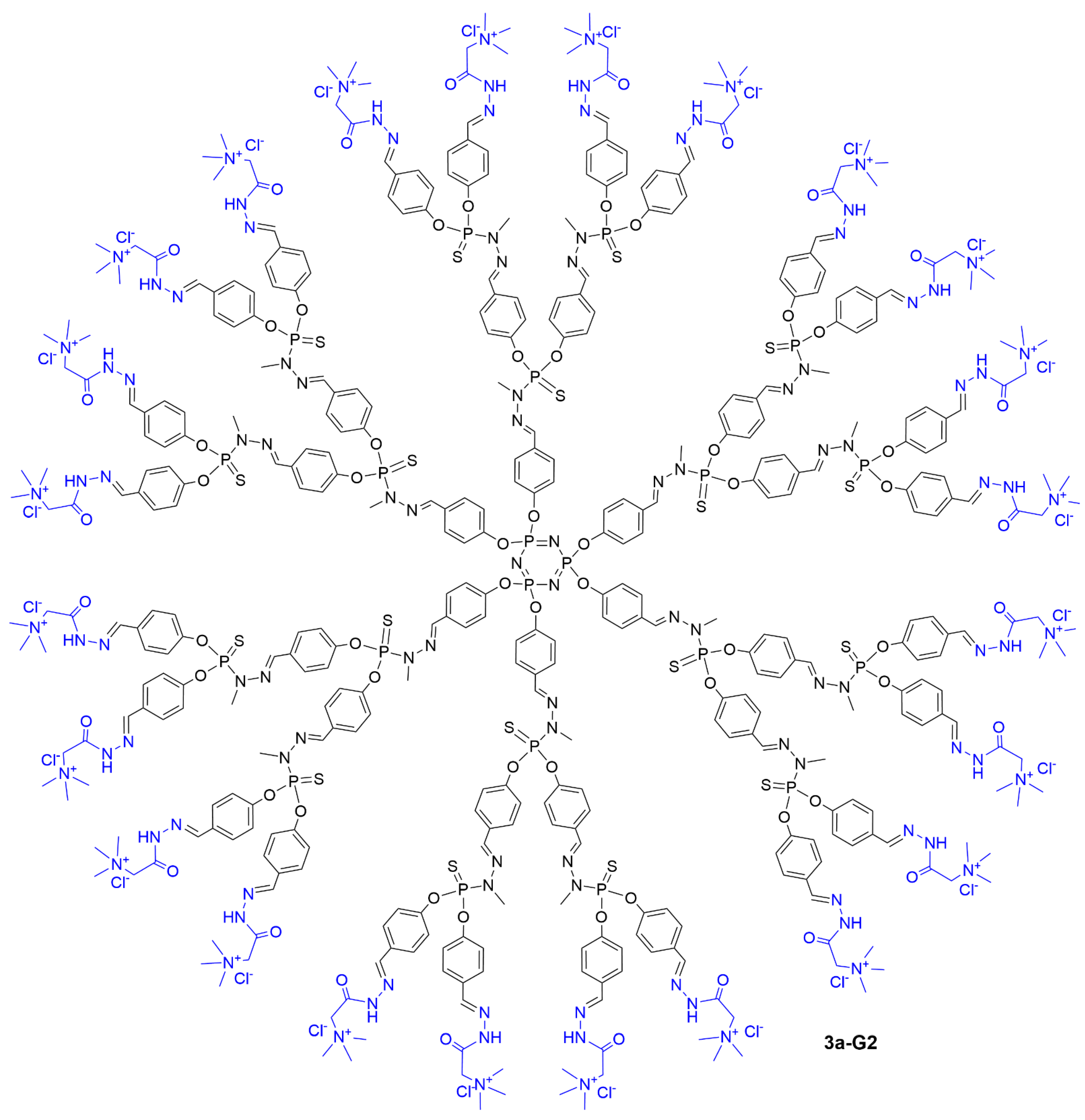
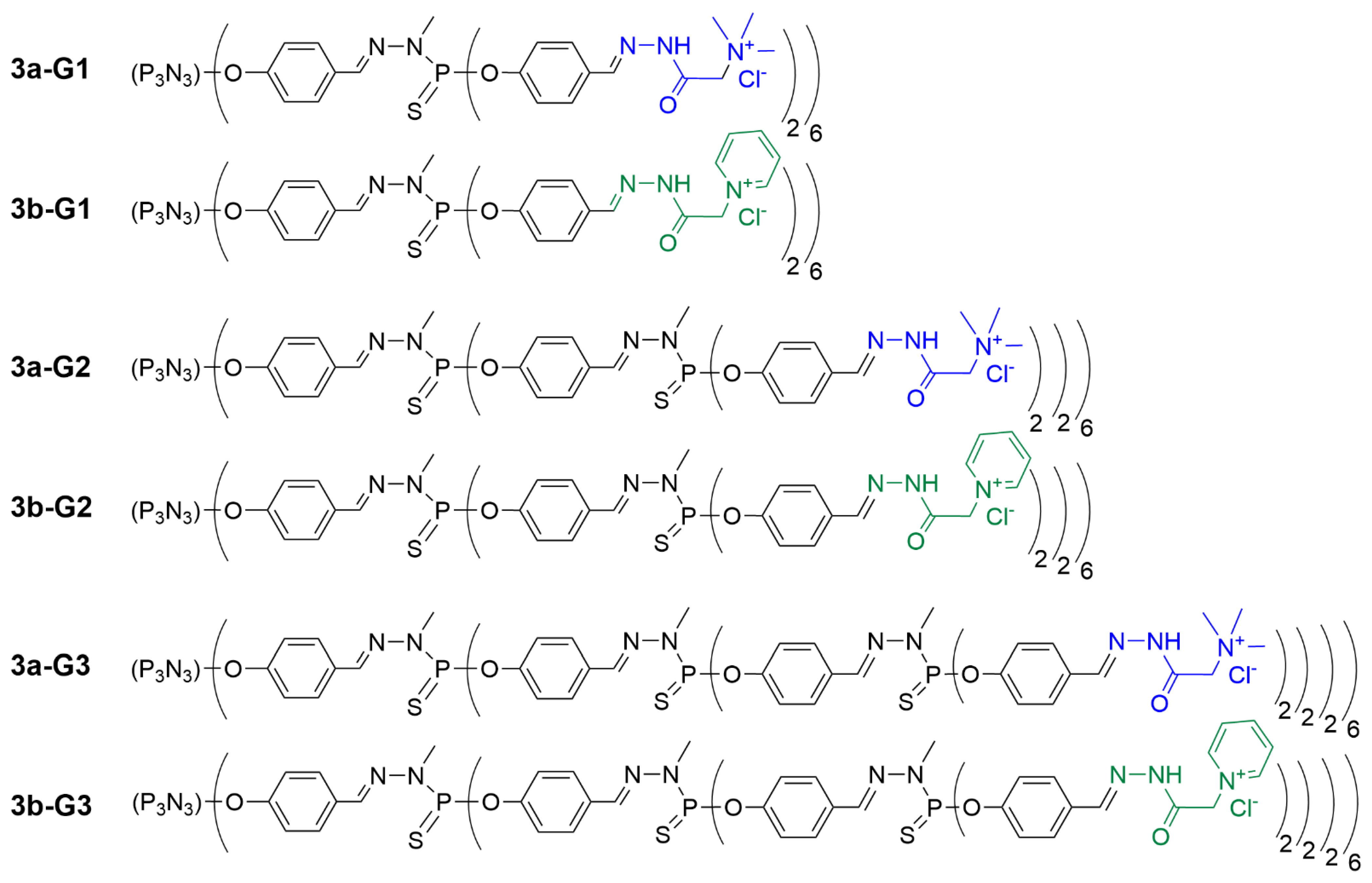
Later on, the applicability of dendrimer hydrogels for biomedical applications was explored. A series of trimethylammonium acetohydrazone-terminated or pyridinium acetohydrazone-terminated phosphorus dendrimers of generations 1, 2, and 3, based on the cyclotriphosphazene core (12, 24 or 48 surface groups, respectively), was synthesized (see
Figure 5 for the full structure of dendrimer
3a-G2 and
Figure 6 for the linear representation of all the dendrimers synthesized in this series). The use of such a hexafunctional core enables one to multiply by two the number of terminal functions at a given generation, compared to the trichlorothiophosphine core shown in
Figure 2, and is particularly useful for the synthesis of a variety of dendritic structures
[4]. The gelation properties of dendrimers
3a-Gn and
3b-Gn were assessed in media suitable for biological studies
[5]. The gelation was carried out either in water or phosphate-buffered saline (a common solution for the handling of biomacromolecules and biological samples) in the presence of biocompatible additives (glucose, glycine or polyethyleneglycol PEG4000). The formation of light-colored gels (colorless to light yellow) was reported to depend on the concentration of the dendrimer, the dendrimer generation and the type of terminal functions, the presence of a salt, and the concentration and nature of the additive. The general conclusions supported those drawn previously
[1]. However, using glucose, glycine, or polyethyleneglycol as additives generally required a longer gelation time (5–10 days), and water content in those dendrimers was lower than reported before (3300–51000 water molecules per dendrimer molecule from generation 1 to generation 3). Dendritic pyridinium salts (
3b-Gn family) were found to be more efficient than trimethylammonium salts (
3a-Gn family)
[5], as observed previously.
Figure 5. Full chemical structure of dendrimer 3a-G2.
Figure 6. Linear representation of phosphorus dendrimers built from a hexafunctional core and functionalized with the Girard T reagent (in blue) or the Girard P reagent (in green) as terminal groups.
Importantly, in this work, the authors succeeded in obtaining representative TEM images of structural units of hydrogels. Dendrimer hydrogels were fixed by paraformaldehyde and cut into ultrathin sections (~70 nm thick). The TEM observation revealed random porous networks, with the network porosity and electron density of structural units correlating with the water content in hydrogels. There were two types of structural units: spherical particles and fibers co-assembled in the hydrogel network. The presence of both types of units in gels depended on the dendrimer content in the hydrogel network and on the nature of the additive [5].
Owing to the polycationic structure of acetohydrazone-terminated dendrimers, hydrogels efficiently bound therapeutic oligonucleotides. In general, oligonucleotide binding capacity depends rather on the nature of the additive (glucose > polyethyleneglycol > glycine) than on the nature of the cation. The loading capacity was 1.5–3 µmol/g. Once bound, oligonucleotides could be partially released from hydrogels. The release was pH-dependent; the release rate increased at pH < 6. Nevertheless, the overall release did not exceed 10% after 24 h [5]. This gives the idea that dendrimer hydrogels can be used as depots of therapeutic oligonucleotides for a sustained release over weeks.
Thus, phosphorus dendritic molecules present a convenient platform for obtaining functional hydrogels. By modulating dendrimer topology, generation and surface chemistry, one can produce materials with controllable properties.
2. Phosphorus Dendrimers in Hydrogels
A good example of a dendrimer inside a hydrogel is an agarose gel containing a polyelectrolyte dendrimer complexed with nucleic acids. In this case, dendrimers themselves are generally not involved in the formation of the hydrogel network. Such complexes, so-called dendriplexes
[6], are frequently analyzed by the agarose gel electrophoresis assay, and these studies clearly demonstrated the compatibility of polycationic phosphorus dendrimers with the agarose hydrogel scaffold. Different examples of such analyses of dendriplexes by gel electrophoresis were reported. The fluorescent dendron
4-G2 (
Figure 7) was associated with the plasmid DNA coding the gene of fluorescent fusion protein BACE-GFP and also with the HygEGFP plasmid
[7]. Different types of fourth-generation dendrimers bearing cyclic ammoniums as terminal functions (
5-G4,
6-G4,
7-G4) were tested for their ability to interact with a [
32P]-labelled 20-mer double-stranded oligonucleotide. The pyrrolidinium derivative
5-G4 was found to be the most efficient
[8]. Generations 0 to 2 of dendrimers functionalized with PTA (1,3,5-triaza-7-phosphaadamantane) complexing ruthenium were tested for their interaction with supercoiled DNA to afford relaxed DNA. Generation 0 in this series (
8-G0) was found to be as efficient as cisplatin
[9]. Generations 3 and 4 of phosphorus dendrimers bearing charged diethylethylene diamine terminal functions (
9-G3 and
9-G4 [9]) formed dendriplexes with the supercoiled form of the pUC19 plasmid, issued from
E. coli cells. Agarose gel electrophoresis demonstrated that the dendrimers did not induce the cleavage of the plasmid
[10].
Figure 7. Structure of phosphorus dendrimers forming dendriplexes analyzed by agarose gel electrophoresis assays.
Cationic phosphorus dendrimers were also used to form dendriplexes with small interfering siRNA, which were analyzed by agarose gel electrophoresis assays. Phosphorus dendrimers
9-G3 and
9-G4 were used as nanocarriers for anticancer siBcl-xl, siBcl-2, siMcl-1 siRNAs and a siScrambled sequence
[11]. Phosphorus dendrimers functionalized with piperidine terminal cationic groups of the third and fourth generation (
10-G3 and
10-G4) showed a remarkable ability to bind pro-apoptotic siRNAs
[12].
Phosphorus dendrimers can also be used to bring new functionalities to hydrogels built of conventional structural blocks. The dendrimer content is thus quite low, so it can be considered a dopant enhancing the properties of the bulk, as they are placed randomly in the pores. The potential of the agarose gel to serve as a depot for the slow release of dendriplexes containing therapeutically relevant siRNA Mcl-1 was studied
[13]. Third-generation polycationic phosphorus dendrimers bearing 48 cationic acetohydrazone groups (
3a-G3 and
3b-G3) or piperidinium groups (
10-G3) on the surface efficiently complexed fluorescein-labelled siRNA to form nanosized dendriplexes of ~100 nm diameter. Dendriplexes were cast into a hot agarose solution before gelation, and the release of siRNA-containing dendriplexes was monitored. Interestingly, the release rate appeared to strongly depend on the dendriplexes composition. Whereas dendriplexes containing a piperidinium-terminated dendrimer (
10-G3) were released for 80% after 3 h, those containing acetohydrazone-terminated dendrimers (
3a-G3 and
3b-G3) were released only for 20% after 24 h. This occurred most likely because of multiple hydrogen bonding between hydrazone moieties on the dendrimers’ periphery and agarose scaffold. It should be reminded that this type of dendrimer was able to form hydrogels themselves
[5]. Remarkably, using mixtures of dendrimers, it was possible to manipulate the dendriplexes’ release rate in quite a wide range. This observation yielded the important insight that using such a kind of dendriplex anchoring in the neutral hydrogel scaffold, one can achieve a sustained local release of therapeutic oligonucleotides. This is especially important for designing local drug delivery systems and tissue engineering tools. It is also worth noting that oligonucleotides were released from a hydrogel not as individual molecules but as dendriplexes, which would facilitate their accumulation in cells of a surrounding tissue
[14].
3. Conclusion and Perspectives
Physical hydrogels attract the attention of researchers due to the simplicity of their preparation as well as their attractive physicochemical and mechanical properties. Dendrimers, being inherent multivalent species, are highly promising building blocks for physical hydrogels due to their high number of functional residues available for gelation. Importantly, the dendritic scaffold takes part in the gelation along with surface groups. Therefore, the use of dendritic molecules for gelation gives room for precise engineering of hydrogel properties by finding an optimal balance between the hydrophilicity/hydrophobicity of the dendritic scaffold and surface functional groups, with the choice of functional group chemistry defining the gelation mode.
Phosphorus dendrimer-based hydrogels have been used as functional materials and drug-delivery vehicles. Quite limited data available to date suggests, nevertheless, that the use of dendrimers and dendrons should be developed for the adjustment of material properties. However, there are several challenges to be addressed in further explorations, namely the large-scale production of hydrogels for sustained drug release or tissue engineering. A better understanding of the relationships between dendritic gelator structure and the following modulation of hydrogel properties can serve as key parameters to construct the next generation of therapeutic systems or biomaterials.