Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Rita Xu and Version 1 by Ruoyao Mei.
Diseases induced by bacterial and viral infections are common occurrences in our daily life, and the main prevention and treatment strategies are vaccination and taking antibacterial/antiviral drugs. Vaccines can only be used for specific viral infections, and the abuse of antibacterial/antiviral drugs will create multi−drug−resistant bacteria and viruses. Therefore, it is necessary to develop more targeted prevention and treatment methods against bacteria and viruses. Proteins on the surface of bacteria and viruses can specifically bind to sugar, so glycopolymers can be used as potential antibacterial and antiviral drugs.
- glycopolymer
- antibacterial
- antivirus
1. Introduction
In recent decades, a variety of infectious diseases have developed caused by bacteria, including Staphylococcus aureus, Streptococcus haemolyticus type α, Streptococcus haemolyticus type β and viruses including Ebola, Zika, coronavirus (COV) [1]. As a result, different regions are experiencing the spread of diseases caused by these pathogens. With the emergence of superbugs, as well as the global epidemic of COVID−19, wresearchers are facing significant challenges in the prevention and treatment of these diseases due to drug resistance and genetic mutations. At present, the effective prevention and treatment strategy for bacterial diseases is to take antibiotic drugs, and the prevention and treatment strategy for viral diseases is mainly vaccination or taking antiviral drugs. However, vaccines can only be used for specific types of viral infections, and antivirals or antimicrobials can make viruses or bacteria resistant. Therefore, it is important to develop more targeted prevention and treatment methods against highly drug−resistant bacteria and viruses. There are a lot of glycoproteins on the surface of bacteria, viruses and other life forms [2]. Different kinds of sugars can specifically combine with pathogens that contain different types of recognition proteins, such as mannose and Escherichia coli, L−Fucose and Vibrio cholerae, salivary lactose and mumps virus/influenza virus, etc. In addition, the specific binding of sugars and proteins is also affected by a variety of factors, such as the “multivalent effect” of polysaccharides [3[3][4][5][6],4,5,6], topology [7,8,9][7][8][9] (star−shaped, dendritic), heterogeneity [10,11[10][11][12][13][14][15][16],12,13,14,15,16], etc. Therefore, glycopolymers with various structures have been developed to specifically recognize and bind with different bacteria and viruses, so as to achieve the application of inhibiting bacterial infection, virus infection, bacterial detection, etc.
2. Glycopolymers Targeting Viruses
There are a large number of sugars on the surface of cells, which facilitate the transmission of biological information between cells by interacting with specific proteins. In contrast, pathogens also recognize their specific host cells through glycoprotein interactions, that is, glycorecognition proteins (lectins) on the surface of pathogens interact with sugar units on the cell surface to cause viral infection. Based on these interaction mechanisms, the antiviral application of sugar has been heavily studied to develop specific targeting systems that can act as inhibitors of the virus.2.1. Glycopolymers Targeting Cell Surface Proteins
C−type lectin receptor, dendritic cell−specific intercellular adhesion molecule 3 grabbing nonintegrin (DC−SIGN), is a pattern recognition receptor expressed on macrophages and dendritic cells. It has been identified as a receptor for many pathogens, such as SARS−CoV−2 and HIV. These viruses spread and escape through the binding of DC−SIGN captured by sugar molecules on their surface and dendritic cell−specific ICAM−3 [53][17]. In the context of the recent SARSCoV−2 epidemic, DC−SIGN−mediated viral transmission and innate immune responses have been identified as a potential factor in the pathogenesis of COVID−19 [54][18]. Therefore, the design of glycopolymers with stronger binding capacity to DC−SIGN to inhibit viral binding to DC−SIGN is an attractive strategy to attenuate excessive innate immune responses and prevent disease progression. The ability of glycopolymers binding to DC−SIGN is influenced by multiple factors. Different types and structures of sugars have different degrees of binding capacity to the virus. In addition, wresearchers can also strengthen the binding ability to virus by simulating the glycoproteins. Becer et al. synthesized a mannose−based glycopolymer using copper−mediated living radical polymerization and azide−alkyne [3 + 2] Huisgen cycloaddition reaction to interact with DC−SIGN and inhibit the binding of HIV envelope glycoprotein gp120 to DC−SIGN [55][19]. The binding affinity of the glycocopolymer to DC−SIGN was investigated by multi−channel surface plasmon resonance (MC−SPR). They used a DC−SIGN functionalized surface to evaluate the binding affinity of glycopolymers (Figure 1a), and used gp120 functionalized surfaces for competitive binding studies (Figure 1b). It proved that the increase in mannose content was the key to high affinity; by contrast, the increase in galactose density decreased the affinity for DC−SIGN.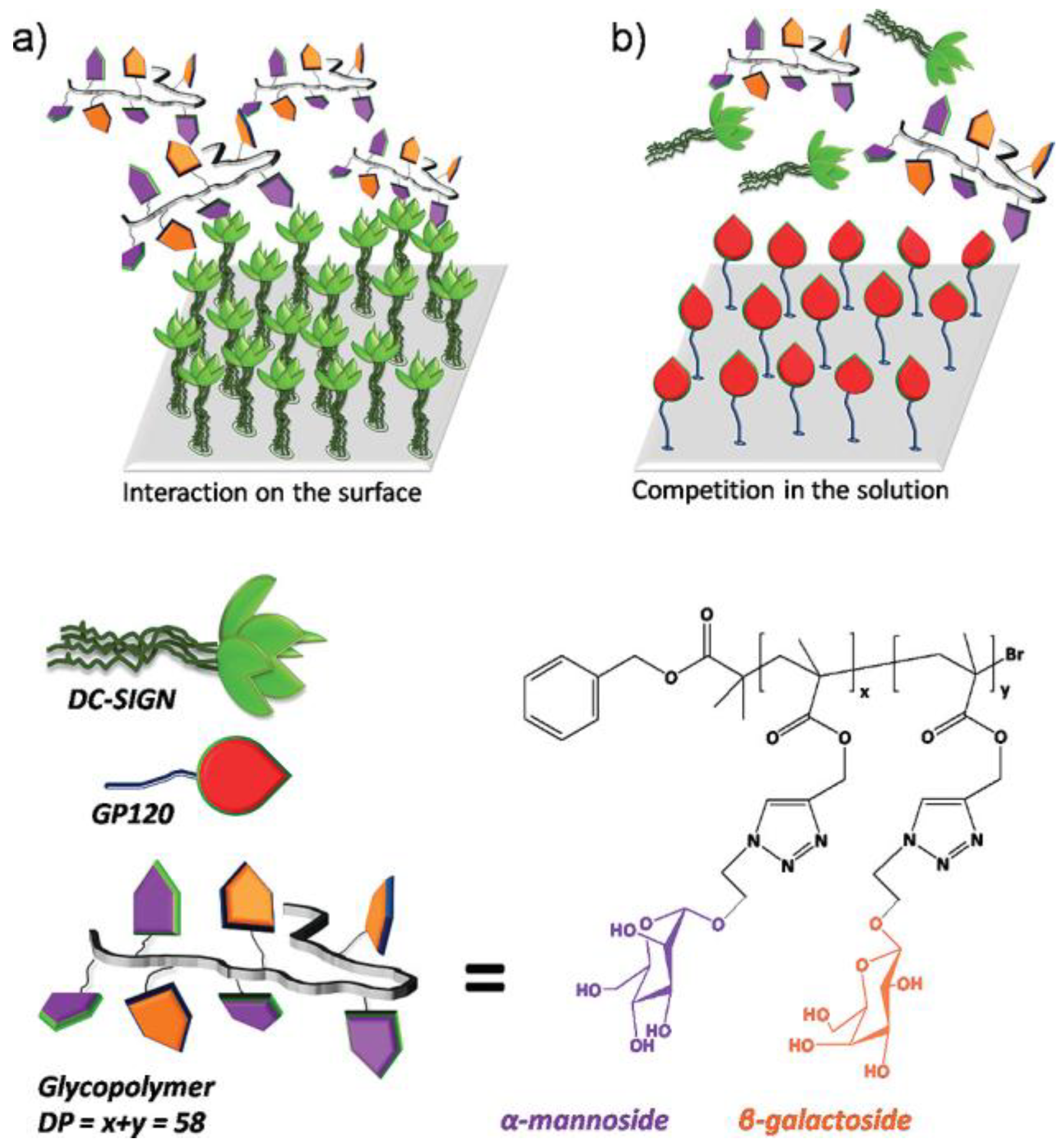
Figure 1. (a) DC−SIGN−functionalized surfaces were used to evaluate the binding affinity of glycopolymers; (b) gp120−functionalized surfaces are used for competitive binding studies. (Bottom) Schematic diagram of DC−SIGN and gp120 structure and chemical structure of glycopolymer.
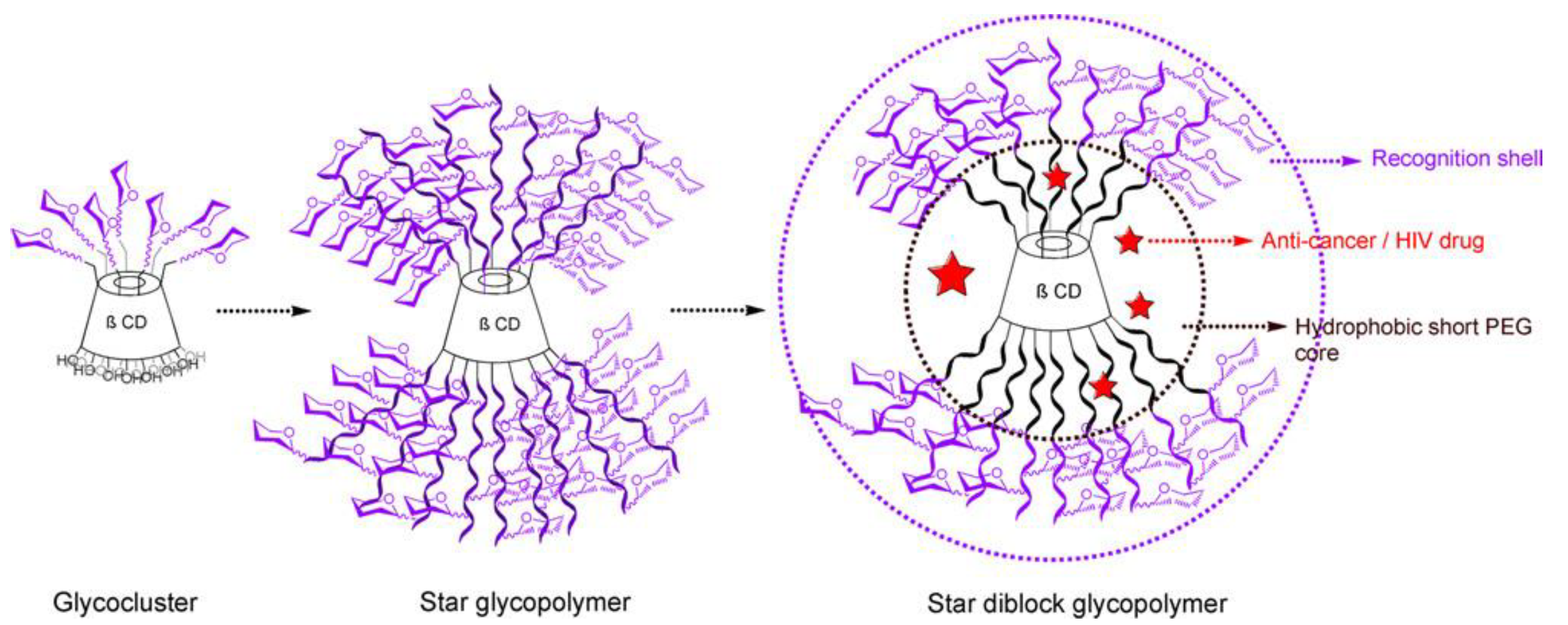
Figure 2. Sugar clusters, star type glycopolymers and star block type glycopolymers.
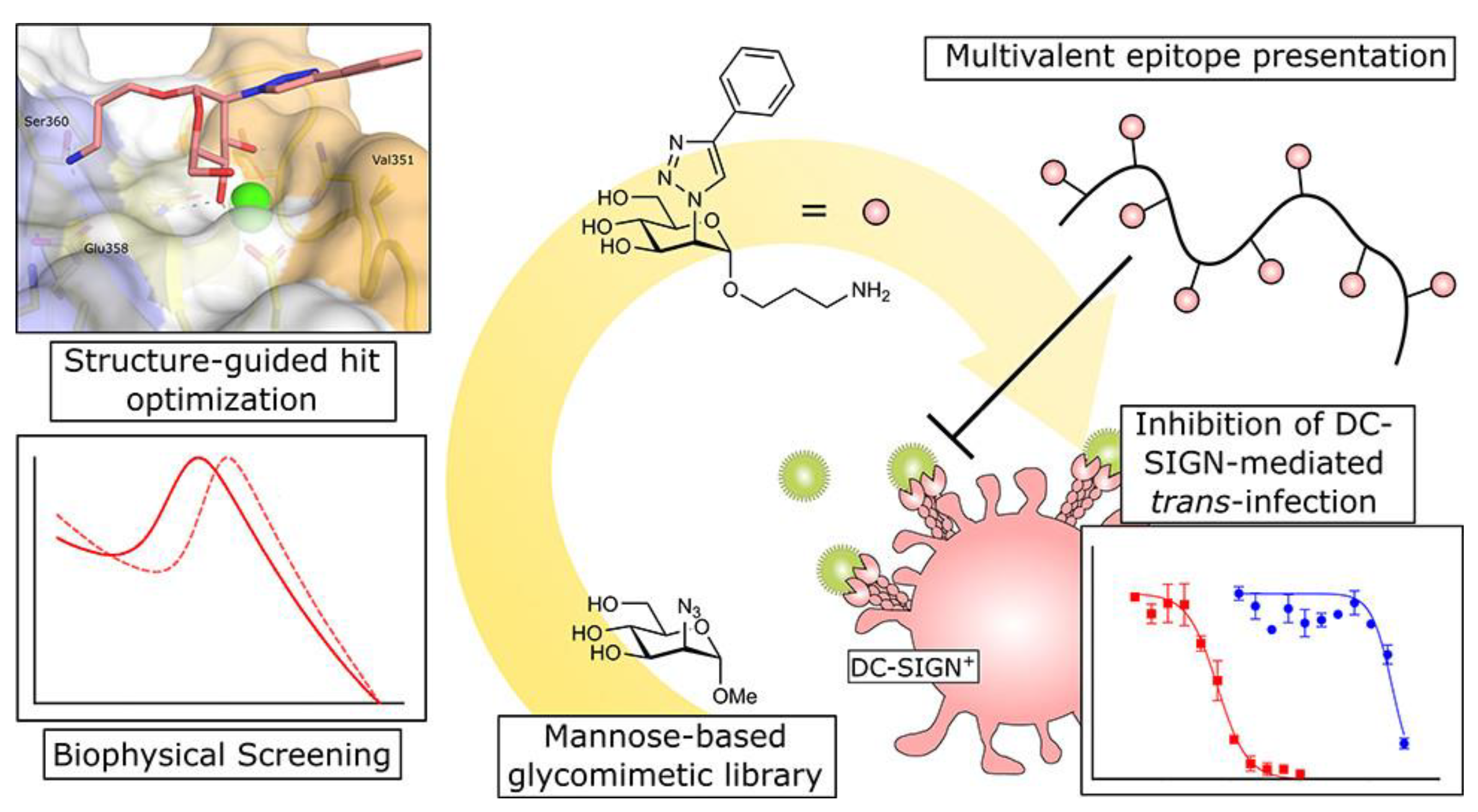
Figure 3. Interaction of triazole−based mannose analogues with DC−SIGN cells.
2.2. Glycopolymers Targeting Virus Surface Lectin
It is difficult to produce vaccines that are effective against multiple existing and emerging strains of viruses due to virus mutations [57,58,59][21][22][23]. To prevent infection, preventing the virus from attaching to the surface of the cell is the general approach to preventing infection with many viruses, including influenza [60,61,62][24][25][26]. Viruses can adhere to cells by binding to glycans on the cell surface; however, by regulating sugar species, density, topology, etc., glycopolymers can have a stronger virus−binding capacity. Therefore, using glycopolymers to recognize viruses can inhibit viral binding to cells. Viruses can recognize various glycans, such as glycans terminating sialic acids (Neu5Ac), and glycosaminoglycans (GAGs), such as heparan sulfates (HS). However, the use of natural polysaccharides as inhibitors for virus recognition may present many challenges to the safety of clinical applications [63,64][27][28]. Firstly, the molecules are heterogeneous. The preparations may be mixed with glycans and contain a variety of impurities. Secondly, as natural glycans, they may also cause biological side effects due to their inconsistent quality and traces of contamination. In search of substitutes, synthetic glycomimetics provide the compound more controllability over its structure. Glycomimetics have been shown to improve stability, bioavailability and half−life. Moreover, the activities of glycomimetics are comparable or even higher than their corresponding natural polysaccharides [65][29]. Based on this, Soria−Martinez et al. synthesized highly sulfated synthetic glycomimetics designed to mimic heparin and other natural polysaccharides with high sulfation degree as viral binding/infection inhibitors (Figure 4). The synthetic glycomimetics can effectively inhibit human papillomavirus (HPV16) infection in vitro and maintain the antiviral activity in vivo [66][30].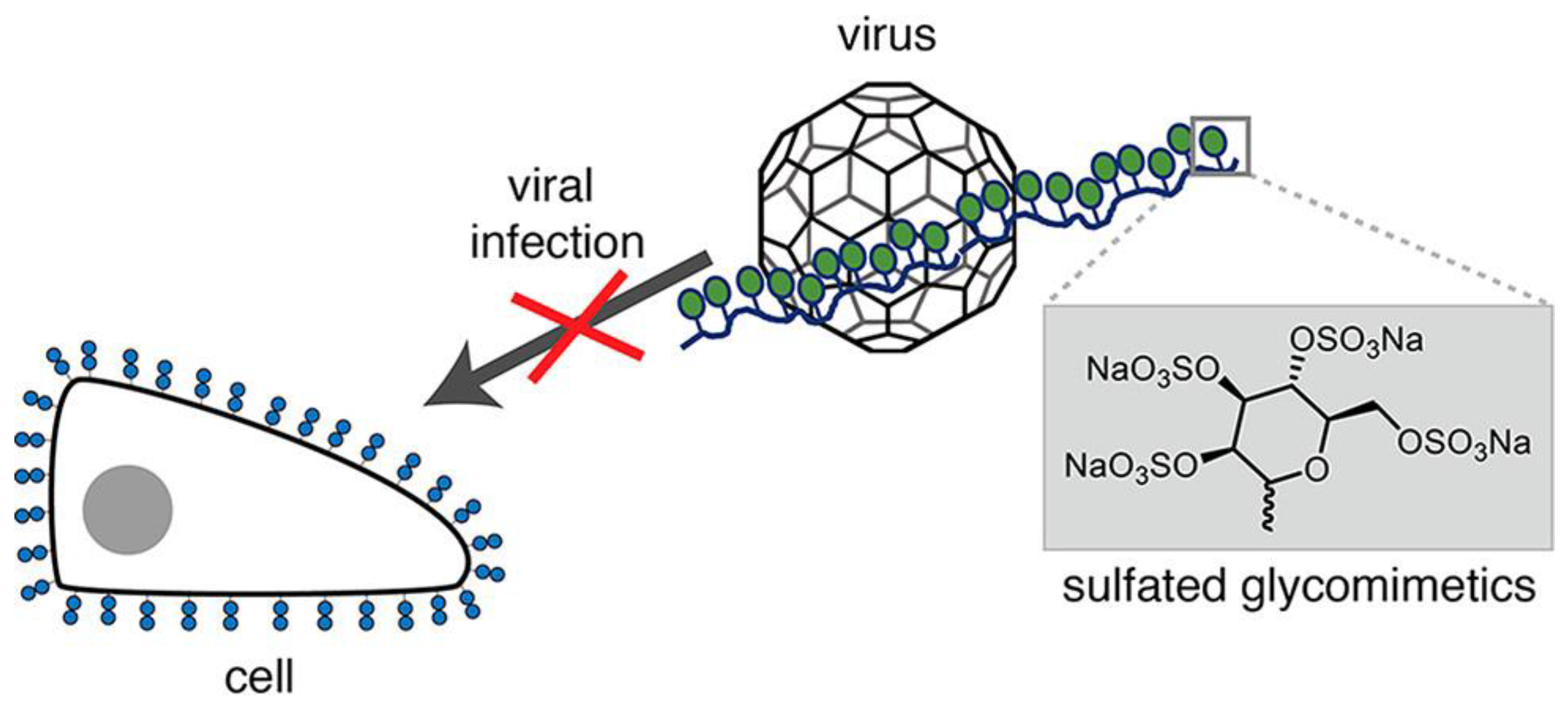
Figure 4. A model of competition between sulfate−like polymers and cellular glycans to inhibit virus invasion.
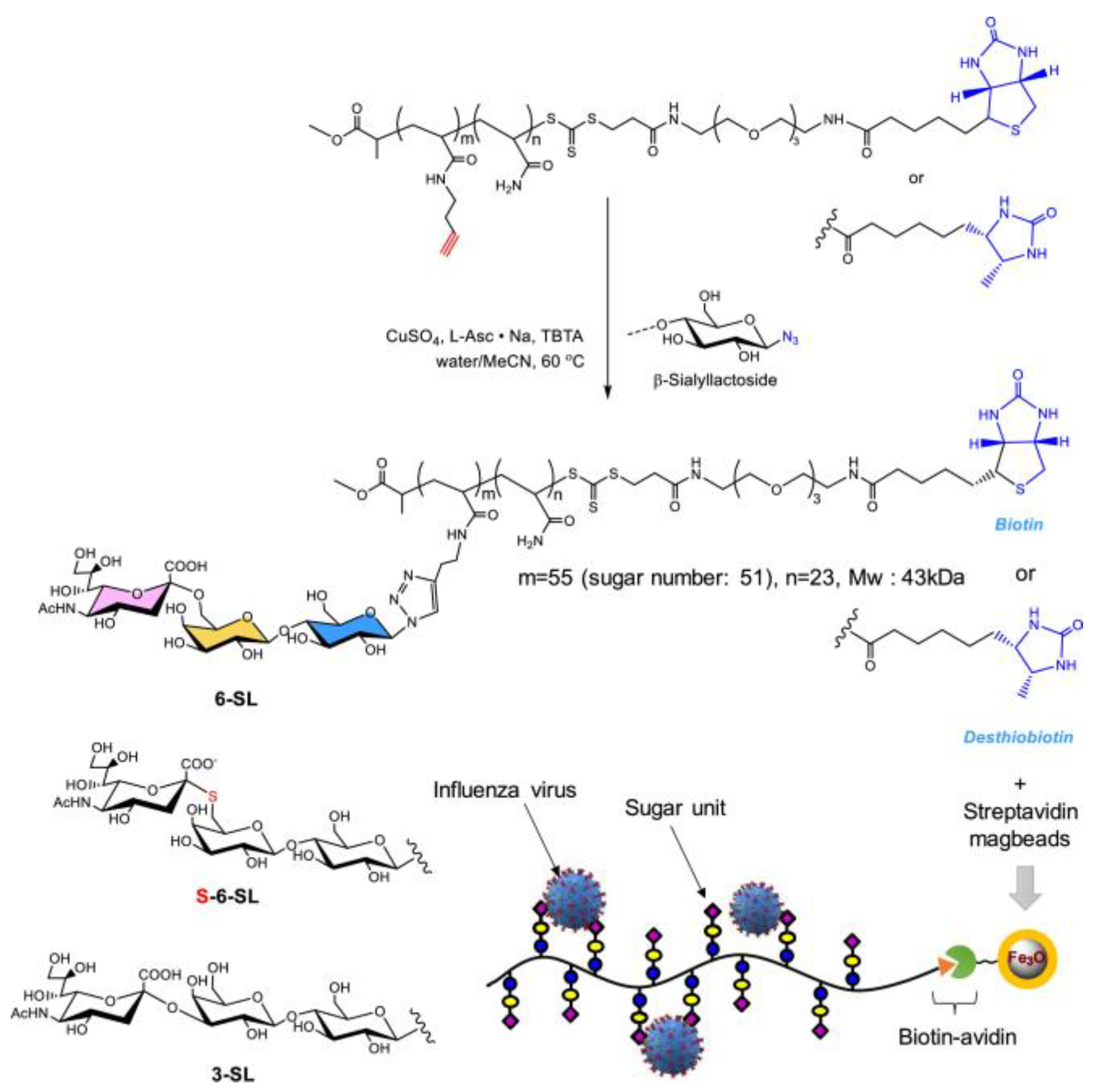
Figure 5. Chemical structures and schematic procedure for the preparation of the glyco−magbeads.
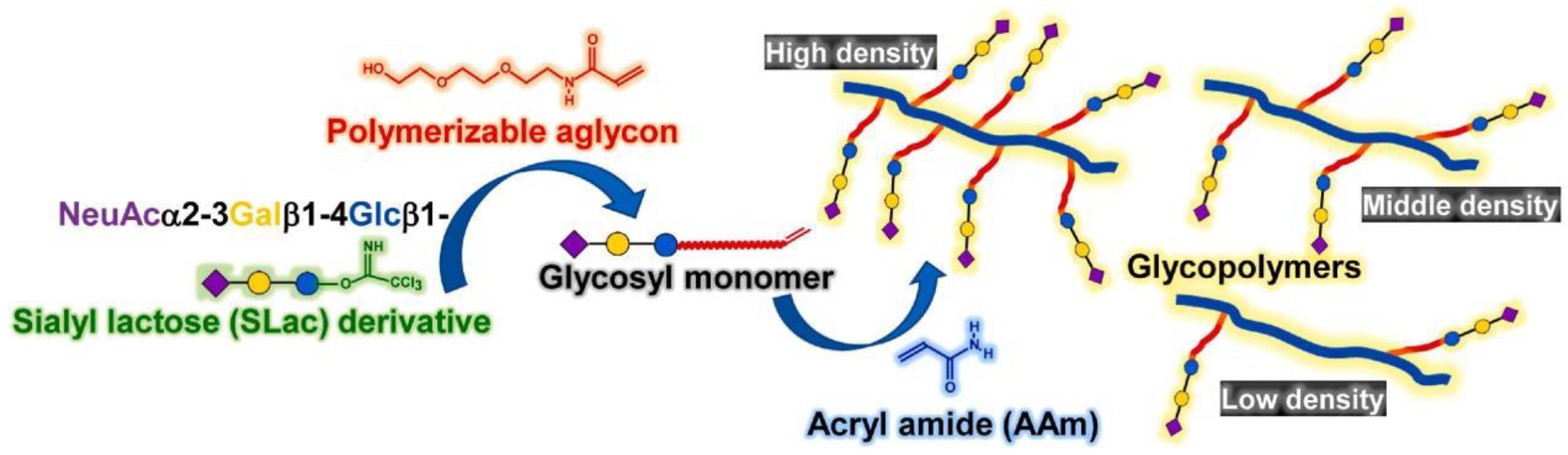
Figure 6. Construction of a synthetic scheme for multivalent glycopolymer containing SLac part.
Figure 7. Topological design of star polymers with glycounits (left) and hemagglutinin (right).
References
- Dhama, K.; Khan, S.; Tiwari, R.; Sircar, S.; Bhat, S.; Malik, Y.S.; Singh, K.P.; Chaicumpa, W.; Bonilla-Aldana, D.K.; Rodriguez-Morales, A.J. Coronavirus disease 2019-COVID-19. Clin. Microbiol. Rev. 2020, 33, 48.
- Miura, Y.; Hoshino, Y.; Seto, H. Glycopolymer Nanobiotechnology. Chem. Rev. 2016, 116, 1673–1692.
- Behren, S.; Westerlind, U. Novel approaches to design glycan-based antibacterial inhibitors. Eur. J. Org. Chem. 2022, 26, e202200795.
- Mammen, M.; Choi, S.K.; Whitesides, G.M. Polyvalent interactions in biological systems: Implications for design and use of multivalent ligands and inhibitors. Angew. Chem. Int. Ed. 1998, 37, 2755–2794.
- Kiessling, L.L.; Pontrello, J.K.; Schuster, M.C. Synthetic multivalent carbohydrate ligands as effectors or inhibitors of biological processes. In Carbohydrate-Based Drug Discovery; Wong, C.-H., Ed.; Wiley: Hoboken, NJ, USA, 2003; pp. 575–608.
- Kiessling, L.L.; Gestwicki, J.E.; Strong, L.E. Synthetic multivalent ligands in the exploration of cell-surface interactions. Curr. Opin. Chem. Biol. 2000, 4, 696–703.
- Ting, S.R.S.; Chen, G.J.; Stenzel, M.H. Synthesis of glycopolymers and their multivalent recognitions with lectins. Polym. Chem. 2010, 1, 1392–1412.
- Gestwicki, J.E.; Cairo, C.W.; Strong, L.E.; Oetjen, K.A.; Kiessling, L.L. Influencing receptor-ligand binding mechanisms with multivalent ligand architecture. J. Am. Chem. Soc. 2002, 124, 14922–14933.
- Becer, C.R. The Glycopolymer Code: Synthesis of glycopolymers and multivalent carbohydrate-Lectin interactions. Macromol. Rapid Commun. 2012, 33, 742–752.
- Ladmiral, V.; Mantovani, G.; Clarkson, G.J.; Cauet, S.; Irwin, J.L.; Haddleton, D.M. Synthesis of neoglycopolymers by a combination of “click chemistry” and living radical polymerization. J. Am. Chem. Soc. 2006, 128, 4823–4830.
- Nishida, Y.; Uzawa, H.; Toba, T.; Sasaki, K.; Kondo, H.; Kobayashi, K. A facile synthetic approach to L- and P-selectin blockers via copolymerization of vinyl monomers constructing the key carbohydrate modules of sialyl lewisX mimics. Biomacromolecules 2000, 1, 68–74.
- Gomez-Garcia, M.; Benito, J.M.; Butera, A.P.; Mellet, C.O.; Fernandez, J.M.G.; Blanco, J.L.J. Probing carbohydrate-lectin recognition in heterogeneous environments with monodisperse cyclodextrin-based glycoclusters. J. Org. Chem. 2012, 77, 1273–1288.
- Gomez-Garcia, M.; Benito, J.M.; Rodriguez-Lucena, D.; Yu, J.X.; Chmurski, K.; Mellet, C.O.; Gallego, R.G.; Maestre, A.; Defaye, J.; Fernandez, J.M.G. Probing secondary carbohydrate-protein interactions with highly dense cyclodextrin-centered heteroglycoclusters: The heterocluster effect. J. Am. Chem. Soc. 2005, 127, 7970–7971.
- Vico, R.V.; Voskuhl, J.; Ravoo, B.J. Multivalent interaction of cyclodextrin vesicles, carbohydrate guests, and lectins: A kinetic investigation. Langmuir 2011, 27, 1391–1397.
- Liang, C.H.; Wang, S.K.; Lin, C.W.; Wang, C.C.; Wong, C.H.; Wu, C.Y. Effects of neighboring glycans on antibody-carbohydrate interaction. Angew. Chem. Int. Ed. 2011, 50, 1608–1612.
- Xue, L.L.; Xiong, X.H.; Chen, K.; Luan, Y.F.; Chen, G.J.; Chen, H. Modular synthesis of glycopolymers with well-defined sugar units in the side chain via Ugi reaction and click chemistry: Hetero vs. homo. Polym. Chem. 2016, 7, 4263–4271.
- Cramer, J.; Lakkaichi, A.; Aliu, B.; Jakob, R.P.; Klein, S.; Cattaneo, I.; Jiang, X.; Rabbani, S.; Schwardt, O.; Zimmer, G.; et al. Sweet drugs for bad bugs: A glycomimetic strategy against the DC-SIGN-mediated dissemination of SARS-CoV-2. J. Am. Chem. Soc. 2021, 143, 17465–17478.
- Watanabe, Y.; Allen, J.D.; Wrapp, D.; Mclellan, J.S.; Crispin, M.J.S. Site-specific glycan analysis of the SARS-CoV-2 spike. Science 2020, 369, 330–333.
- Becer, C.R.; Gibson, M.I.; Geng, J.; Ilyas, R.; Wallis, R.; Mitchell, D.A.; Haddleton, D.M. High-affinity glycopolymer binding to human DC-SIGN and disruption of DC-SIGN interactions with HIV envelope glycoprotein. J. Am. Chem. Soc. 2010, 132, 15130–15132.
- Zhang, Q.; Su, L.; Collins, J.; Chen, G.; Wallis, R.; Mitchell, D.A.; Haddleton, D.M.; Becer, C.R. Dendritic cell lectin-targeting sentinel-like unimolecular glycoconjugates to release an anti-HIV drug. J. Am. Chem. Soc. 2014, 136, 4325–4332.
- Peck, K.M.; Lauring, A.S. Complexities of viral mutation rates. J. Virol. 2018, 92, 8.
- Sanjuan, R.; Domingo-Calap, P. Mechanisms of viral mutation. Cell. Mol. Life Sci. 2016, 73, 4433–4448.
- Duffy, S. Why are RNA virus mutation rates so damn high? PLoS Biol. 2018, 16, 6.
- Griffiths, C.; Drews, S.J.; Marchant, D.J. Respiratory syncytial virus: Infection, detection, and new options for prevention and treatment. Clin. Microbiol. Rev. 2017, 30, 277–319.
- Suthar, M.S.; Diamond, M.S.; Gale, M. West nile virus infection and immunity. Nat. Rev. Microbiol. 2013, 11, 115–128.
- Jung, K.; Saif, L.J. Porcine epidemic diarrhea virus infection: Etiology, epidemiology, pathogenesis and immunoprophylaxis. Vet. J. 2015, 204, 134–143.
- Xu, D.; Esko, J.D. Demystifying heparan sulfate–protein interactions. Annu. Rev. Biochem. 2014, 83, 129–157.
- Katoh, M. FGFR inhibitors: Effects on cancer cells, tumor microenvironment and whole-body homeostasis (review). Int. J. Mol. Med. 2016, 38, 3–15.
- Zhang, G.-L.; Ye, X.-S. Synthetic glycans and glycomimetics: A promising alternative to natural polysaccharides. Chem. Eur. J. 2018, 24, 6696–6704.
- Soria-Martinez, L.; Bauer, S.; Giesler, M.; Schelhaas, S.; Materlik, J.; Janus, K.; Pierzyna, P.; Becker, M.; Snyder, N.L.; Hartmann, L.; et al. Prophylactic antiviral activity of sulfated glycomimetic oligomers and polymers. J. Am. Chem. Soc. 2020, 142, 5252–5265.
- Lees, W.J.; Spaltenstein, A.; Kingery-Wood, J.E.; Whitesides, G.M. Polyacrylamides bearing pendant.alpha.-sialoside groups strongly inhibit agglutination of erythrocytes by influenza A virus: Multivalency and steric stabilization of particulate biological systems. J. Med. Chem. 1994, 37, 3419–3433.
- Liu, H.-P.; Meng, X.; Yu, Q.; Tao, Y.-C.; Xu, F.; He, Y.; Yu, P.; Yang, Y. Synthesis of S-sialyl polymers as efficient polyvalent influenza inhibitors and capturers. J. Carbohydr. Chem. 2018, 37, 18–29.
- Li, G.; Ma, W.; Mo, J.; Cheng, B.; Shoda, S.I.; Zhou, D.; Ye, X.S. Influenza virus precision diagnosis and continuous purification enabled by neuraminidase-resistant glycopolymer-coated microbeads. ACS Appl. Mater. Interfaces 2021, 13, 46260–46269.
- Nagao, M.; Fujiwara, Y.; Matsubara, T.; Hoshino, Y.; Sato, T.; Miura, Y. Design of glycopolymers carrying sialyl oligosaccharides for controlling the interaction with the influenza virus. Biomacromolecules 2017, 18, 4385–4392.
- Matsuoka, K.; Kaneshima, T.; Adachi, R.; Sasaki, J.; Hashiguchi, T.; Koyama, T.; Matsushita, T.; Hatano, K. Preparation of glycopolymers having sialyl alpha2 --> 3 lactose moieties as the potent inhibitors for mumps virus. Bioorgan. Med. Chem. Lett. 2021, 52, 128389.
- Nagao, M.; Matsubara, T.; Hoshino, Y.; Sato, T.; Miura, Y. Topological design of star glycopolymers for controlling the interaction with the influenza virus. Bioconjug. Chem. 2019, 30, 1192–1198.
More
