Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Camila Xu and Version 1 by Aadil Javed.
Gastric cancer predominantly occurs in adenocarcinoma form and is characterized by uncontrolled growth and metastases of gastric epithelial cells. The growth of gastric cells is regulated by the action of several major cell cycle regulators including Cyclins and Cyclin-dependent kinases (CDKs), which act sequentially to modulate the life cycle of a living cell. It has been reported that inadequate or over-activity of these molecules leads to disturbances in cell cycle dynamics, which consequently results in gastric cancer development.
- gastric cancer
- Cyclins
- Cyclin-dependent kinases (CDKs)
- cell signaling
- microRNAs
- Non-coding RNAs
1. Introduction to Gastric Cancer
1.1. General Epidemiology of Gastric Cancer
Among malignancies, gastric cancer is considered the fifth most commonly occurring disease, with a million new annual cases. As the late diagnosis of the disease is common, the mortality rate is higher, and for this reason, gastric cancer is the third deadliest cancer worldwide [1]. South America, Eastern Europe, and East Asia are considered hotspots for mortality and incidence of gastric cancer [2]. Cases occur twice as often in men as in women. The recent trend highlights a decreasing mortality rate. However, due to aging populations, the incidence rate of the disease is expected to increase [3].
1.2. General Risk Factors for Gastric Cancer
Infection rates for the major cause of the disease, H. pylori, have decreased due to economic development and improved living standards [4,5][4][5]. Non-cardia gastric cancer is associated with H. pylori infection, where the gastric mucosa is subjected to chronic infection leading to atrophic gastritis, which further results in intestinal metaplasia [6]. Most H. pylori infections remain asymptomatic. However, gastric cancer occurrence due to infection is associated with the virulence, host, and environmental factors along with oncogene activation causing pathogenicity due to Cytotoxin-Associated Gene A (Cag-A) protein capable of affecting a cohort of cellular signaling pathways [7,8,9][7][8][9]. Treatment of the infection can help reduce the burden of gastric cancer transformation; however, it is dependent on the degree of pre-existing damage [10]. High-risk population environments, familial predisposition, alcohol consumption, cigarette smoking, and older age are other risk factors for non-cardia gastric cancer beyond H. pylori infection [11]. H. pylori infection has been associated with salted food intake and gastric cancer development; therefore, dietary modification involving food intake with less salt is one of the preventive measures against gastric cancer. High intake of vegetables and fruits is also recommended [12].
1.3. Genetic Parameters Associated with Gastric Cancer
The genetic background as an etiological feature is also dominant in gastric cancer, as 1–3% of the cases possess germline mutations and approximately 10% of the patients present familial association [13,14,15][13][14][15]. The three major types of gastric cancer involving autosomal dominance as the hereditary element include gastric adenocarcinoma with proximal polyposis (stomach), familial intestinal gastric cancer, and hereditary diffuse type gastric cancer (HDGC). HDGC cases show a representation of 30–40% of the patients with CDH1 (E-Cadherin) mutations with screening for CDH1 mutations now available at expert centers with clinical geneticists. The pathogenic CDH1 mutation-carrying patients are recommended prophylactic total gastrectomy [16,17,18][16][17][18]. A germline mutation in CTNNA1 (encoding alpha-E-catenin) is associated with three families of HDGC [19,20][19][20]. HDGC also exhibits mutations in DOT1-L, FBXO24, IR and PALB2 [21,22][21][22]. The proximal polyposis carrying gastric adenocarcinoma is linked with APC promoter 1 gene mutations [23]. There are genetic disorders that have been associated with the development of gastric cancer and include PeutzJegher syndrome, (STK11), MUTYH-associated adenomatous polyposis, Li-Fraumeni syndrome (TP53), juvenile polyposis (SMAD4, BMPR1A), Cowden Syndrome (PTEN), Lynch syndrome (PMS2, MLH1/2, MSH6), and familial adenomatous polyposis (APC) [24].
1.4. General Classification of Gastric Cancer
Borrmann classified early gastric cancers according to microscopic appearance, comprising four types, namely: Type I (broad base and polyploid without ulceration), Type II (sharp margins and elevated borders with ulceration), Type III (infiltration at the base with ulceration), and Type IV (thickened wall with diffusive infiltration) [25]. The therapy response along with prognosis according to histopathological phenotypes is a subject of conflict among researchers as variations in histopathological classifications remain [26]. For example, the World Health Organization (WHO) classification leads to complexity due to the distribution into multiple subtypes even for rare cases. The WHO system includes Paneth cell type, hepatoid, medullary carcinoma with lymphoid stroma, signet ring cell, poorly cohesive, mucinous, mucoepidermoid, micropapillary, papillary, parietal cells, and tubular subtypes [27]. Another classification system employs the Lauren system, consisting of distinguishing unclassifiable, indeterminate, diffuse type, and intestinal type gastric cancer. However, many subtypes remain outside the Lauren classification [28]. Furthermore, the Nakamura system of classification is based on undifferentiated and differentiated types, which can inform the decision for endoscopic resection in the cases of early forms of gastric cancer [29].
1.5. General Diagnosis and Therapies for Gastric Cancer
The diagnosis of gastric cancer is based on digital image analysis and liquid biopsies [30], with symptoms at the time of presentation including abdominal pain, weight loss, early satiety, anorexia, and indigestion. Late-stage symptoms often include anemia or dysphagia [31]. Surgery remains the foremost defense against gastric cancer and involves endoscopic resection and gastrectomy along with lymphadenectomy and minimally invasive surgery [32]. Other curative options available to patients of gastric cancer are neoadjuvant and perioperative chemotherapy along with preoperative and postoperative radiation therapy, and postoperative adjuvant chemotherapy [33]. In advanced cases of gastric cancer, chemotherapy leads to improvement in quality of life along with survival, especially in cases of metastatic or unresectable forms of gastric cancer [34]. Supportive care provides a 3–4-month survival advantage, whereas combination chemotherapy in patients has been shown to give an approximately 1-year median overall survival [35,36][35][36]. Chemotherapy is recommended for patients exhibiting organ function and adequate performance function [37]. The chemotherapeutics routinely used against gastric cancer include Oteracil (orotate phosphoribosyltransferase), Gimeracil (dihydropyrimidine dehydrogenase inhibitor), and S-1 (oral 5-fluorouracil prodrug) along with Irinotecan, Taxanes, and platinum. These therapies can be employed in combination as well [38,39][38][39].
2. Overview of Cell Cycle Regulation
The cell cycle of a eukaryotic cell has an interphase containing three phases, the G1, S, and G2 phases, and the mitosis (M) phase carrying cytokinesis [40]. During the interphase of the cell cycle, the growth of cells occurs due to the accumulation of the nutrients required for subsequent mitosis, along with the replication of cellular contents including organelles and DNA. Mitosis then occurs, dividing the cells into two daughter cells carrying genetic material and organelles [41]. There are several cell cycle checkpoints ensuring the correct progression of the cell cycle in terms of cellular components and division [42]. Cyclin-dependent kinases (CDKs) are activated by different Cyclins due to their sequential expression throughout the cell cycle [43].
Mitogenic signaling gives rise to the activation of Cyclin D in the G1 phase of the cell cycle, which in turn functions in activating CDK4/6, causing the phosphorylation of retinoblastoma protein (RB) [44]. The E2F transcription factors are then released to activate Cyclin E and Cyclin A transcriptions, leading to further phosphorylation of RB. There are internal CDK inhibitors in cells with families including INK4 and CIP/KIP, which bind the complexes of Cyclin-CDKs to inhibit phosphorylation of RB and cause eventual prevention of E2F release. These mechanisms cause the replication machinery to be arrested, hence acting as a checkpoint for the cell cycle control [45,46][45][46]. The mitotic checkpoint is triggered due to various interactions of Cyclin B-CDK1 complexes, which inhibit the RB by phosphorylations from the S to G2/M transition. The Cyclin B/CDK1 complex assists in nuclear envelope breakdown, chromosome condensation, and mitotic spindle formations. The phosphorylations of tumor suppressor RB control the checkpoints, because hyperphosphorylations due to altered signaling in tumor cells render the growth of cells independent of outside or mitogenic signals [47]. A general overview of the cell cycle pathway involving various Cyclins, CDKs, E2F and Rb states is given in Figure 1. In diseased states such as cancers, these pathways are dysregulated, and this knowledge can be utilized to perform diagnosis and targeted therapy [48].
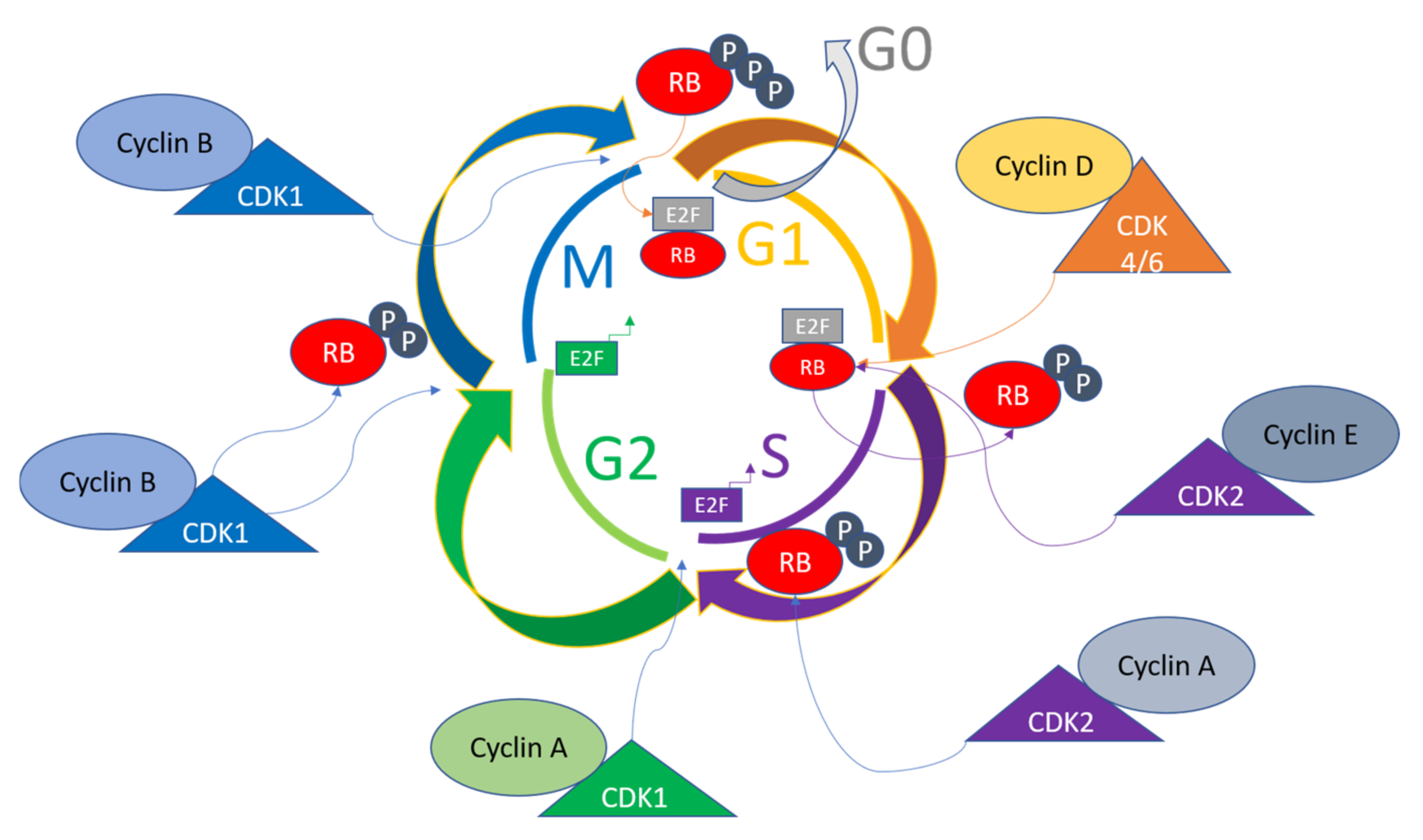
Figure 1. General pathway of cell cycle regulation. Various Cyclins activate different CDKs sequentially to progress the cell cycle and determine the fate of the cells. The CDKs in turn act to phosphorylate various substrates for transitioning the cell cycle phases. For example, RB is one of the major regulators of the cell cycle, and is the target of CDK4/6, CDK2, and CDK1, and together with activated E2F and its target genes, leads to the transition of cell cycle phases.
3. The Role of CDKs in Cell Cycle Control
The cells in adult tissues carrying diploid DNA usually reside in a state of quiescence marked by the G0 phase that is either permanent or temporary depending on the functionality of the cells. When cells enter a new cell cycle after mitosis, growth factors or hormones trigger a cascade of signaling events that converge on CDK4 or CDK6 to cause the cells to go through another round of cell cycle upon entry into the S phase. The Cyclin D protein with fluctuating levels throughout the cell cycle binds the CDK4/6 to form complexes [49,50][49][50]. Activated Cyclin D-CDK4/6 complexes phosphorylate RB along with p130 and p107, which in hypophosphorylated forms function in the recruitment of repressors of E2F transcription factors to inhibit the G1 to S transition. Cyclin C/CDK3 complexes are also involved in RB phosphorylations, which can lead to entry into the S phase directly from Go, which is either a reversible or permanent phase depending on the cell type [51,52][51][52]. The downstream target genes of E2F activated upon RB phosphorylated by CDK4/6 include CCNE, CCNA and CCNB, encoding Cyclin E, Cyclin A, and Cyclin B, respectively. Other proteins activated upon this cascade include dihydrofolate reductase (DHFR), ribonucleotide reductase M1 (RRM1), RRM2, and Polo-like kinase 1 (PLK1), spindle checkpoint protein MAD2, and mitotic checkpoint serine-threonine kinase (BUB1). These proteins function in the progression of the cell cycle [53,54][53][54].
In late G1, E2F factors activate Cyclin E1 and Cyclin E2, which bind CDK2 to activate the Cyclin E/CDK2 complex, activating CDK2, which before Cyclin E binding is kept sequestered in p27 and p21 bound forms. These inhibitors are also marked for ubiquitin-mediated degradation upon activation of CDK2. CDK2 is further activated by cell division cycle 25A (CDC25A), which dephosphorylates CDK2 [55]. CDK2 phosphorylates a range of substrates to progress the cell cycle and carry out different functions such as histone synthesis (it is a nuclear protein activator of histone transcription), centrosome duplication, and DNA replication [56]. The schematic regulation of the cell cycle by CDKs has recently been reviewed in detail [57]. The activation of CDK4/6 and CDK2 and their downstream targets render the fate of the cell cycle independent of the mitogenic signals; therefore, the restriction point is passed at this point. When the S phase nears its end, Cyclin A displaces Cyclin E from CDK2 and F-box/WD repeats carrying protein 7 (FBXW7), leading to proteasomal degradation of Cyclin E [58].
The termination of the S phase is led by E2F1, and CDC6 phosphorylations by CDK2 bound with Cyclin A, causing entry into the G2 phase of the cell cycle. CDK2 is also involved in the activation of CDK1, which drives the G2/M transition. Cyclin B activates CDK1 and CDK1 phosphorylations, causing nuclear envelope breakdown, chromosome condensation, and mitotic spindle assembly during mitosis. Anaphase-promoting complex/cyclosome (APC/C) degrades Cyclin B and decreases CDK1 activity in the anaphase at the spindle assembly checkpoint (SAC) [59,60][59][60]. A high fidelity of genomic integrity is ensured through the realization of the critical events that are dependent on cell cycle progression and mitotic onset, which is enabled by CDK1 rendering it the most important of the Cyclin-dependent kinases [61]. CDK1 activity is further controlled by Myelin transcription factor 1 (MYT1), G2 checkpoint kinase (WEE1), and CDC25C phosphatase. CDK1 is inhibited by phosphorylations at Tyr15 by Wee1, and Thr14 and Tyr15 by MYT1, and these phosphates are removed by CDC25C for activation of CDK1 [62,63][62][63].
DNA damage during the cell cycle causes cell arrest to repair the damage before entering mitosis. These checkpoints occur before and after DNA synthesis in G1 and G2 phases. DNA damage signaling involves checkpoint kinases CHK1 and CHK2, along with Ataxia telangiectasia mutated kinase (ATM), Ataxia telangiectasia, and Rad3 related kinase (ATR), and phosphatidylinositol 3-kinase (PI3K) [64]. CHK1/CHK2 axis is activated upon phosphorylation of ATM/ATR kinases, which detect DNA damage [65]. CHK2, upon activation by upstream kinases, leads to p53 activation, causing the G1 arrest to repair the damaged DNA [66]. Cyclin E-CDK2 complex activity is reduced upon activation of p21 by p53, as DNA repair machinery is upregulated upon p21 activation. Eventually, apoptosis is triggered if the stress of DNA damage is not repaired properly [67,68][67][68]. CHK1 also triggers G2 arrest by phosphorylating CDC25A to induce proteolysis by ubiquitination. Another substrate of CHK1 is WEE1, which is another mechanism for G2 arrest of the cell cycle [69,70][69][70].
The mitotic checkpoint (also known as the spindle assembly checkpoint (SAC)), functions to monitor the metaphase plate in order to correct the chromosome alignment. The checkpoint is regulated by monopolar spindle 1 (MPSI), also known as TTK protein kinase, which regulates the recruitment of checkpoint proteins to the kinetochore complexes. The genome integrity and appropriate chromosome segregation are regulated by the phosphorylation of substrates of TTK [71,72][71][72]. The passage of SAC brings the APC/C complex into play, which stimulates the degradation of securing and Cyclin B to initiate mitosis [73,74][73][74]. The checkpoints present at various time points throughout the cell cycle ensure the genomic and mitotic fidelity of the cells entering the new cell cycle, and the convergence of various signaling pathways and these checkpoints on CDKs indicate the importance of Cyclins and CDKs in the cell cycle progression. The cell cycle checkpoints and the major regulators of CDKs are depicted in Figure 2. Since dysregulated cell cycle regulation is considered one of the hallmarks of cancer, CDK inhibition for therapeutic purposes, especially CDK4/6 using small molecule inhibitors, presents a convincing solution for overcoming resistance to conventional drugs [75].
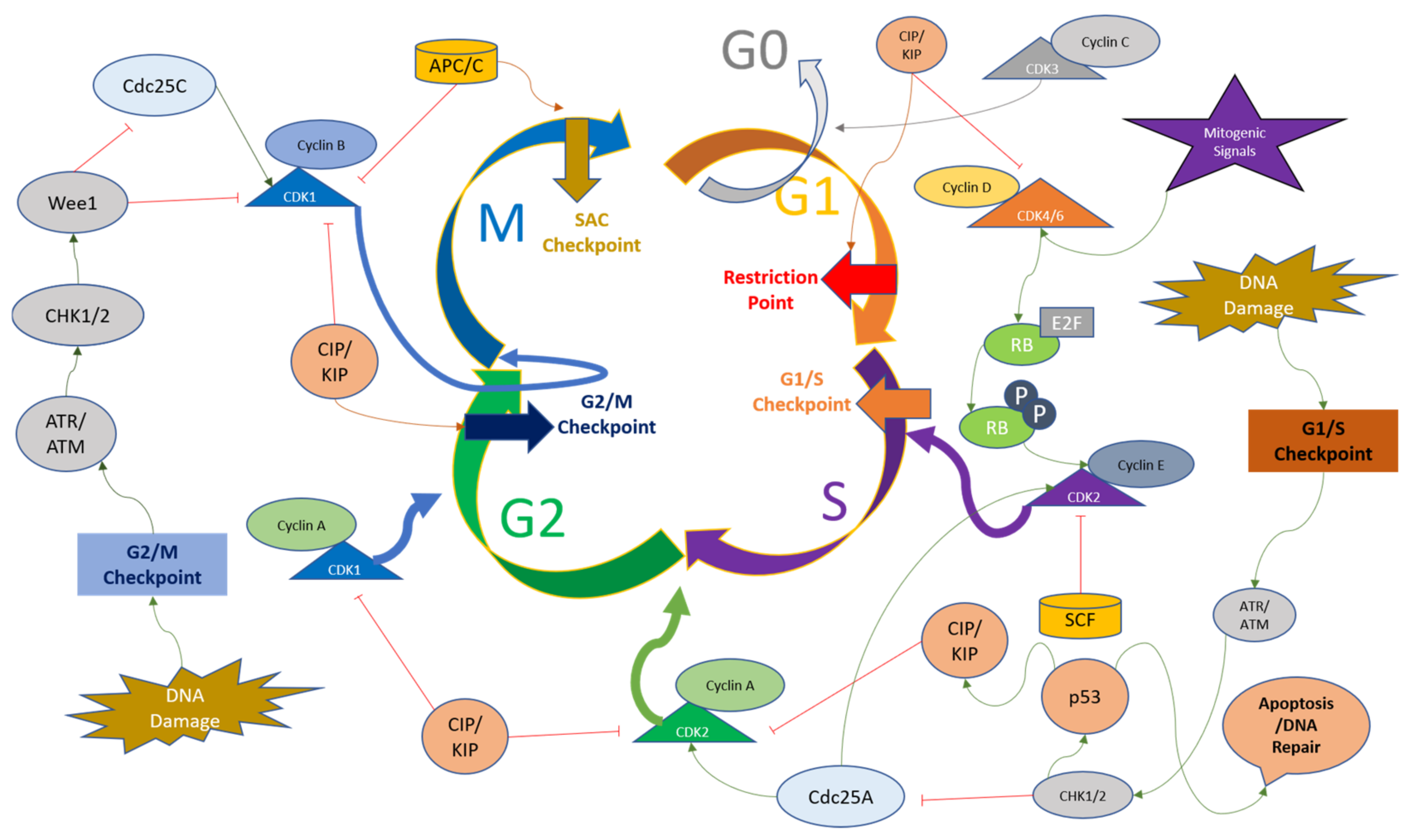
Figure 2. Overview of cell cycle regulation with respect to CDKs and their major regulators. The upstream factors of Cyclins and CDKs can either activate or downregulate their respective functions and cause the cell cycle to halt at various time points including the G1 restriction point, G1/S checkpoint, G2/M checkpoint, and Spindle Assembly Checkpoint (SAC). The description of these factors and their action on Cyclins and CDKs are detailed in the main text.
4. The Regulation of Cyclins and CDKs in Gastric Cancer
As mentioned previously, cell cycle regulators such as Cyclins and CDKs can be utilized as biomarkers, prognostic markers, and diagnostic factors, and targeted using small molecule inhibitors [76,77][76][77]. However, there is a need to develop a thorough understanding of not only the downstream effects of inactivation of these regulators, but also the upstream regulators functioning to either activate or inhibit Cyclins and CDKs in specific contexts such as solid tumors or hematological malignancies [78].
4.1. The Regulation of Cyclin D1 in Gastric Cancer
Cyclin D and its isoforms are the first major type of Cyclin taking part in sequentially organizing the order of the events of cell cycle regulation [79]. It activates CDK4/6 upon transcription signals activated via mitogenic or growth factors and is dysregulated in various cancers [80]. Cyclin D1 expression at high levels has been associated significantly with poor prognosis of gastric cancer, as observed in gastric cancer samples in which CD44 expression was also higher, indicating a coexpression of these proteins could be a potential biomarker for the severity of the disease [81].
4.1.1. Factors Downregulating Cyclin D1 in Gastric Cancer
In the natural paradigm of cellular signaling, the events converge at major regulators to keep the cell fates in check. Various factors downregulate the activity of Cyclin D1 in gastric cancer cells and are discussed here in detail. The carbohydrate response element binding protein (CHREBP) is downregulated in gastric cancer and inhibits Cyclin D1 levels in gastric cancer cells in order to suppress growth via negative modulation of the Cyclin D1-Rb-E2F1 signaling mechanism [82]. A ring finger domain-containing protein (RN181), which functions as an E3 ubiquitin ligase, was shown to be downregulated in gastric cancer as compared to normal tissues and regulates Cyclin D1-CDK4 activity via inhibition of ERK/MAPK signaling and G1 to S phase transition [83]. The miR-129-5p induces cell cycle arrest in vitro and in vivo conditions in gastric cancer and targets HOXC10 directly for its downregulation, which further functions in regulating the expression of Cyclin D1 [84]. In another study, involving gastric cancer cell line SGC-7901, curcumin treatment led to the stabilization of miR-34a, which in turn inhibited Cyclin D1 and CDK4 from inducing cell cycle arrest and apoptosis [85]. miR-623 is downregulated in gastric cancer and can sensitize invasive cancer cells to 5-FU for induction of apoptosis. miR-623 directly targets Cyclin D1 mRNA for its down regulation, and CCND1 overexpression reverses the effects of miR-623 [86].
An Interleukin 6 cytokine family member known as Leukemia inhibitory factor (LIF) downregulates Cyclin D1 and upregulates p21 in gastric cancer cells under both in vivo and in vitro conditions [87]. Tripartite motif-containing 58 protein (TRIM58) is a potential tumor suppressor protein in gastric cancer, where it is presented in the downregulated form. Its overexpression in gastric cancer cell lines led to a reduction of survivin, Cyclin D1, c-myc, and β-catenin. It was concluded that TRIM58 increases β-catenin degradation to inhibit the progression of gastric cancer through ubiquitination-mediated mechanisms [88]. A potential antitumor compound, Glycyrrhizic acid, has been tested for its efficacy in gastric cancer cell lines, and led to G1 arrest and induction of apoptosis, along with reduction of Cyclin D1, D2, D3, E1, and E2 and an increase in pro-apoptotic cleavage patterns of pro-caspases [89]. Dihydroartemisinin (DHA) exerts its anti-tumor activities through inhibition of CDK4 activity and targets Cyclin D1 negatively for induction of cell cycle arrest under in vivo and in vitro conditions in gastric cancer [90]. Table 1 illustrates and summarizes the upstream molecules or inhibitors that act in negative regulation of Cyclin D1 in gastric cancer cells or tissues.
Table 1.
The upstream molecules or inhibitors downregulating Cyclin D1 in gastric cancer are shown, along with their mode of action.
| Regulator Molecule | Cyclin/CDK | Mode of Action | References | |
|---|---|---|---|---|
| CHREBP | Cyclin D1 | Negative 242 modulation of the Cyclin D1-Rb-E2F1 signaling mechanism | [82] | |
| RN181 | Cyclin D1/CDK4 | E3 ubiquitin ligase regulates Cyclin 245 D1-CDK4 activity via inhibition of ERK/MAPK | [83] | |
| Circumin | Cyclin D1/CDK4 | Stabilization of miR-34a, which inhibits Cyclin D1 and CDK4 | [85] | |
| LIF | Cyclin D1 | Downregulates Cyclin D1 and upregulates p21 | [87] | |
| Upregulation | [ | 103 | ] | |
| Hsa_circ00000647 | Circular RNA | Cyclin D1 | Upregulation | [104] |
| HOTAIR | Long Non-coding RNA | Cyclin D1 | Upregulation | [106] |
| GCRL1 | Long Non-coding RNA | CDK4 | Downregulation | [115] |
| miR-885-3p |
H. pylori-infected cells exhibiting proliferative advantage leads to the downregulation of Cyclin E1, c-myc, β-catenin, and p-GSK3b to inhibit cell proliferation [155]. A long non-coding RNA, GHET1, is upregulated in gastric cancer, and its inhibition in gastric cancer cells leads to reductions in CDK2, Cyclin E1, CDK6, CDK4, and Cyclin D1 [156]. Human ribosomal protein 6 (RPL6) is expressed more highly in gastric cancer and confers multidrug resistance to gastric cancer cells, as observed in cells overexpressing RPL6. Gain-of-function and loss-of-function experiments with RPL6 indicated Cyclin E to be the main target for promoting G1 to S transition for promoting the growth of gastric cancer cells [157].
Since Cyclin E is overexpressed in gastric cancer, its coexpression network analysis provides a unique tool to select the potential oncogenes involved in the progression of gastric cancer. Nuclear transcription factor Y alpha (NF-YA) is found as coexpressed with Cyclin E. Therefore, it was further evaluated in gastric cancer cell lines, and it was observed that NF-YA increases the Cyclin E transcription in gastric cancer to assist gastric cancer progression, and this unique signaling axis can be targeted for therapy as well [158]. An oncogene named 14-3-3ε functions as an upstream factor for Cyclin E activation, as the suppression of 14-3-3ε leads to Cyclin E downregulation in gastric cancer cells [159]. The Enhancer of Zeste Homolog 2 (EZH2), functioning as a polycomb protein, also upregulates Cyclin E in gastric cancer cells, as its inhibition leads to Cyclin D1 and Cyclin E downregulation. EZH2 is found in overexpressed forms in gastric carcinomas and regulates cell cycle-related proteins for the development of invasive phenotypes [160]. Bruceine D (BD) extracted from Brucea javanica treatment leads to the inhibition of a long non-coding RNA (LINC01667). LINC01667 is upregulated in gastric cancer, where it sponges miR-138-p to upregulate Cyclin E1 expression. It was observed that BD sensitizes gastric cancer cells to Doxorubicin by targeting LINC01667/miR-138-p/Cyclin E1 axis, and could be a potential drug candidate against gastric cancer [161]. Various factors mentioned here are depicted in Figure 5 to elucidate the findings related to Cyclin E conducted in gastric cancer cells.
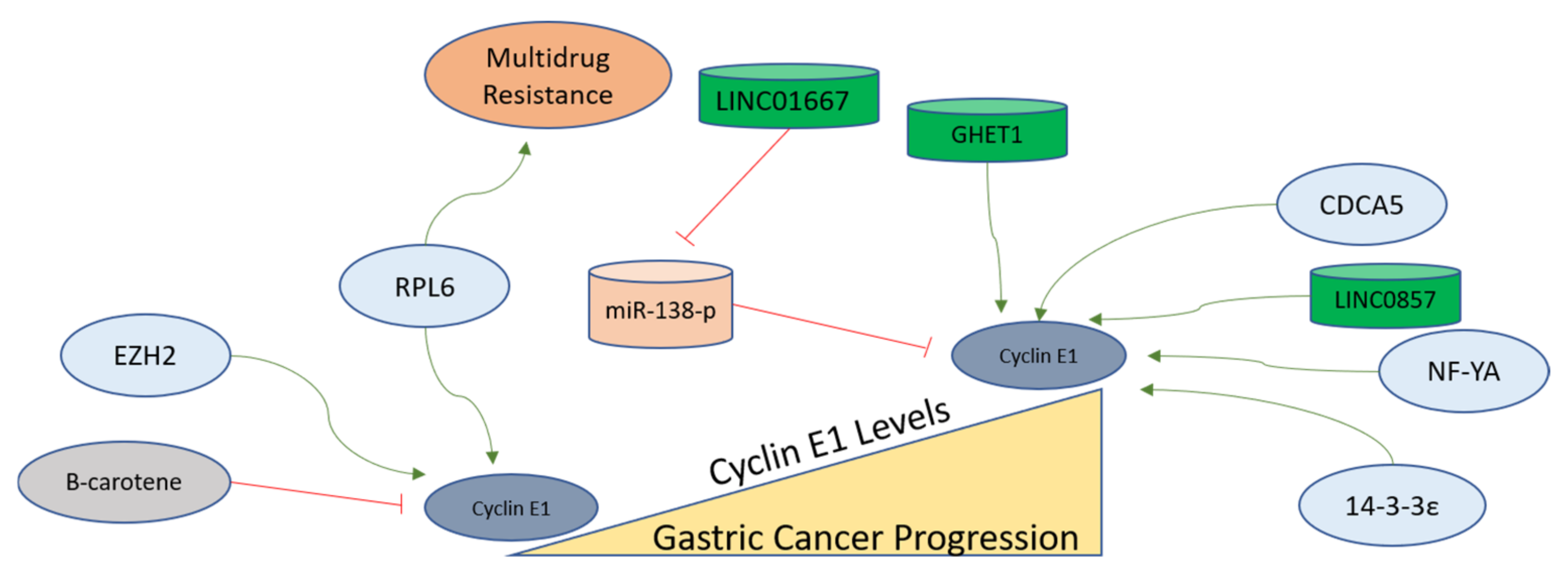
Figure 5. Cyclin E1 regulation in gastric cancer progression. Cyclin E functions to progress cells from G1 to the S phase and remains upregulated in the S phase. In gastric tumors, Cyclin E1 expression is higher than in normal tissues. The factors activating the functions of Cyclin E in gastric cells are shown, with green arrows indicating activation and red lines showing inhibition. The mechanisms of these regulators are described in detail in the main text.
4.4. Cyclin A and CDK2 Regulation in Gastric Cancer
4.4.1. Cyclin A Expression in Gastric Cancer
Cyclin A expression has been associated with the progression of various cancers. The protein levels of Cyclin A are controlled tightly in order to achieve progression of the cell cycle, and these mechanisms of proteolysis and protein stability demonstrate the involvement of Cyclin A in the progression of normal cells to carcinogenesis [162,163][162][163]. In gastric cancer patients, higher expression of Cyclin A has been correlated with HuR (mRNA stability factor). Furthermore, Cyclin A expression and its association with poor survival in gastric cancer are not correlated with distant metastases, but were significantly associated with nodal metastases, penetration depth, noncurative resection, intestinal type, proximal location, high stage, and old age of the patients. The same study concluded that Cyclin A expression was correlated with cytoplasmic localization of HuR and not nuclear HuR, and could be the mechanism for Cyclin A-involvement in the progression or development of gastric cancer [164]. Not many studies have been conducted on the direct involvement of Cyclin A protein involving gastric cancer cells or tissues. However, the Cyclin A partner CDK2 [165] has been studied, and is reviewed in detail.
4.4.2. The Regulation of CDK2 in Gastric Cancer
Generally, Cyclin E and Cyclin A bind to CDK2 sequentially to activate the CDK2, with both Cyclins heading toward ubiquitin-mediated proteolysis. However, CDK2 in unbound form remains inactive and is degraded via a lysosome-autophagy-mediated pathway [166]. As mentioned previously, CDK2 is activated via binding with Cyclin E and Cyclin A for the progression from G1 to S and from S onward in order to maintain cellular integrity [167]. In gastric cancer cells, a long non-coding RNA named LINC01021, which is upregulated in cancer cells, functions in the regulation of KISS1, which is further stabilized via CDK2 activity. CDK2 phosphorylates CDX2 and causes its nuclear export. The mechanism involves LINC01021-mediated binding of CDX2 and CDK2 to promote angiogenesis, invasion, and migration of gastric cancer cells [168]. Moreover, Poly (rC) binding protein 2 (PCBP2), acting as an oncogene in gastric cancer cells, promotes the CDK2 activity by direct interaction and is overexpressed in gastric cancer, where the higher expression is associated with poor survival of the patients [169]. Furthermore, another gastric cancer promoter protein named a DEAD cassette helicase 21 (DDX21), which is an ATP-dependent RNA helicase, is involved in upregulating the expression of Cyclin D1 and CDK2. It provides a rationale for the involvement of CDK2 in the development of gastric cancer as a novel upstream factor for gene regulation of CDK2 [170].
Apart from regular cell cycle regulatory pathways, CDK2 is also a major regulator of cancer cell metabolism in gastric cancer. In gastric cancer cell lines, CDK2 modulates the aerobic glycolytic capacity via suppression of SIRT5 which acts as a tumor suppressor in gastric cancer cells [171]. Another long non-coding RNA, hepatocyte nuclear factor 1 homeobox A antisense RNA 1 (HNF1A-AS1), is upregulated in gastric cancer cells, where early-growth response protein 1 (EFR1) activates the transcription of HNF1A-AS1. HNF1A-AS1 functions as competing-endogenous RNA (ceRNA) to enhance CDC34 expression by binding to miR-661. Subsequently, both EGR1 and HNF1A-AS1 inhibit p21 via activation of CDK2 and CDK4, adding another layer of regulatory mechanisms involved in the function of CDK2 in the development of gastric cancer [172]. Sulforaphane (SFN) exerts antiproliferative effects in gastric effects via inhibition of cell cycle progression and inducing apoptosis and the mechanism includes decreased CDK2 activity p-53-mediated apoptosis to prevent the progression of cells into developing tumorigenic capabilities [173]. Another long non-coding RNA GHET1, which is highly expressed in gastric cancer cells, promotes the cell cycle regulators including CDK2 for inducing the invasive phenotype including the G1-to-S transition and the inhibition of p21 [156].
In gastric cancer cells, TGF-b1 activates caspase-3 to initiate the transition from cell cycle arrest to apoptosis through Rb, p27, and p21 cleavage. These events lead to the activation of CDK2, which has been implicated as a downstream target of caspase to execute the apoptosis induced by TGF-b1 [174]. Furthermore, miR-638 in gastric cancer cells directly regulates the expression of CDK2 [175]. Another micro-RNA, miR-302b, targets CDK2 in gastric cancer cells through ERK signaling [176]. The regulatory factors of Cyclin E-A/CDK2 axis are listed in Table 5, and non-coding RNAs targeting this axis are listed in Table 6. The regulatory factors affecting CDK2 in gastric cancer are displayed in Figure 6.
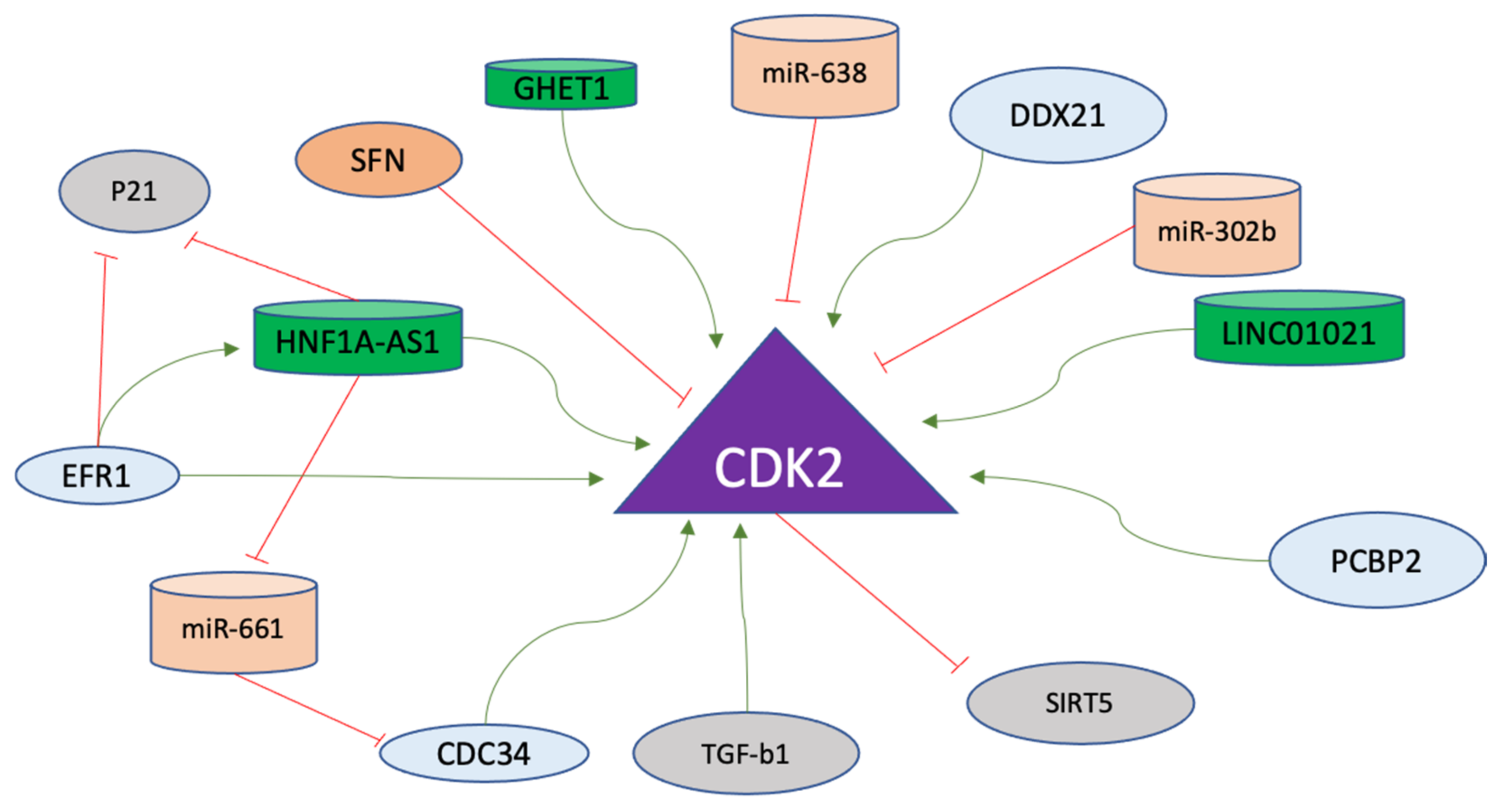
Figure 6. CDK2 regulation in gastric cancer progression. CDK2 is the major driver of the S phase in the cell cycle and promotes various pathways. Positive regulators (green arrows) including long non-coding RNAs (green) and negative regulators of CDK2 (red lines), including micro-RNAs (orange), are shown. The details of the mode of action of these molecules on CDK2 are explained in the text.
Table 5.
The molecules acting as regulators of Cyclin E-A/CDK2 axis in gastric cancer.
| Regulator Molecule | Cyclin/CDK | Effect on Cyclin/CDK | Reference |
|---|
Table 6.
Non-coding RNAs targeting the Cyclin E/CDK2 axis in gastric cancer.
| Non-Coding RNA | Category | Cyclin/CDK | Effect on Cyclin/CDK | Reference | ||||
|---|---|---|---|---|---|---|---|---|
| CDCA5 | Cyclin E | Induces proliferative phenotype via Cyclin E activation upregulation | ||||||
| LINC0857 | Long Non-coding RNA | [ | 154] | |||||
| Cyclin E1 | Upregulation | [ | 103 | ] | β-carotene | Cyclin E | Inhibits cell proliferation and downregulates Cyclin E in H. pylori infected gastric cells | [155] |
| RPL6 | ||||||||
| GHET1 | Long Non-coding RNA | Cyclin E1/CDK2 | Upregulation | [156] | Cyclin E | Upregulates Cyclin E, confers multi-drug resistance and progresses cells from G1 to S-phase | [157] | |
| LINC01667 | Long Non-coding RNA | Cyclin E1 | Upregulation | [161] | ||||
| miR-138-p | MicroRNA | Cyclin E1 | Downregulation | [161] | ||||
| LINC01021 | Long Non-coding RNA | CDK2 | Upregulation | [168] | ||||
| HNF1A-AS1 | Long Non-coding RNA | CDK2 | Upregulation | [172] | ||||
| miR-638 | MicroRNA | CDK2 | Downregulation | [175] | ||||
| miR-302b | MicroRNA | CDK2 | Downregulation | [176] |
4.5. The Regulation of Cyclin B/CDK1 Axis in Gastric Cancer
4.5.1. Cyclin B1 levels in Gastric Cancer
Cyclin B1, also known as mitotic Cyclin, regulates the progression of the cell cycle at G2/M and is implicated as a driver of tumorigenesis [177]. The lymph node metastasis of gastric cancer has been associated with overexpression of Cyclin B1, with one study showcasing the role of CDK1 and Cyclin B1 pathways in the loss of p27 and progression of gastric cancer [178]. Thirty-two percent of the 61 patients studied exhibited higher levels of Cyclin B1 levels in gastric cancer using immunohistopathological screening [179]. The mRNA of CCNB1 (Cyclin B1) was also significantly higher in gastric cancer tissues compared to normal tissues, as evidenced by The Cancer Genome Atlas (TCGA) and Oncomine datasets. The same study highlighted the association of higher Cyclin B1 levels with poor overall survival of the disease [180]. The poor prognosis and lymph node metastasis for intestinal-type carcinomas were also linked with the expression of Cyclin B1, as both pRB and Cyclin B1 were positive for diffuse carcinomas [181].
4.5.2. Cyclin B1 Regulation in Gastric Cancer
Cyclin B1 is another Cyclin that functions to controll the cell cycle. It has been implicated in various cancers as a modulator of tumorigenesis and in some resistance to therapy [182]. In gastric cancer cells, Cyclin B1 expression is mechanistically regulated via the interaction of Aurora B with CREPT/RPRD1B. Gastric carcinogenesis is critically regulated through the transcription of Cyclin B1, which is further promoted via Aurora B, which phosphorylates CREPT/RPRD1B. This mode of Cyclin B1 stabilization is known to exhibit the potential to be used as a therapeutic target [183]. The transcription factors involved in Cyclin B1 regulation, determined on the basis of enrichment analysis, are E2F4, NFYA, SIN3A, and FOXM1 [180]. The promoter activity and subcellular distribution of Cyclin B1 are regulated via another oncogene product, Pak1, which is overexpressed in gastric cancer. Pak1 regulates the mRNA and protein levels of Cyclin B1 for the promotion of progression and metastasis of gastric cancer as its knockdown resulted in the inhibition of the growth of gastric cancer cells and xenograft tumors. The mechanism of Cyclin B1 regulation via Pak1 involves the recruitment of NF-kB to the Cyclin B1 promoter site [184]. Cyclin B1 levels are also regulated post-transcriptionally via miR-663, which is a micro-RNA contributing to the suppression of the growth of gastric cancer cells [185]. A transcription factor named Islet-1 (ISL-1), containing a LIM-home domain, is known to function in the progression of gastric cancer. CDK1 phosphorylates ISL1 at S269 in vivo, which further strengthens its binding to Cyclin B1 and Cyclin B2 promoters to accelerate transcription. Moreover, the phosphorylation of ISL-1 via the activity of CDK1 stabilizes ISL-1 in gastric cancer cells [186]. The factors regulating Cyclin B1 in gastric cancer are further presented and summarized in Figure 7.
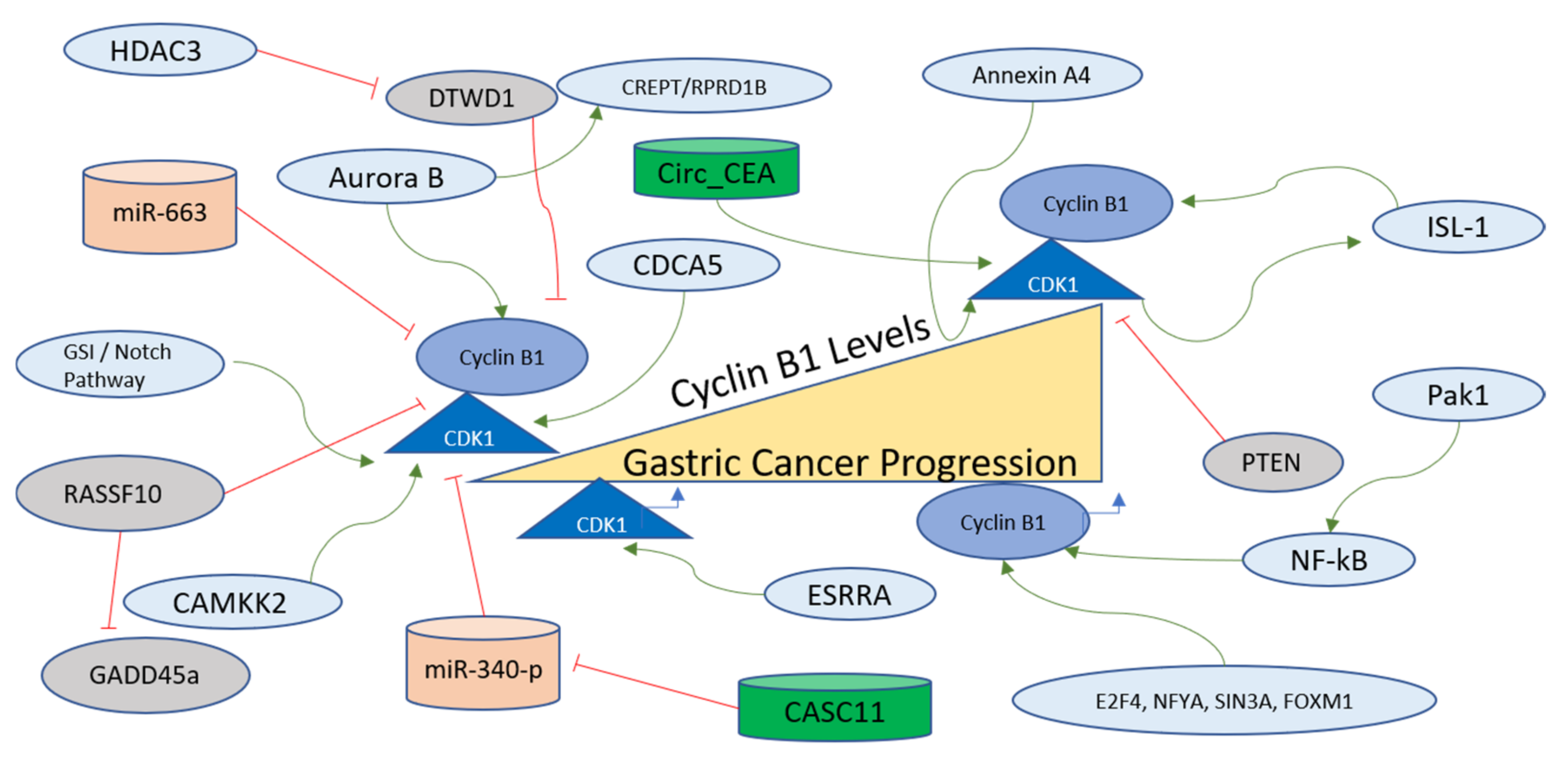
Figure 7. Cyclin B1/CDK1 regulation in gastric cancer progression. The Cyclin B1/CDK1 axis is at the critical conjuncture of G2-M transition and mitotic exit, and is therefore an important component of the cell division pathway. Various factors, including long non-coding RNAs (green), among others (green arrows), activate this axis and are involved in the progression of gastric cancer. The two miRNAs (orange), however, negatively affect the Cyclin B1 and CDK1 in gastric cells, inhibiting their functions and oncogenic properties. Further details of the regulating factors affecting the Cyclin B1/CDK1 axis are described in the main text.
4.5.3. Regulation of CDK1 in Gastric Cancer
CDK1 is the mitotic CDK that functions to control several important steps of prior to cell division, and has been reported as a target for therapy for various cancers [63,187][63][187]. The paracancerous tissues exhibit lower expression of CDK1 compared to gastric cancer tissues, with high CDK1 expressing patients displaying lower survival rates, and CDK1 has been termed an independent prognostic factor for Prostate cancer [188]. Calcium/calmodulin-dependent protein kinase 2 (CAMKK2) works as an upstream factor for CDK1, CDK2, and ERK1 in gastric cancer, as evidenced by bioinformatics analysis. It has been shown that CAMKK2 works in the MEK/ERK1 signaling cascade alongside CDK1 to achieve the progression of gastric cancer [189]. One of the biomarkers of gastric cancer, known as Annexin A4, which functions as an intracellular Ca2+ in gastric cancer cells, has been shown to be one of the upstream factors for upregulating the CDK1 mRNA along with other factors such as hyaluronan-mediated motility receptor (RHAMM) and an inhibitor of p21. The major cellular phenotype observed in Annexin A4-expressing cells was the upregulation of epithelial cell proliferation [190].
A long non-coding RNA named CASC11 is also involved in gastric cancer development, with the prime target being miR-340-p. The miR-340-p targets CDK1 directly in gastric cancer cells. Therefore, a mechanism involving the axis of CASC11/miR-340-p/CDK1 has been proposed in which CDK1 functions as an inhibitor of apoptosis and a promoter of the cell cycle [191]. Another transcription factor named Estrogen-related receptor-α (ESRRA) manifests higher in the gastric cancer cell line, and is also known to be an orphan nuclear receptor that targets the DSN1 gene and subsequently enhanced the migration and cell viability of cancer cells via CDC25/Cyclin B1/CDK1 pathway [192]. Cyclin B1-CDK1 complex formation is inhibited as a function of the activity of a tumor suppressor named Ras-associated domain family protein RASSF10, which in gastric cancer is silenced due to hypermethylation of its promoter. RASSF10 functions by promoting GADD45a nuclear accumulation and the induction of mitotic arrest due to the inhibition of the Cyclin B1-CDK1 pathway [193].
Circ_CEA, obtained from Cell Adhesion Molecule 5 (CEA), is another oncogene involved in the progression of gastric cancer with a mechanism involving activation of CDK1 and its subsequent phosphorylation of p53. Circ_CEA functions as a scaffold for enhancing the interaction between CDK1 and p53 and p53S315 levels increase as a result of this interaction, which leads to suppression of p53 activity due to reduced nuclear retention and inhibition of the activation of the targets of p53 that can induce apoptosis [194]. In gastric cancer cells, γ-secretase inhibitor (GSI) or Notch inhibition can lead to cell death or mitotic arrest due to the induction of PTEN dephosphorylation at the C-terminus, causing nuclear localization of PTEN. The mechanism involves CDK1, which is one of the substrates of PTEN, where PTEN activation leads to nuclear accumulation of Cyclin B1-CDK1 along with apoptosis [195]. In gastric cancer tissues, CDK1 and CDCA5 expressions are correlated with each other, and this coexpression has been observed in MGC-803 cells, with the inhibition of one or both of these genes leading to suppression of invasion, migration, colony formation, and proliferation [196]. Cyclin B1 is inhibited by the DTW Domain-Containing 1 (DTWD1) tumor suppressor in gastric cancer cells, which is further inhibited via the action of histone deacetylase 3 [197]. The non-coding RNAs targeting the Cyclin B1/CDK1 axis in gastric cancer are listed in Table 7. The upstream factors regulating the Cyclin B1/CDK1 axis are listed in Table 8, and all regulators are shown in Figure 7.
Table 7.
Non-coding RNAs targeting the Cyclin B1CDK1 axis in gastric cancer are listed.
| Non-Coding RNA | Category | Cyclin/CDK | Effect on Cyclin/CDK | Reference |
|---|
Table 8.
The upstream regulators of the Cyclin B1/CDK1 axis studied in gastric cancer are listed with their mode of action.
| Regulator Molecule | Cyclin/CDK | Effect on Cyclin/CDK | Reference | |||||
|---|---|---|---|---|---|---|---|---|
| miR-663 | MicroRNA | Cyclin B1 | Downregulation | [185] | ||||
| miR-340-p | MicroRNA | CDK1 | ||||||
| NFkB | Cyclin B1 | Recruited to promoter of Cyclin B1 via Pak1 activity for upregulation of Cyclin B1 | [184] | Downregulation | [ | |||
| E2F4, NFYA, SIN3A, FOXM1 | 191 | ] | ||||||
| Cyclin B1 | Increase the transcription of Cyclin B1 via transcription factor activity | [ | 180] | CASC11 | Long Non-coding RNA | CDK1 | Upregulation | |
| Aurora B | [ | 191 | NF-YA | Cyclin E | TRIM58 | Cyclin D1 | Reduction of survivin, Cyclin D1, c-myc, and β-catenin via degradation | [88] |
| Glycyrrhizic acid | Cyclin D1 | An anti-tumor compound induces apoptosis along with reduction of Cyclin D1, D2, D3, E1, and E2 | [89] | |||||
| DHA | Cyclin D1/ CDK4 |
Anti-tumor activities through inhibition of CDK4 activity and targets Cyclin D1 negatively | [90] | |||||
| AZD1152 | Cyclin D1 | Specifically inhibits Aurora B also reduces Cyclin D1 for inhibiting tumorigenesis | [91] | |||||
| SHR6390 | Cyclin D1/ CDK4/6 |
Reduces refraction from Pyrotinib and increases efficacy against gastric cancer | [92] |
4.1.2. Factors Upregulating Cyclin D1 in Gastric Cancer
There are various upstream regulators of Cyclin D1 that act as oncogenes for the progression of normal gastric cells to gastric carcinoma. Cytotoxin-associated gene A (CagA) has been recognized as one of the factors associated with gastric cancer and Cag+ cells exhibit higher expression of Regeneration gene 3 (Reg3). Both genes function in the regulation of the cell cycle via Cyclin D1-CDK4 complex formation to induce proliferation and G1 to S transition in gastric cells [93]. Kruppel-like factor 5 (KLF5) is correlated with a worse prognosis of gastric cancer and is positively associated with Cyclin D1 in gastric cancer, as observed in MGC803 and SGC7901 cell lines [94]. Aurora B functions to promote cytokinesis and cell division and has been implicated as one of the promoters of gastric cancer. It facilitates the phosphorylation of H3 at S10, which in turn leads to the transcription of CCND1 and upregulation of Cyclin D1 under in vitro and in vivo conditions in gastric cancer. Moreover, AZD1152, which specifically inhibits Aurora B, also inhibits Cyclin D1 to inhibit tumorigenesis and helps target gastric cancer [91].
An m6A methyltransferase (METTL16) that modifies RNA via N6-methyladenosine (m6A) functions to upregulate Cyclin D1 transcription through its methyltransferase activity in gastric cancer cell proliferation [95]. Epstein–Barr virus has been associated with gastric carcinomas and EBV oncogene BARF1 stabilizes and interacts with Cyclin D1 at transcriptional and protein levels in gastric cancer [96]. Cyclin-dependent kinase 5-regulatory subunit-associated protein 3 (CDK5-RAP3), also known as C53, forming different isoforms, has also been implicated in various cancers, and its isoform d (IC53d) is found upregulated in gastric cancer. IC53d promotes gastric cancer cell proliferation and invasive phenotypes via the promotion of GSK3b and Akt phosphorylations, further increasing Cyclin D1 and G1 to S phase transition [97]. Another oncogene giving rise to WDR5 protein is increased in gastric cancer, where it induces H3K4me3 and Cyclin D1 for the progression of the cell cycle and tumorigenesis [98]. Cyclin D1 protects gastric cancer cells from Doxorubicin, where p73 modulates Cyclin D1 levels positively with the help of activator protein 1 (AP-1) [99].
Another oncogene named Sine oculis homeobox homolog 1 (SIX1) is involved in positively regulating the expression of Cyclin D1 along with epithelial–mesenchymal transition (EMT)-related proteins and p-ERK and MMP2 in gastric cancer cells. SIX1 regulation of Cyclin D1 implies that Cyclin D1 is at the crossroads of multiple cellular signaling pathways involved in the progression of gastric cancer [100]. In MGC-803 cells, Resveratrol treatment leads to the inhibition of Cyclin D1, along with c-myc and β-catenin, indicating that Cyclin D1 regulation is influenced by c-myc and β-catenin in gastric cancer cells [101]. Methyltransferase-like 3 (METTL3) modifying mRNA via N6-methyladenosine is involved in gastric cancer, whereby it upregulates Cyclin D1 levels and activates the Akt signaling pathway, as shown in loss-of-function experiments in gastric cancer cells [102].
LINC0857 is a long non-coding RNA that upregulates Cyclin D1 and Cyclin E1 for the progression of gastric cancer and has been indicated as a potential biomarker for the prognosis and diagnosis of the disease [103]. A circular endogenous RNA, has-circ00000647, interacts with and inhibits miR-326-3p and upregulates Cyclin D1 in gastric cancer cells (SGC-7901), showcasing another layer of gene expression regulation upstream of Cyclin D1 for the progression of gastric cancer [104]. Cheng et al. reported that a coronin-like actin-binding protein 1C (CORO1C) functioning in the assembly of F-actin via actin-dependent processes promotes Cyclin D1 and Vimentin for the induction of tumorigenesis in gastric cancer cells [105]. A long non-coding RNA (HOTAIR) targets miR-454-3p to upregulate the STAT3/Cyclin D1 in gastric cancer cells to promote the proliferation and progression of cancer [106]. Table 2 summarizes the molecules involved in the positive regulation of Cyclin D1 in gastric cancer cells or tissues. A schematic representation of factors regulating Cyclin D1 specifically in the context of gastric cancer is provided in Figure 3.
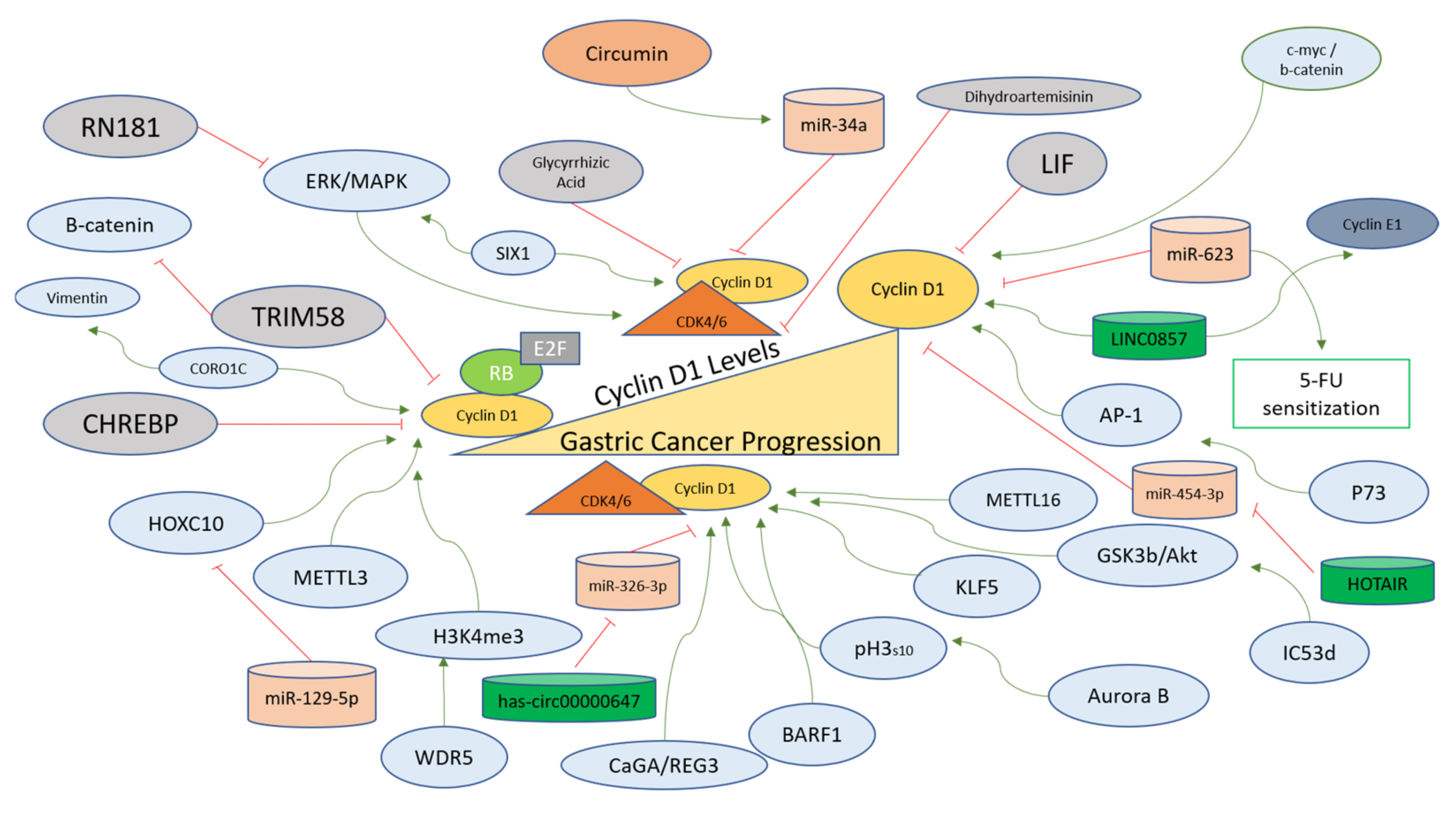
Figure 3. Cyclin D1 regulation in gastric cancer progression. Cyclin D1 levels are upregulated in gastric cancer and are affected positively (green arrows) for the oncogenic transformation of gastric cells via the action of upstream factors (blue). In normal gastric cells, the levels of Cyclin D1 remain tightly controlled via the negative regulators (red lines), which directly inhibit the transcription of the Cyclin D1 gene or inhibit the binding of Cyclin D1 with CDK4/6 directly or indirectly. The modes of action of these regulators are described in the main text.
Table 2.
The upstream molecules upregulating or activating Cyclin D1 in gastric cancer are shown, along with their mode of action.
| Regulator Molecule | Mode of Action on Cyclin D1 | Reference |
|---|---|---|
| KLF5 | Correlated with Cyclin D1 and higher expression showed worse prognosis | [94] |
| CagA | Assists in formation of Cyclin D1-CDK4 complex formation | [93] |
| Aurora B | Phosphorylates H3 and induces Cyclin D1 transcription | [91] |
| METTL16 | Upregulates Cyclin D1 transcription through its methyltransferase activity | [95] |
| BARF1 | Stabilizes and interacts with Cyclin D1 | [96] |
| IC53d | Promotes GSK3b/Akt signaling and induces Cyclin D1 | [97] |
| WDR5 | Induces H3K4me3 and Cyclin D1 for the progression of the cell cycle | [98] |
| p73 | Regulates activator protein 1 (AP-1) for promoting Cyclin D1 | [99] |
| SIX1 | Stabilizes p-ERK and MMP2 for upregulating Cyclin D1 | [100] |
| METTL3 | Modifying mRNA via N6-methyladenosine to upregulate Cyclin D1 and activates the Akt signaling pathway | [102] |
| CORO1C | Assembles F-actin via actin-dependent processes to promote Cyclin D1 and Vimentin | [105] |
4.2. The Regulation of CDK4/6 in Gastric Cancer
CDK4/6 inhibitors have been utilized in various pre-clinical and clinical studies to circumvent the progression of various cancers. However, the treatments lead to resistance, which is one of the major problems that needs to be rectified [107]. The mechanisms of resistance arising against CDK4/6 inhibition in Breast cancer have been reviewed [108]. The status of various inhibitors and clinical outcomes, along with mechanisms of sensitivity and resistance, have also been reviewed in detail [109]. Therefore, an evaluation of literature regarding the regulation of CDK4/6 in gastric cancer is also necessary in order to understand the field of small molecule inhibitors and the ways in which gastric cancer cells may overcome the drug effects using alternative mechanisms of the regulation of these players.
4.2.1. The Regulation of CDK4 in Gastric Cancer
Cyclin-dependent kinase 4 is the first CDK that becomes active as a result of mitogenic signaling upon binding with Cyclin D1. CDK4 inhibition has been at the forefront of the treatments against solid tumors [110]. Gastric inflammatory myofibroblastic tumors can lead to invasion into the diaphragm and spleen, with double amplification of MDM2 and CDK4. The data suggest that CDK4 could be the driver of carcinogenesis in gastric tissues [111]. Pyrotinib is a chemotherapeutic agent explored in clinical trials against gastric cancer. The refraction from Pyrotinib has been associated with the Cyclin D1-CDK4/6 axis; therefore, CDK4/6 inhibitor SHR6390 has also been investigated as a therapeutic option. The combined treatment of the aforementioned agents provided a better response against gastric cancer [92]. P21-activated kinase 1 (PAK1) is involved in gastric cancer progression. The silencing of PAK1 sensitizes cells to CDK4/6 inhibitor in gastric cancer cells in a PDK1-AKT1-dependent pathway [112]. The orphan nuclear receptor (Nurr1) is induced in H. pylori infections via PI3K/AKT-Sp1 mediated fashion and in gastric cancer progression, Nurr1 directly binds with CDK4 promoter site for promoting its transcription and facilitating proliferation [113]. H. pylori infection in gastric cells leads to the production of Progranulin which autocrine growth factor and it further upregulates CDK4 via PI3K/Akt signaling [114]. A long non-coding RNA GCRL1 promotes the metastasis and proliferation of gastric cancer cells under in vitro and in vivo conditions, where it sponges miR-885-3p to positively regulate the CDK4 levels and its invasion and proliferation-related properties and can be targeted for therapy [115]. CDK4 regulators are summarized in Figure 4.
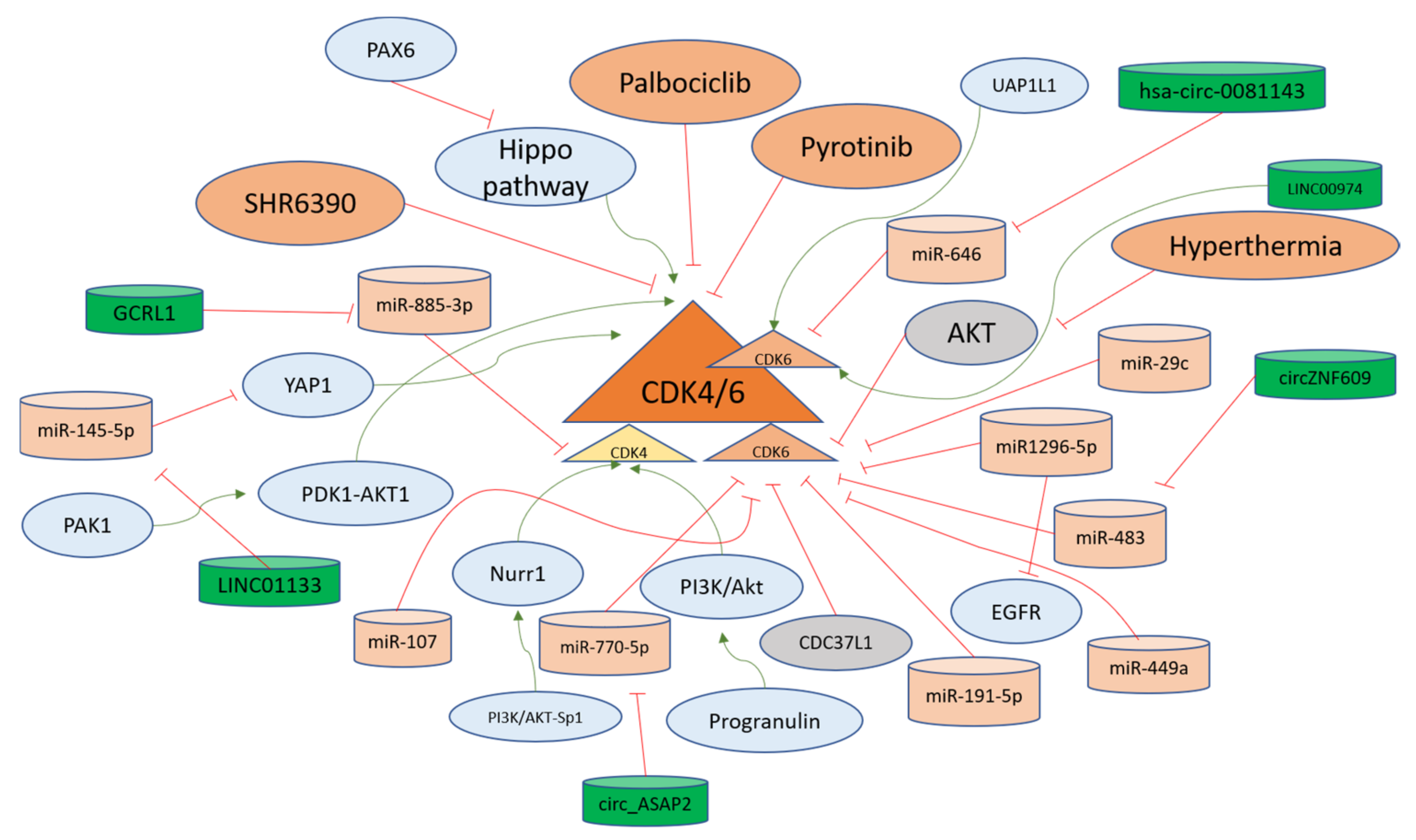
Figure 4. CDK4/6 regulation in gastric cancer progression. CDK4/6 are kinases that accelerate the cell cycle progression from the G1 phase. Different long non-coding RNAs (green) facilitate the upregulation of these CDKs via sponging (red lines) microRNAs (orange). Moreover, other pathways such as PI3K-Akt and Hippo function in activating (green arrows) the CDK4/6 for developing gastric cancer. The negative upstream regulators of CDK4/6, such as CDC37L1 and AKT, are shown in grey and keep the levels of CDK4/6 down in gastric cells. The overall mechanisms for the regulation of CDK4/6 in the gastric cells are explained in the main text.
4.2.2. The Regulation of CDK6 in Gastric Cancer
CDK6 is overexpressed in stomach cancer tissues and is associated with poor survival from the disease. CDK4/6 inhibitor (PD-0332991) targeting CDK6 inhibited development or proliferation and induced apoptosis in stomach cancer cells [116,117][116][117]. CDK6 expression has been recorded to be higher in gastric cancer tissues than in normal gastric tissues. Moreover, miR-449a is downregulated in gastric cancer and can target CDK6 in gastric cancer cells directly, as indicated in the gain of function experiments [118]. CDK6 is upregulated in gastric cancer and miR-107 regulates the levels of CDK6 as evidenced by the CDK6 3′UTR luciferase activity in gastric cancer cells [119]. CDK6-specific inhibitor PD0332991 in the gastric cancer cell line led to cell cycle arrest in the G1 phase of the cell cycle with inhibition of pRb at ser780 along with induction of p27 and p53, and therefore has been implicated as a potential therapeutic option [120].
Another study found that Cyclin D1 is upregulated via the Hippo pathway, which is inactivated through LATS2 the promoter of which is hypermethylated due to PAX6 activity. PAX6 activity induces resistance in gastric cancer cells towards Palbociclib targeting CDK4/6. Therefore, it has been suggested to target multiple pathways in order to overcome PAX6-mediated chemoresistance [121]. Cell division cycle 37 like 1 (CDC37L1) is downregulated in gastric cancer and inhibits CDK6, as demonstrated from the experiments showcasing that Palbociclib inversed the effects of CDC37L1 silenced gastric cancer cells [122].
A tumor suppressor miRNA miR1296-5p in gastric cancer cells targets both EGFR and CDK6 for inhibition of growth, invasion, and migration, as observed in MGC-803 and SGC-7901 cell lines [123]. The miR-191-5p targets mRNA of CDK6 coding mRNA at 5′UTR to inhibit the expression of CDK6 and its oncogenic functions in gastric cancer cells [124]. Another microRNA miR-29c that has been shown to be expressed to low extents in gastric cancer tissues and cells targets CDK6 directly, inhibiting its translation and subsequent activity in order to promote the development of gastric cancer [125]. Hyperthermia is one of the adjuvant therapeutic options for gastric cancer treatment [126]. However, gastric cancer cells exhibit resistance to hyperthermia, and CDK6 is upregulated through the induction of AKT pathway inhibition and has been implicated as the protector of cells against apoptosis induced through hyperthermia. Therefore, CDK6 inhibition is considered a relevant option to overcome resistance to hyperthermia-related therapies for advanced gastric cancer [127]. Table 3 displays the molecules studied in gastric cancer that act in regulation of CDK4/6.
Table 3.
The regulators of CDK4/6 investigated in the context of gastric cancer are given. (Note: UAP1L1 is a downstream target of CDK6).
| Regulator Molecule | Cyclin/CDK | Effect on Cyclin/CDK | Reference |
|---|---|---|---|
| SHR6390 | CDK4/6 | Reduces refraction from Pyrotinib and increases efficacy against gastric cancer | [92 |
. Another oncogenic long non-coding RNA LINC00974 upregulates CDK6 in gastric cancer cells and is involved in the regulation of cell cycle progression from G1 to S phase [134]. The factors regulating the CDK6 levels and activity in gastric cancer cells are summarized in Figure 4. All the non-coding RNAs studied in gastric cancer that act as direct or indirect regulators of Cyclin D1-CDK4/6 axis are shown in Table 4.
Table 4.
The non-coding RNAs regulating Cyclin D1-CDK4/6 axis in gastric cancer.
| Non-Coding RNA | Category | Cyclin/CDK | Effect on Cyclin/CDK | Reference | ||
|---|---|---|---|---|---|---|
| ] | ||||||
| miR-129-5p | MicroRNA | Cyclin D1 | Downregulation | [84] | ||
| PAK1 | CDK4/6 | PAK1 silenced cells sensitizes gastric cancer cells ina PDK1-AKT1 dependent pathway to CDK4/6 inhibition | [112] | |||
| miR-34a | MicroRNA | Cyclin D1 CDK4 | Nurr1 | CDK4 | Binds to CDK4 promoter to induce transcription and activation of CDK4 | [113] |
| Downregulation | [ | 85 | PD0332991 | CDK6 | Direct inhibition of CDK6 and reduction of Rb-phosphorylation to induce cell cycle arrest | [120] |
| PAX6 | CDK4/6 | Upregulates CDK4/6 and induces chemoresistance in gastric cancer cells against Palbociclib | [121] | |||
| CDC37L1 | CDK6 | Inhibits the activity and expression of CDK6 in gastric cancer cells | [122] | |||
| UAP1L1 | CDK6 | CDK6 regulates UAP1L1-mediated phenotypes (UAP1L1 is a downstream regulator of CDK6) | [128] | |||
| PD-0332991 | CDK4/6 | Direct inhibition of CDK4/6 and induction of apoptosis | [117] |
A long intergenic non-coding RNA (linc01133) is upregulated in gastric cancer through transcriptional activation of c-Fos and c-Jun. Linc01133 targets miR-145-5p directly for upregulation of YES1 which in turn activates YES-1 dependent YAP1 for upregulating Cyclin D1, CDK4, and CDK6 for promoting the cell cycle transition from G1 to S phase [129]. A circular RNA named hsa-circ-0081143, found in higher concentrations in gastric cancer, sponges miR-646 for its downregulation and induces the expression of CDK6, thus promoting cisplatin resistance in gastric cancer through regulation of the miR-646/CDK6 axis [130,131][130][131]. Another circular RNA circZNF609 targeting miR-483 leads to the activation of CDK6 in gastric cancer cells to increase migration and proliferation [132]. Another circular RNA circ_ASAP2 targeting miR-770-5p is upregulated in gastric cancer and leads to activation of CDK6, and has been termed as a potential target for gastric cancer therapy [133]. The UDP-GlcNAc pyrophosphorylase-1 like 1 (UAP1L1) is another upstream driver of CDK6 in gastric cancer, as the phenotypes observed in UAP1L1 overexpressing cells were reversed in CDK6-silenced cells, suggesting that gastric cancer progression depends on CDK6 activity via multiple pathways [128]
225].
Moreover, the CDK7-specific inhibitor BS-181 has also shown promising results against gastric cancer cells, leading to reduced invasion, migration, and proliferation in the gastric cancer cell line [226]. CDK7 expression levels have been correlated with matrix metalloproteinase 14 (MMP14) in gastric cancer, and higher expression of these proteins, along with the mRNA of their genes, has been associated with lymph node matastasis of gastric cancer and worse prognosis of the disease [227]. Moreover, miR-107 also targets CDK8 in gastric cancer cells at its 3′UTR site and regulates the mRNA levels of CDK8 [228]. CDK8 levels and their association with β-catenin delocalization have also been associated with poor prognosis of gastric cancer in a study involving patient tissues and gastric cancer cell lines [229].
CDK9 is another type of CDK that is upregulated in gastric cancer, and is regulated via the activity of miR-613, which functions as a tumor suppressor in gastric cancer cells. Dual luciferase reporter activity demonstrated that miR-613 directly targets the CDK9 gene for its downregulation and suppresses the metastases and progression of gastric cancer cells [230]. CDK10 expression is significantly lower in gastric cancer tissues than in normal tissues. Overexpression of CDK10 led to inhibition of invasion, migration, and proliferation of gastric cancer cells whereas the knockdown of CDK10 demonstrated the opposite phenotypes, indicating that CDK10 is downregulated in gastric cancer. Moreover, loss of CDK10 was also associated with poor survival, tumor differentiation, and metastasis in gastric cancer [231,232][231][232]. CDK10 activity and its expression levels in various cancers have been reviewed and it has been shown that this particular CDK behaves as either an oncoprotein or tumor suppressor depending on the tissues; however, in gastric cancer, the levels of CDK10 are lower than in normal tissues [221].
CDK12 expression is high in some cancers and lower in some cancers, and in the case of gastric cancer, it has been observed to be downregulated, and is negatively correlated with poor outcomes, poorly differentiated adenocarcinoma, and advanced stage; therefore CDK12, could be a tumor suppressor in gastric cancer [233]. The CDK12 gene has been reported to be one of the driver genes that regulates the growth of gastric cancer. In gastric cancer cells, CDK12 phosphorylates PAK2 for activation of the MAPK signaling, and its specific inhibitor Proterol has been approved for clinical trials by the FDA due to positive outcomes obtained from PDX and cell line studies [234]. HMGA2 is an oncogene that promotes gastric cancer through a progression of S-G2/M transitions and targets CDK13. HMGA2 and CDK13 are coexpressed in gastric cancer, and higher expression of both these genes has been associated with poor prognosis. SR-4835 is an inhibitor of CDK12/13, and can target HMGA2, thereby indicating a novel mechanism of transition of gastric cancer cells [235]. CDK14, also known as Pftk1, is also overexpressed in gastric cancer, and is involved in promoting metastasis and invasion of tumors [236]. CDK18 is expressed at higher levels in gastric cancer cells, where CXXC finger protein 4 (CXXC4) overexpression can assist in the inhibition of immune escape via the CDK18-ERK1/2 axis. Therefore, CDK18 has been implicated as an immune modulator for tumor progression [237]. Isochorismatase domain-containing 1 (ISOC1) is upregulated in gastric cancer, where it functions to activate and upregulate CDK19 in order to induce proliferation of gastric cancer cells and increase tumor size [238]. The expression levels of all Cyclins and CDKs investigated under tumor vs. normal conditions in gastric cells or tissues are summarized in Table 9.
Table 9. The levels of Cyclins and CDKs alter upon Tumorigenesis. The upregulation or downregulation of all Cyclins and CDKs investigated in gastric cancer tissues or cells compared to their normal counterparts.
| Cyclin/CDK | Expression in Cancer vs. Normal Cells/Tissues | References | ||||||
|---|---|---|---|---|---|---|---|---|
| Cyclin A | Upregulated | [164,239][164][239] | ||||||
| Cyclin B1 | Upregulated | [178,179,181,240][178][179][181][240] | ||||||
| Cyclin C | Downregulated | [210] | ||||||
| Cyclin D1 | Upregulated | [81,241,81][241]242,[242]243][[243] | ||||||
| Cyclin E | ||||||||
| Cyclin D2 | Upregulated | An upstream factor of Cyclin E, acts as oncogene in gastric cancer | [244,245[159] | |||||
| ] | [ | 244][245] | EZH2 | Cyclin E | A polycomb protein upregulates Cyclin E and its inhibition leads to Cyclin D1 and Cyclin E downregulation | |||
| Cyclin E | Upregulated | [135,147,243,246,247][135][147][243][246][247] | [160] | |||||
| PCBP2 | CDK2 | Interacts with CDK2 and directly activates it, higher expression is associated with poor prognosis | ||||||
| Cyclin G2 | Downregulated | [212,213,214,215][212 | [169] | MicroRNA | CDK4 | Upregulation | [115] | |
| ] | [ | 213][214][215] | DDX21 | CDK2 | An ATP-dependent RNA helicase directly upregulates Cyclin D1 and CDK2 | [170] | miR-449a | |
| Cyclin L2 | Downregulated | [219] | MicroRNA | CDK6 | Downregulation | [118] | ||
| miR-107 | ||||||||
| EGR1 | CDK2 | Activates CDK2 and leads to inhibition of p21 | [172] | MicroRNA | CDK6 | Downregulation | [119] | |
| SFN | CDK2 | Downregulates CDK2 and induces apoptosis via p53 mediated pathway | [173] | miR1296-5p | MicroRNA | CDK6 | Downregulation | |
| TGF-b1 | CDK2 | [ | 123 | ] | ||||
| Activates caspase mediated apoptosis and CDK2 | [ | 174 | ] | miR-191-5p | MicroRNA | CDK6 | Downregulation | [124] |
| miR-29c | MicroRNA | CDK6 | Downregulation | [125] | ||||
| LINC01133 | Long Non-coding RNA | Cyclin D1-CDK4/6 | Upregulation | [129] | ||||
| circZNF609 | Circular RNA | CDK6 | Upregulation | [132] | ||||
| circ_ASAP2 | Circular RNA | CDK6 | Upregulation | [133] | ||||
| LINC00974 | Long Non-coding RNA | CDK6 | Upregulation | [134] | ||||
| circ-0081143 | Circular RNA | CDK6 | Upregulation | [131] |
4.3. Cyclin E Regulation in Gastric Cancer
4.3.1. Cyclin E Expression Analyses in Gastric Cancer
Cyclin E1-expressing tumors have been reported to be more invasive compared to those with lower levels of Cyclin E1. Moreover, gastric cancer with aberrant p53 and higher Cyclin E1 can be termed a separate sub-group of the disease, displaying poor prognosis [135]. The lymph node metastases of gastric cancer are correlated significantly with gene amplification in the Cyclin E1 gene. The gastric cancer cell line MKN-7 also exhibits amplification of Cyclin E1 with other cancer lines exhibiting higher protein levels of Cyclin E1. Therefore, it has been suggested that the progression of gastric carcinoma and abnormal growth of gastric cells occur due to events leading to Cyclin E1 overexpression [136]. Cyclin E overexpression and amplification are strongly associated with poor outcomes for gastric cancer patients. HER2 amplification is also correlated with higher Cyclin E expression, and HER2-targeted therapies for gastric cancer lead to Cyclin E-mediated resistance [137].
The Cyclin E1 gene CCNE1 is expressed highly in gastric cancer tissues, along with gastric cancer cell lines. The higher expression of Cyclin E1 is associated with lymphatic metastases and tumor node metastases, and is associated with poor prognosis of the disease. Moreover, it has been shown that Cyclin E1 inhibition can sensitize cells to cisplatin-induced apoptosis in gastric cancer cell lines such as NCI-N87 and MGC-803 [138]. Cyclin D1 and Cyclin E1 expressions were compared for impact on survival for gastric cancer, and it was observed that Cyclin E-negative cancers portrayed good outcomes, while Cyclin E-positive cancers showed worse outcomes, as indicated through survival curves. Cyclin E overexpression was shown to be a better prognostic factor compared to Cyclin D1 [139,140][139][140]. Cyclin E and CDK2 overexpression along with p57 (KIP2) regulation are important factors for metastasis and progression of gastric cancer [141]. Cyclin E-expressing tumors also expressed p53 more than Cyclin E-negative gastric tumors and p53 and Cyclin E-coexpressing tumors were regarded as being more invasive; therefore, Cyclin E is termed an early activator of gastric carcinogenesis [142]. Moreover, the correlation of loss of p21 (WAF1/CIP1) and overexpression of Cyclin E has been recorded as a useful prognostic factor for gastric cancer [143]. Another study discovered that lower p27 and higher Cyclin E expression were correlated with poor survival in gastric cancer patients [144].
The low-molecular-weight isoforms of Cyclin E are overexpressed in early-onset gastric cancer (EOGC), and these forms have been associated with survival in EOGC [145]. Cyclin D1 and Cyclin E2 have been studied in the histopathological screening of gastric carcinoma. Both Cyclin D1 and Cyclin E2 were observed to be frequently overexpressed; however, Cyclin D1 was shown to be more sensitive and specific for survival analysis than Cyclin E2 [146]. Moreover, Cyclin E expression was positively correlated with pRB and negatively correlated with p21 in gastric carcinoma cases [147]. A meta-analysis conducted on the literature regarding Cyclin E concluded that Cyclin E overexpression in clinical settings can be used as an indicator of poor prognosis of Gastrointestinal cancer [148]. Since Cyclin E1 expression is high in gastric cancer, its gene amplification (CCNE1) was recorded at different metastatic locations, and it was observed that CCNE1 amplification was significantly associated with liver metastasis of gastric cancer [149]. H. pylori-induced precancerosis in gerbils led to a significant increase in Cyclin E1 expression, indicating the role of Cyclin E in the early onset of gastric cancer [150].
As mentioned previously, the CDK4/6 inhibitor Palbociclib is a chemotherapeutic agent utilized to target gastric cancer cells. The sensitivity to Palbociclib depends on Cyclin B1 downregulation as Cyclin E-expressing cells become resistant to CDK4/6 inhibition [151]. Cyclin E-overexpressing cells exhibited high sensitivity to Gemcitabine as compared to Cyclin E-silenced cells, indicating that Cyclin E-expressing cells can be targeted for therapy [152].
4.3.2. Factors Regulating Cyclin E in Gastric Cancer Cells
Cyclin E is regulated through a plethora of upstream factors that can lead to the activation of Cyclin E/CDK2 formation and subsequent progression of cell signaling and the cell cycle [153]. Cell division cycle associated 5 (CDCA5) is involved in the proliferation of gastric cancer cells, and its inhibition results in the downregulation of Cyclin E1 (CCNE1). Therefore, it has been suggested that CDCA5 is an upstream activator of Cyclin E1 for the development of gastric cancer [154]. Β-carotene introduction to
| ISL-1 | |||
| Cyclin B1 | CDK1 stabilizes ISL-1, which binds to Cyclin B1 promoter and upregulates its transcription | [ | 186] |
| CAMKK2 | CDK1 | Works in MEK/ERK1 signaling cascade and activates CDK1 | [189] |
| Annexin A4 | CDK1 | Functions as an intracellular Ca2+ regulator and upregulates the transcription of CDK1 | [190] |
| ESRRA | Cyclin B1/CDK1 | Targets DSN1 and increases cell viability via CDC25/Cyclin B1/CDK1 pathway | [192] |
| RASSF10 | Cyclin B1/CDK1 | Promotes the GADD45a nuclear accumulation and inhibits Cyclin B1/CDK1 complex formation | [193] |
| GSI/PTEN | Cyclin B1/CDK1 | GSI dephosphorylates PTEN, which causes nuclear accumulation of Cyclin B1/CDK1 and induces apoptosis | [195] |
| CDCA5 | CDK1 | Coexpressed with CDK1 and also stabilizes it in gastric cancer cells | [196] |
| DTWD1 | Cyclin B1 | Histone deacetylase 3 inhibits DTWD1 which further inhibits Cyclin B1 in gastric cancer | [197] |
4.6. Natural Chemical Compounds against the Cyclin B/CDK1 axis in Gastric Cancer
There are various natural compounds and small molecule inhibitors that have been used to inhibit the Cyclin B1/CDK1 axis to induce mitotic death and cell cycle arrest. Most of these have been reviewed in the context of gastrointestinal cancers [198]. Among natural compounds, flavonoids extracted from Citrus aurantium target Cyclin B1 and CDK1 to induce apoptosis in gastric cancer cells, and have been shown to be potential chemoprevention agents [199]. Under both in vitro and in vivo conditions, another specific flavonoid (Kaempferol) leads to Cyclin B1, CDK1, and CDC25C reduction along with increased PARP cleavage and caspase-3 and caspase-9 levels. The same study reported that the inhibitor treatment causes a reduction of COX-2, p-ERK, and p-Akt, implicating the Cyclin B1/CDK1 axis and its involvement in other signaling pathways regulating the growth of gastric cancer cells [200]. Another flavonoid metabolite (2,4,6-Trihydroxybenzoic Acid) has been investigated and observed to be a multiple CDK inhibitor and can be utilized as a chemo-therapeutic agent against gastric cancer [201]. Menadione has been known as an oxidative damage-inducing agent and has been studied in gastric cancer cell lines, where it resulted in proteasome-mediated degradation of Cyclin B1 and CDK1 without reducing the mRNA levels of these genes, with an overall cell cycle arrest in the G2-M phase [202]. Luteolin is another flavonoid that has been investigated for cellular phenotypes in gastric cancer cells, and it also reduced the protein levels of CDC25C, Cyclin B1, and CDK1, and increased the levels of CDK inhibitor p21 with the induction of apoptosis indicated by levels of p53, Caspase 3, 6, 9, and Bax [203].
Cyclin B1 has been shown to be downregulated upon treatment with Ramson watery extract in AGS cells with no impact on G1-related proteins and induction of G2/M arrest and apoptosis [204]. Oridonin is a natural compound extracted from Rabdosia rubescens and in gastric cancer cells (SGC-7901); it blocks the cells at the G2/M phase, reducing both Cyclin B1 and CDK1 levels [205]. Cytotoxic licorice compounds have shown promising effects as anti-cancer agents, and Licochalcone (LCA) has been studied for its cytotoxic effects in gastric cancer cell lines. This resulted in apoptosis and decreased expressions of Cyclin B1, MDM2, and Cyclin A1 in MKN-45, AGS, and MKN-28 cell lines [206]. Silbinin extracted from milk thistle is a known flavonolignan compound, and in the gastric cancer cell line (MGC-803), it negatively influenced the STAT3 pathway to activate caspase 3 and caspase 9 activities to induce apoptosis with a negative impact on survival, Cyclin B1, and CDK1 [207]. These data indicate that the cell survival-related properties of Cyclin B1 are also linked with the STAT3 pathway. Resveratrol is another natural compound belonging to the polyphenol family that has been investigated in gastric cancer cells (AGS), leading to the activation of various signaling pathways including PERK/eIF2a and ATF4/CHOP and cell cycle arrest at G2/M and endoplasmic reticulum stress-mediated apoptosis. Moreover, the same study demonstrated that resveratrol inhibited Cyclin B1/CDK1 complex and sensitized cells to cisplatin in a synergistic manner [208].
4.7. Other Cyclins and CDKs Investigated in Gastric Cancer Cells
4.7.1. Other Cyclins in Gastric Cancer
Apart from Cyclin D, E, A, and B, as mentioned previously, there are other members of the Cyclin family of proteins that have been studied in the context of gastric cancer. Cisplatin resistance is one of the major hurdles in gastric cancer interventions [209]. Cyclin C is another type of Cyclin that has been studied in the context of drug resistance in gastric cancer. Cisplatin treatment leads to cytoplasmic retention of Cyclin C, which remains anchored at the mitochondria, leading to ROS synthesis and mitochondrial fission. HACE1 adds ubiquitin to Cyclin C for its degradation in gastric cancer cells when treated with cisplatin. Therefore, cisplatin-mediated degradation of Cyclin C is the mechanism of apoptosis in gastric cancer which can be impaired upon mutations in ubiquitinating sites of Cyclin C [210]. Cyclin C regulates cisplatin sensitivity in gastric cancer cells [211].
Cyclin G2 was observed in gastric cancer patients with an exception of female patients, where it was recorded as being lower, and the overall expression was inversely correlated with advanced stages of gastric cancer [212]. Another study explored Cyclin G2 in gastric cancer and observed decreased Cyclin G2 expression in gastric cancer tissues. Overexpression of Cyclin G2 led to a decrease in metastasis and tumor growth under in vitro and in vivo conditions. Cyclin G2 inhibited the β-catenin inhibition with the mechanism depending on the interaction of Dpr1 with Cyclin G2. DPr1 inhibited Wnt/β-catenin signaling via CK1 phosphorylation, which was impacted by Cyclin G2 [213]. GSK-3b is considered to be an upstream factor of Cyclin G2 activity in the aforementioned mechanism of gastric cancer regulation. Cyclin G2 is downregulated in gastric cancer and is regulated via miR-340 which is overexpressed and promotes gastric cancer. In experiments involving the depletion of Cyclin G2, the cellular phenotypes of silenced miR-340 were eradicated whereas CCNG2 3′UTR luciferase activity exhibited direct regulation of Cyclin G2 via miR-340 [214]. Therefore, Cyclin G2 downregulation via miR-340 is one of the mechanisms of how Cyclins are regulated to progress the normal cells to cancerous cells in gastric cancer. Another study investigated the effects of Cyclin G2 overexpression in gastric cancer cells and found that it causes the inhibition of proliferation. Moreover, mutational data were investigated with no major mutations observed; however, there were four synonymous SNPs in Cyclin G2 in gastric cancer samples. It was concluded that Cyclin G2 downregulation in gastric cancer could be the result of other signaling events involved in the development of gastric cancer [215].
Cyclin H is another type of Cyclin that forms a complex with CDK7. CDK7 functions by forming a complex with the RING finger protein known as Mat1 to regulate the cell cycle along with transcription machinery [216,217][216][217]. CDK7 inhibition using a specific inhibitor named THZ2 results in the generation of ROS, induction of cell cycle arrest, and apoptosis in gastric cancer cells in in vitro and in vivo conditions. Therefore, CDK7 has been implicated as a therapeutic target for gastric cancer [218]. Cyclin L2 is another Cyclin implicated in cell cycle regulation and transcription of gastric cancer cells. Interestingly, Cyclin L2 overexpression results in cell cycle arrest and inhibition of growth in gastric cancer cells. It also sensitizes gastric cancer cells to cisplatin, docetaxel, and fluorouracil [219]. Cyclin T2 is another type of Cyclin, and has been observed in gastric cancer samples in overexpressed form as compared to normal counterparts. miR-216 is depleted in gastric cancer patients and its overexpression results in the inhibition of invasion, migration, and proliferation along with the induction of apoptosis and cell cycle arrest in gastric cancer cells. Cyclin T2 overexpression reversed the effects of miR-216b mimicking effects in experiments on gastric cancer cells, implicating a mechanism of Cyclin T2 regulation in the development of gastric cancer [220]. Therefore, Cyclin G2 and Cyclin L2, along with Cyclin C, are involved in negatively influencing the growth of gastric cancer cells.
4.7.2. Other CDKs in Gastric Cancer
CDK4/6, 2, and 1 are not the only CDKs regulating the cell cycle. There are other CDKs that depend on various Cyclins to activate their kinase domains [221,222][221][222]. CDK5 has been specifically observed to be coexpressed with chromosomal maintenance 1 (CRM1) in gastric cancer, and this coexpression has been described as a promising prognostic model for gastric cancer [223]. In gastric cancer tissues, CDK5 levels are downregulated, and this downregulation is correlated with the severity of the disease as observed in lymph node metastases of gastric cancer. Moreover, the nuclear accumulation or localization of CDK5 is also reduced in gastric cancer cells. When nuclear-targeted CDK5 was ectopically expressed in gastric cancer cells, it led to the inhibition of the proliferation of these cells in a xenograft model. CDK5 inhibition upon treatment with small molecule inhibitor NS-0011, which increases the CDK5 localization in the nucleus, causes suppression of tumorigenesis and proliferation in xenografts [224]. CDK5 interacts with Protein phosphatase 2A (PP2A), which is downregulated in gastric cancer, and for which lower expression is associated with poor survival of the patients. PP2A inhibition in cells stably expressing CDK5 showcased the opposite effects of CDK5-mediated inhibition of gastric cancer progression [
| Cyclin T2 | ||
| Upregulated | ||
| [ | ||
| 220 | ||
| ] | ||
| CDK1 | Upregulated | [186,188,196,240,248][186][188][196][240][248] |
| CDK4 | Upregulated | [83,249][83][249] |
| CDK5 | Downregulated | [224] |
| CDK6 | Upregulated | [250] |
| CDK7 | Upregulated | [218,251][218][251] |
| CDK8 | Upregulated | [229] |
| CDK9 | Upregulated | [230] |
| CDK10 | Downregulated | [231,232][231][232] |
| CDK12 | Upregulated/Downregulated | [233,234,252][233][234][252] |
| CDK13 | Upregulated | [235] |
| CDK14 | Upregulated | [236] |
| CDK18 | Upregulated | [237] |
| CDK19 | Upregulated | [238] |
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953.
- Thrift, A.P.; El-Serag, H.B. Burden of Gastric Cancer. Clin. Gastroenterol. Hepatol. 2020, 18, 534–542.
- Hamashima, C. The burden of gastric cancer. Ann. Transl. Med. 2020, 8, 734.
- Xie, Y.; Shi, L.; He, X.; Luo, Y. Gastrointestinal cancers in China, the USA, and Europe. Gastroenterol. Rep. 2021, 9, 91–104.
- Qadri, Q.; Rasool, R.; Gulzar, G.M.; Naqash, S.; Shah, Z.A. H. pylori Infection, Inflammation and Gastric Cancer. J. Gastrointest. Cancer 2014, 45, 126–132.
- Mentis, A.-F.A.; Boziki, M.; Grigoriadis, N.; Papavassiliou, A.G. Helicobacter pylori infection and gastric cancer biology: Tempering a double-edged sword. Cell. Mol. Life Sci. 2019, 76, 2477–2486.
- Chen, S.-Y.; Zhang, R.-G.; Duan, G.-C. Pathogenic mechanisms of the oncoprotein CagA in H. pylori-induced gastric cancer. Oncol. Rep. 2016, 36, 3087–3094.
- Alipour, M. Molecular Mechanism of Helicobacter pylori-Induced Gastric Cancer. J. Gastrointest. Cancer 2021, 52, 23–30.
- Zhang, F.; Chen, C.; Hu, J.; Su, R.; Zhang, J.; Han, Z.; Chen, H.; Li, Y. Molecular mechanism of Helicobacter pylori-induced autophagy in gastric cancer (Review). Oncol. Lett. 2019, 18, 6221–6227.
- Kumar, S.; Metz, D.C.; Ellenberg, S.; Kaplan, D.E.; Goldberg, D.S. Risk Factors and Incidence of Gastric Cancer After Detection of Helicobacter pylori Infection: A Large Cohort Study. Gastroenterology 2020, 158, 527–536.e7.
- Machlowska, J.; Baj, J.; Sitarz, M.; Maciejewski, R.; Sitarz, R. Gastric Cancer: Epidemiology, Risk Factors, Classification, Genomic Characteristics and Treatment Strategies. Int. J. Mol. Sci. 2020, 21, 4012.
- Vahid, F.; Davoodi, S.H. Nutritional Factors Involved in the Etiology of Gastric Cancer: A Systematic Review. Nutr. Cancer 2021, 73, 376–390.
- Weiss, J.; Zakas, A.; Hause, J. Gastrointestinal disorders. In Handbook of Clinical Adult Genetics and Genomics: A Practice-Based Approach; Elsevier: Amsterdam, The Netherlands, 2020; pp. 185–211. ISBN 9780128173442.
- Hata, K.; Yamamoto, Y.; Kiyomatsu, T.; Tanaka, T.; Kazama, S.; Nozawa, H.; Kawai, K.; Tanaka, J.; Nishikawa, T.; Otani, K.; et al. Hereditary gastrointestinal cancer. Surg. Today 2016, 46, 1115–1122.
- Gurzu, S.; Jung, I.; Orlowska, J.; Sugimura, H.; Kadar, Z.; Turdean, S.; Bara, T. Hereditary diffuse gastric cancer—An overview. Pathol. Res. Pract. 2015, 211, 629–632.
- Lynch, H.T.; Lynch, J.F.; Shaw, T.G. Hereditary gastrointestinal cancer syndromes. Gastrointest. Cancer Res. 2011, 4, S9–S17.
- Tan, R.Y.C.; Ngeow, J. Hereditary diffuse gastric cancer: What the clinician should know. World J. Gastrointest. Oncol. 2015, 7, 153–160.
- Gamble, L.A.; Heller, T.; Davis, J.L. Hereditary Diffuse Gastric Cancer Syndrome and the Role of CDH1: A Review. JAMA Surg. 2021, 156, 387.
- Majewski, I.J.; Kluijt, I.; Cats, A.; Scerri, T.S.; de Jong, D.; Kluin, R.J.C.; Hansford, S.; Hogervorst, F.B.L.; Bosma, A.J.; Hofland, I.; et al. An α-E-catenin (CTNNA1) mutation in hereditary diffuse gastric cancer. J. Pathol. 2013, 229, 621–629.
- Taja-Chayeb, L.; Vidal-Millán, S.; Trejo-Becerril, C.; Pérez-Cárdenas, E.; Chávez-Blanco, A.; Domínguez-Gómez, G.; González-Fierro, A.; Romo-Pérez, A.; Dueñas-González, A. Hereditary diffuse gastric cancer (HDGC). An overview. Clin. Res. Hepatol. Gastroenterol. 2022, 46, 101820.
- Donner, I.; Kiviluoto, T.; Ristimäki, A.; Aaltonen, L.A.; Vahteristo, P. Exome sequencing reveals three novel candidate predisposition genes for diffuse gastric cancer. Fam. Cancer 2015, 14, 241–246.
- Fewings, E.; Larionov, A.; Redman, J.; Goldgraben, M.A.; Scarth, J.; Richardson, S.; Brewer, C.; Davidson, R.; Ellis, I.; Evans, D.G.; et al. Germline pathogenic variants in PALB2 and other cancer-predisposing genes in families with hereditary diffuse gastric cancer without CDH1 mutation: A whole-exome sequencing study. Lancet Gastroenterol. Hepatol. 2018, 3, 489–498.
- Li, J.; Woods, S.L.; Healey, S.; Beesley, J.; Chen, X.; Lee, J.S.; Sivakumaran, H.; Wayte, N.; Nones, K.; Waterfall, J.J.; et al. Point Mutations in Exon 1B of APC Reveal Gastric Adenocarcinoma and Proximal Polyposis of the Stomach as a Familial Adenomatous Polyposis Variant. Am. J. Hum. Genet. 2016, 98, 830–842.
- van der Post, R.S.; Oliveira, C.; Guilford, P.; Carneiro, F. Hereditary gastric cancer: What’s new? Update 2013–2018. Fam. Cancer 2019, 18, 363–367.
- Hosoda, K.; Watanabe, M.; Yamashita, K. Re-emerging role of macroscopic appearance in treatment strategy for gastric cancer. Ann. Gastroenterol. Surg. 2019, 3, 122–129.
- Mariette, C.; Carneiro, F.; Grabsch, H.I.; van der Post, R.S.; Allum, W.; de Manzoni, G.; on behalf of European Chapter of International Gastric Cancer Association. Consensus on the pathological definition and classification of poorly cohesive gastric carcinoma. Gastric Cancer 2019, 22, 1–9.
- Nagtegaal, I.D.; Odze, R.D.; Klimstra, D.; Paradis, V.; Rugge, M.; Schirmacher, P.; Washington, K.M.; Carneiro, F.; Cree, I.A.; The WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology 2020, 76, 182–188.
- Chen, Y.-C.; Fang, W.-L.; Wang, R.-F.; Liu, C.-A.; Yang, M.-H.; Lo, S.-S.; Wu, C.-W.; Li, A.F.-Y.; Shyr, Y.-M.; Huang, K.-H. Clinicopathological Variation of Lauren Classification in Gastric Cancer. Pathol. Oncol. Res. 2016, 22, 197–202.
- Gotoda, T.; Jung, H.-Y. Endoscopic resection (endoscopic mucosal resection/endoscopic submucosal dissection) for early gastric cancer. Dig. Endosc. 2013, 25, 55–63.
- Necula, L.; Matei, L.; Dragu, D.; Neagu, A.I.; Mambet, C.; Nedeianu, S.; Bleotu, C.; Diaconu, C.C.; Chivu-Economescu, M. Recent advances in gastric cancer early diagnosis. World J. Gastroenterol. 2019, 25, 2029–2044.
- Maconi, G.; Manes, G.; Porro, G.B. Role of symptoms in diagnosis and outcome of gastric cancer. World J. Gastroenterol. 2008, 14, 1149–1155.
- Tan, Z. Recent Advances in the Surgical Treatment of Advanced Gastric Cancer: A Review. Med. Sci. Monit. 2019, 25, 3537–3541.
- Ilson, D.H. Advances in the treatment of gastric cancer: 2019. Curr. Opin. Gastroenterol. 2019, 35, 551–554.
- Lordick, F. Chemotherapy for resectable microsatellite instability-high gastric cancer? Lancet Oncol. 2020, 21, 203.
- Johnston, F.M.; Beckman, M. Updates on Management of Gastric Cancer. Curr. Oncol. Rep. 2019, 21, 67.
- Irino, T.; Matsuda, S.; Wada, N.; Kawakubo, H.; Kitagawa, Y. Essential updates 2019/2020: Perioperative and surgical management of gastric cancer. Ann. Gastroenterol. Surg. 2021, 5, 162–172.
- Muro, K.; Van Cutsem, E.; Narita, Y.; Pentheroudakis, G.; Baba, E.; Li, J.; Ryu, M.-H.; Wan Zamaniah, W.I.; Yong, W.-P.; Yeh, K.-H.; et al. Pan-Asian adapted ESMO Clinical Practice Guidelines for the management of patients with metastatic gastric cancer: A JSMO–ESMO initiative endorsed by CSCO, KSMO, MOS, SSO and TOS. Ann. Oncol. 2019, 30, 19–33.
- Wagner, A.D.; Syn, N.L.X.; Moehler, M.; Grothe, W.; Yong, W.P.; Tai, B.-C.; Ho, J.; Unverzagt, S. Chemotherapy for advanced gastric cancer. Cochrane Database Syst. Rev. 2017, 8, CD004064.
- Liu, Y.; Chen, L.; Zhang, R.; Chen, B.; Xiang, Y.; Zhang, M.; Huang, X.; Zhang, W.; Chen, X.; Pan, T.; et al. Efficacy and safety of elemene combined with chemotherapy in advanced gastric cancerr: A Meta-analysis. Medicine 2020, 99, 19481.
- Stallaert, W.; Kedziora, K.M.; Taylor, C.D.; Zikry, T.M.; Ranek, J.S.; Sobon, H.K.; Taylor, S.R.; Young, C.L.; Cook, J.G.; Purvis, J.E. The structure of the human cell cycle. Cell Syst. 2022, 13, 230–240.e3.
- Carlton, J.G.; Jones, H.; Eggert, U.S. Membrane and organelle dynamics during cell division. Nat. Rev. Mol. Cell Biol. 2020, 21, 151–166.
- Ben-Salem, S.; Venkadakrishnan, V.B.; Heemers, H.V. Novel insights in cell cycle dysregulation during prostate cancer progression. Endocr.-Relat. Cancer 2021, 28, R141–R155.
- Satyanarayana, A.; Kaldis, P. Mammalian cell-cycle regulation: Several Cdks, numerous cyclins and diverse compensatory mechanisms. Oncogene 2009, 28, 2925–2939.
- Giacinti, C.; Giordano, A. RB and cell cycle progression. Oncogene 2006, 25, 5220–5227.
- Lim, S.; Kaldis, P. Cdks, cyclins and CKIs: Roles beyond cell cycle regulation. Development 2013, 140, 3079–3093.
- Ekholm, S.V.; Reed, S.I. Regulation of G1 cyclin-dependent kinases in the mammalian cell cycle. Curr. Opin. Cell Biol. 2000, 12, 676–684.
- Moser, J.; Miller, I.; Carter, D.; Spencer, S.L. Control of the Restriction Point by Rb and p21. Proc. Natl. Acad. Sci. USA 2018, 115, E8219–E8227.
- Suski, J.M.; Braun, M.; Strmiska, V.; Sicinski, P. Targeting cell-cycle machinery in cancer. Cancer Cell 2021, 39, 759–778.
- Malumbres, M.; Barbacid, M. To cycle or not to cycle: A critical decision in cancer. Nat. Rev. Cancer 2001, 1, 222–231.
- Bai, J.; Li, Y.; Zhang, G. Cell cycle regulation and anticancer drug discovery. Cancer Biol. Med. 2017, 14, 348–362.
- Ren, S.; Rollins, B.J. Cyclin C/Cdk3 Promotes Rb-Dependent G0 Exit. Cell 2004, 117, 239–251.
- Lee, P.-H.; Osley, M.A. Chromatin structure restricts origin utilization when quiescent cells re-enter the cell cycle. Nucleic Acids Res. 2021, 49, 864–878.
- Asghar, U.; Witkiewicz, A.K.; Turner, N.C.; Knudsen, E.S. The history and future of targeting cyclin-dependent kinases in cancer therapy. Nat. Rev. Drug Discov. 2015, 14, 130–146.
- Visconti, R.; Della Monica, R.; Grieco, D. Cell cycle checkpoint in cancer: A therapeutically targetable double-edged sword. J. Exp. Clin. Cancer Res. 2016, 35, 153.
- Ditano, J.P.; Sakurikar, N.; Eastman, A. Activation of CDC25A phosphatase is limited by CDK2/cyclin A-mediated feedback inhibition. Cell Cycle 2021, 20, 1308–1319.
- Chi, Y.; Carter, J.H.; Swanger, J.; Mazin, A.V.; Moritz, R.L.; Clurman, B.E. A novel landscape of nuclear human CDK2 substrates revealed by in situ phosphorylation. Sci. Adv. 2020, 6, eaaz9899.
- Ding, L.; Cao, J.; Lin, W.; Chen, H.; Xiong, X.; Ao, H.; Yu, M.; Lin, J.; Cui, Q. The Roles of Cyclin-Dependent Kinases in Cell-Cycle Progression and Therapeutic Strategies in Human Breast Cancer. Int. J. Mol. Sci. 2020, 21, 1960.
- Siu, K.T.; Rosner, M.R.; Minella, A.C. An integrated view of cyclin E function and regulation. Cell Cycle 2012, 11, 57–64.
- Gavet, O.; Pines, J. Progressive Activation of CyclinB1-Cdk1 Coordinates Entry to Mitosis. Dev. Cell 2010, 18, 533–543.
- Holt, J.E.; Weaver, J.; Jones, K.T. Spatial regulation of APCCdh1-induced cyclin B1 degradation maintains G2 arrest in mouse oocytes. Development 2010, 137, 1297–1304.
- Santamaría, D.; Barrière, C.; Cerqueira, A.; Hunt, S.; Tardy, C.; Newton, K.; Cáceres, J.F.; Dubus, P.; Malumbres, M.; Barbacid, M. Cdk1 is sufficient to drive the mammalian cell cycle. Nature 2007, 448, 811–815.
- Potapova, T.A.; Daum, J.R.; Byrd, K.S.; Gorbsky, G.J. Fine Tuning the Cell Cycle: Activation of the Cdk1 Inhibitory Phosphorylation Pathway during Mitotic Exit. Mol. Biol. Cell 2009, 20, 1737–1748.
- Sunada, S.; Saito, H.; Zhang, D.; Xu, Z.; Miki, Y. CDK1 inhibitor controls G2/M phase transition and reverses DNA damage sensitivity. Biochem. Biophys. Res. Commun. 2021, 550, 56–61.
- Bensimon, A.; Aebersold, R.; Shiloh, Y. Beyond ATM: The protein kinase landscape of the DNA damage response. FEBS Lett. 2011, 585, 1625–1639.
- Zhao, H.; Piwnica-Worms, H. ATR-Mediated Checkpoint Pathways Regulate Phosphorylation and Activation of Human Chk1. Mol. Cell. Biol. 2001, 21, 4129–4139.
- Mustofa, M.K.; Tanoue, Y.; Tateishi, C.; Vaziri, C.; Tateishi, S. Roles of Chk2/CHEK2 in guarding against environmentally induced DNA damage and replication-stress. Environ. Mol. Mutagen. 2020, 61, 730–735.
- Bačević, K.; Lossaint, G.; Achour, T.N.; Georget, V.; Fisher, D.; Dulić, V. Cdk2 strengthens the intra-S checkpoint and counteracts cell cycle exit induced by DNA damage. Sci. Rep. 2017, 7, 13429.
- He, G.; Siddik, Z.H.; Huang, Z.; Wang, R.; Koomen, J.; Kobayashi, R.; Khokhar, A.R.; Kuang, J. Induction of p21 by p53 following DNA damage inhibits both Cdk4 and Cdk2 activities. Oncogene 2005, 24, 2929–2943.
- Otto, T.; Sicinski, P. Cell cycle proteins as promising targets in cancer therapy. Nat. Rev. Cancer 2017, 17, 93–115.
- Schmidt, M.; Rohe, A.; Platzer, C.; Najjar, A.; Erdmann, F.; Sippl, W. Regulation of G2/M Transition by Inhibition of WEE1 and PKMYT1 Kinases. Molecules 2017, 22, 2045.
- Musacchio, A. The Molecular Biology of Spindle Assembly Checkpoint Signaling Dynamics. Curr. Biol. 2015, 25, R1002–R1018.
- Serafim, R.A.M.; Da Silva Santiago, A.; Schwalm, M.P.; Hu, Z.; dos Reis, C.V.; Takarada, J.E.; Mezzomo, P.; Massirer, K.B.; Kudolo, M.; Gerstenecker, S.; et al. Development of the First Covalent Monopolar Spindle Kinase 1 (MPS1/TTK) Inhibitor. J. Med. Chem. 2022, 65, 3173–3192.
- Thu, K.L.; Soria-Bretones, I.; Mak, T.W.; Cescon, D.W. Targeting the cell cycle in breast cancer: Towards the next phase. Cell Cycle 2018, 17, 1871–1885.
- Yatskevich, S.; Kroonen, J.S.; Alfieri, C.; Tischer, T.; Howes, A.C.; Clijsters, L.; Yang, J.; Zhang, Z.; Yan, K.; Vertegaal, A.C.O.; et al. Molecular mechanisms of APC/C release from spindle assembly checkpoint inhibition by APC/C SUMOylation. Cell Rep. 2021, 34, 108929.
- Vijayaraghavan, S.; Moulder, S.; Keyomarsi, K.; Layman, R.M. Inhibiting CDK in Cancer Therapy: Current Evidence and Future Directions. Target. Oncol. 2018, 13, 21–38.
- Zhang, M.; Zhang, L.; Hei, R.; Li, X.; Cai, H.; Wu, X.; Zheng, Q.; Cai, C. CDK inhibitors in cancer therapy, an overview of recent development. Am. J. Cancer Res. 2021, 11, 1913–1935.
- Ettl, T.; Schulz, D.; Bauer, R.J. The Renaissance of Cyclin Dependent Kinase Inhibitors. Cancers 2022, 14, 293.
- Liu, J.; Peng, Y.; Wei, W. Cell cycle on the crossroad of tumorigenesis and cancer therapy. Trends Cell Biol. 2022, 32, 30–44.
- Pauklin, S.; Madrigal, P.; Bertero, A.; Vallier, L. Initiation of stem cell differentiation involves cell cycle-dependent regulation of developmental genes by Cyclin D. Genes Dev. 2016, 30, 421–433.
- Gao, X.; Leone, G.W.; Wang, H. Cyclin D-CDK4/6 functions in cancer. In Advances in Cancer Research; Academic Press: Cambridge, MA, USA, 2020; ISBN 9780128203279.
- Ibrahim, H.M.; AbdElbary, A.M.; Mohamed, S.Y.; Elwan, A.; Abdelhamid, M.I.; Ibrahim, A. Prognostic Value of Cyclin D1 and CD44 Expression in Gastric Adenocarcinoma. J. Gastrointest. Cancer 2019, 50, 370–379.
- Zhang, J.; Zhang, J.; Fu, Z.; Zhang, Y.; Luo, Z.; Zhang, P.; Xu, Y.; Huang, C. CHREBP suppresses gastric cancer progression via the cyclin D1-Rb-E2F1 pathway. Cell Death Discov. 2022, 8, 300.
- Wang, S.; Wang, X.; Gao, Y.; Peng, Y.; Dong, N.; Xie, Q.; Zhang, X.; Wu, Y.; Li, M.; Li, J. RN181 is a tumour suppressor in gastric cancer by regulation of the ERK/MAPK–cyclin D1/CDK4 pathway. J. Pathol. 2019, 248, 204–216.
- He, J.; Ge, Q.; Lin, Z.; Shen, W.; Lin, R.; Wu, J.; Wang, B.; Lu, Y.; Chen, L.; Liu, X.; et al. MiR-129-5p induces cell cycle arrest through modulating HOXC10/Cyclin D1 to inhibit gastric cancer progression. FASEB J. 2020, 34, 8544–8557.
- Sun, C.; Zhang, S.; Liu, C.; Liu, X. Curcumin Promoted miR-34a Expression and Suppressed Proliferation of Gastric Cancer Cells. Cancer Biother. Radiopharm. 2019, 34, 634–641.
- Jiang, L.; Yang, W.; Bian, W.; Yang, H.; Wu, X.; Li, Y.; Feng, W.; Liu, X. MicroRNA-623 Targets Cyclin D1 to Inhibit Cell Proliferation and Enhance the Chemosensitivity of Cells to 5-Fluorouracil in Gastric Cancer. Oncol. Res. Featur. Preclin. Clin. Cancer Ther. 2018, 27, 19–27.
- Xu, G.; Wang, H.; Li, W.; Xue, Z.; Luo, Q. Leukemia inhibitory factor inhibits the proliferation of gastric cancer by inducing G1-phase arrest. J. Cell. Physiol. 2019, 234, 3613–3620.
- Liu, X.; Long, Z.; Cai, H.; Yu, S.; Wu, J. TRIM58 suppresses the tumor growth in gastric cancer by inactivation of β-catenin signaling via ubiquitination. Cancer Biol. Ther. 2020, 21, 203–212.
- Wang, H.; Ge, X.; Qu, H.; Wang, N.; Zhou, J.; Xu, W.; Xie, J.; Zhou, Y.; Shi, L.; Qin, Z.; et al. Glycyrrhizic Acid Inhibits Proliferation of Gastric Cancer Cells by Inducing Cell Cycle Arrest and Apoptosis. Cancer Manag. Res. 2020, 12, 2853–2861.
- Fan, H.-N.; Zhu, M.-Y.; Peng, S.-Q.; Zhu, J.-S.; Zhang, J.; Qu, G.-Q. Dihydroartemisinin inhibits the growth and invasion of gastric cancer cells by regulating cyclin D1-CDK4-Rb signaling. Pathol. Res. Pract. 2020, 216, 152795.
- Nie, M.; Wang, Y.; Yu, Z.; Li, X.; Deng, Y.; Wang, Y.; Yang, D.; Li, Q.; Zeng, X.; Ju, J.; et al. AURKB promotes gastric cancer progression via activation of CCND1 expression. Aging 2020, 12, 1304–1321.
- Chen, Z.; Xu, Y.; Gong, J.; Kou, F.; Zhang, M.; Tian, T.; Zhang, X.; Zhang, C.; Li, J.; Li, Z.; et al. Pyrotinib combined with CDK4/6 inhibitor in HER2-positive metastatic gastric cancer: A promising strategy from AVATAR mouse to patients. Clin. Transl. Med. 2020, 10, e148.
- Liu, B.; Li, X.; Sun, F.; Tong, X.; Bai, Y.; Jin, K.; Liu, L.; Dai, F.; Li, N. HP-CagA+ Regulates the Expression of CDK4/CyclinD1 via reg3 to Change Cell Cycle and Promote Cell Proliferation. Int. J. Mol. Sci. 2020, 21, 224.
- Chen, P.; Qian, X.-K.; Zhang, Y.-F.; Sun, X.-G.; Shi, X.-J.; Gao, Y.-S. KLF5 promotes proliferation in gastric cancer via regulating p21 and CDK4. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 4224–4231.
- Wang, X.-K.; Zhang, Y.-W.; Wang, C.-M.; Li, B.; Zhang, T.-Z.; Zhou, W.-J.; Cheng, L.-J.; Huo, M.-Y.; Zhang, C.-H.; He, Y.-L. METTL16 promotes cell proliferation by up-regulating cyclin D1 expression in gastric cancer. J. Cell. Mol. Med. 2021, 25, 6602–6617.
- Wiech, T.; Nikolopoulos, E.; Lassman, S.; Heidt, T.; Schöpflin, A.; Sarbia, M.; Werner, M.; Shimizu, Y.; Sakka, E.; Ooka, T.; et al. Cyclin D1 expression is induced by viral BARF1 and is overexpressed in EBV-associated gastric cancer. Virchows Arch. 2008, 452, 621–627.
- Lin, J.-X.; Xie, X.-S.; Weng, X.-F.; Qiu, S.-L.; Xie, J.-W.; Wang, J.-B.; Lu, J.; Chen, Q.-Y.; Cao, L.-L.; Lin, M.; et al. Overexpression of IC53d promotes the proliferation of gastric cancer cells by activating the AKT/GSK3β/cyclin D1 signaling pathway. Oncol. Rep. 2019, 41, 2739–2752.
- Sun, W.; Guo, F.; Liu, M. Up-regulated WDR5 promotes gastric cancer formation by induced cyclin D1 expression. J. Cell. Biochem. 2018, 119, 3304–3316.
- Ji, Z.-P.; Qiang, L.; Zhang, J.-L. Transcription activated p73-modulated cyclin D1 expression leads to doxorubicin resistance in gastric cancer. Exp. Ther. Med. 2018, 15, 1831–1838.
- Xie, Y.; Jin, P.; Sun, X.; Jiao, T.; Zhang, Y.; Li, Y.; Sun, M. SIX1 is upregulated in gastric cancer and regulates proliferation and invasion by targeting the ERK pathway and promoting epithelial-mesenchymal transition. Cell Biochem. Funct. 2018, 36, 413–419.
- Dai, H.; Deng, H.-B.; Wang, Y.-H.; Guo, J.-J. Resveratrol inhibits the growth of gastric cancer via the Wnt/β-catenin pathway. Oncol. Lett. 2018, 16, 1579–1583.
- Lin, S.; Liu, J.; Jiang, W.; Wang, P.; Sun, C.; Wang, X.; Chen, Y.; Wang, H. METTL3 promotes the proliferation and mobility of gastric cancer cells. Open Med. 2019, 14, 25–31.
- Pang, K.; Ran, M.-J.; Zou, F.-W.; Yang, T.-W.; He, F. Long non-coding RNA LINC00857 promotes gastric cancer cell proliferation and predicts poor patient survival. Oncol. Lett. 2018, 16, 2119–2124.
- Mo, W.L.; Jiang, J.T.; Zhang, L.; Lu, Q.C.; Li, J.; Gu, W.D.; Cheng, Y.; Wang, H.T. Circular RNA hsa_circ_0000467 Promotes the Development of Gastric Cancer by Competitively Binding to MicroRNA miR-326-3p. BioMed Res. Int. 2020, 2020, 4030826.
- Cheng, X.; Wang, X.; Wu, Z.; Tan, S.; Zhu, T.; Ding, K. CORO1C expression is associated with poor survival rates in gastric cancer and promotes metastasis in vitro. FEBS Open Bio 2019, 9, 1097–1108.
- Jiang, D.; Li, H.; Xiang, H.; Gao, M.; Yin, C.; Wang, H.; Sun, Y.; Xiong, M. Long Chain Non-Coding RNA (lncRNA) HOTAIR Knockdown Increases miR-454-3p to Suppress Gastric Cancer Growth by Targeting STAT3/Cyclin D1. Med. Sci. Monit. 2019, 25, 1537–1548.
- McCartney, A.; Migliaccio, I.; Bonechi, M.; Biagioni, C.; Romagnoli, D.; De Luca, F.; Galardi, F.; Risi, E.; De Santo, I.; Benelli, M.; et al. Mechanisms of Resistance to CDK4/6 Inhibitors: Potential Implications and Biomarkers for Clinical Practice. Front. Oncol. 2019, 9, 666.
- Pandey, K.; An, H.J.; Kim, S.K.; Lee, S.A.; Kim, S.; Lim, S.M.; Kim, G.M.; Sohn, J.; Moon, Y.W. Molecular mechanisms of resistance to CDK4/6 inhibitors in breast cancer: A review. Int. J. Cancer 2019, 145, 1179–1188.
- Álvarez-Fernández, M.; Malumbres, M. Mechanisms of Sensitivity and Resistance to CDK4/6 Inhibition. Cancer Cell 2020, 37, 514–529.
- Du, Q.; Guo, X.; Wang, M.; Li, Y.; Sun, X.; Li, Q. The application and prospect of CDK4/6 inhibitors in malignant solid tumors. J. Hematol. Oncol. 2020, 13, 41.
- He, J.; Zhao, X.; Huang, C.; Zhou, X.; You, Y.; Zhang, L.; Lu, C.; Yao, F.; Li, S. Double amplifications of CDK4 and MDM2 in a gastric inflammatory myofibroblastic tumor mimicking cancer with local invasion of the spleen and diaphragm. Cancer Biol. Ther. 2018, 19, 967–972.
- Qian, Y.; Wu, X.; Wang, H.; Hou, G.; Han, X.; Song, W. PAK1 silencing is synthetic lethal with CDK4/6 inhibition in gastric cancer cells via regulating PDK1 expression. Hum. Cell 2020, 33, 377–385.
- Shang, W.; Liang, X.; Li, S.; Li, T.; Zheng, L.; Shao, W.; Wang, Y.; Liu, F.; Ma, L.; Jia, J. Orphan nuclear receptor Nurr1 promotes Helicobacter pylori-associated gastric carcinogenesis by directly enhancing CDK4 expression. eBioMedicine 2020, 53, 102672.
- Yang, D.; Li, R.; Wang, H.; Wang, J.; Han, L.; Pan, L.; Li, X.; Kong, Q.; Wang, G.; Su, X. Clinical implications of progranulin in gastric cancer and its regulation via a positive feedback loop involving AKT and ERK signaling pathways. Mol. Med. Rep. 2017, 16, 9685–9691.
- Lin, Z.; Zhou, Z.; Guo, H.; He, Y.; Pang, X.; Zhang, X.; Liu, Y.; Ao, X.; Li, P.; Wang, J. Long noncoding RNA gastric cancer-related lncRNA1 mediates gastric malignancy through miRNA-885-3p and cyclin-dependent kinase 4. Cell Death Dis. 2018, 9, 607.
- Isinger-Ekstrand, A.; Johansson, J.; Ohlsson, M.; Francis, P.; Staaf, J.; Jönsson, M.; Borg, Å.; Nilbert, M. Genetic profiles of gastroesophageal cancer: Combined analysis using expression array and tiling array–comparative genomic hybridization. Cancer Genet. Cytogenet. 2010, 200, 120–126.
- Huang, S.; Ye, H.; Guo, W.; Dong, X.; Wu, N.; Zhang, X.; Huang, Z. CDK4/6 inhibitor suppresses gastric cancer with CDKN2A mutation. Int. J. Clin. Exp. Med. 2015, 8, 11692–11700.
- Li, L.-P.; Wu, W.-J.; Sun, D.-Y.; Xie, Z.-Y.; Ma, Y.-C.; Zhao, Y.-G. miR-449a and CDK6 in gastric carcinoma. Oncol. Lett. 2014, 8, 1533–1538.
- Feng, L.; Xie, Y.; Zhang, H.; Wu, Y. miR-107 targets cyclin-dependent kinase 6 expression, induces cell cycle G1 arrest and inhibits invasion in gastric cancer cells. Med. Oncol. 2012, 29, 856–863.
- Wang, D.; Sun, Y.; Li, W.; Ye, F.; Zhang, Y.; Guo, Y.; Zhang, D.Y.; Suo, J. Antiproliferative effects of the CDK6 inhibitor PD0332991 and its effect on signaling networks in gastric cancer cells. Int. J. Mol. Med. 2018, 41, 2473–2484.
- Zhang, Y.; He, L.; Huang, L.; Yao, S.; Lin, N.; Li, P.; Xu, H.; Wu, X.; Xu, J.; Lu, Y.; et al. Oncogenic PAX6 elicits CDK4/6 inhibitor resistance by epigenetically inactivating the LATS2-Hippo signaling pathway. Clin. Transl. Med. 2021, 11, e503.
- Li, L.; Tao, X.; Li, Y.; Gao, Y.; Li, Q. CDC37L1 acts as a suppressor of migration and proliferation in gastric cancer by down-regulating CDK6. J. Cancer 2021, 12, 3145–3153.
- Jia, Y.; Zhao, L.-M.; Bai, H.-Y.; Zhang, C.; Dai, S.-L.; Lv, H.-L.; Shan, B.-E. The tumor-suppressive function of miR-1296-5p by targeting EGFR and CDK6 in gastric cancer. Biosci. Rep. 2019, 39, BSR20181556.
- Cao, N.J.; Hou, H.H.; Li, F.; Guo, S.T.; Wang, Y. miRNA-191-5p represses cell growth by targeting CDK6 in gastric cancer. Zhonghua Yi Xue Za Zhi 2020, 100, 3689–3693.
- Jiang, H.; Liu, Z.-N.; Cheng, X.-H.; Zhang, Y.-F.; Dai, X.; Bao, G.-M.; Zhou, L.-B. MiR-29c suppresses cell invasion and migration by directly targeting CDK6 in gastric carcinoma. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 7920–7928.
- Fang, H.; Zhang, Y.; Wu, Z.; Wang, X.; Wang, H.; Wang, Y.; Chai, F.; Jiang, Y.; Jin, Z.; Wan, Y.; et al. Regional hyperthermia combined with chemotherapy in advanced gastric cancer. Open Med. 2019, 14, 85–90.
- Liu, G.; Zhao, H.; Ding, Q.; Li, H.; Liu, T.; Yang, H.; Liu, Y. CDK6 is stimulated by hyperthermia and protects gastric cancer cells from hyperthermia-induced damage. Mol. Med. Rep. 2021, 23, 240.
- Qi, J.; Liu, S.; Liu, W.; Cai, G.; Liao, G. Identification of UAP1L1 as tumor promotor in gastric cancer through regulation of CDK6. Aging 2020, 12, 6904–6927.
- Sun, Y.; Tian, Y.; He, J.; Tian, Y.; Zhang, G.; Zhao, R.; Zhu, W.-J.; Gao, P. Linc01133 contributes to gastric cancer growth by enhancing YES1-dependent YAP1 nuclear translocation via sponging miR-145-5p. Cell Death Dis. 2022, 13, 51.
- Chen, H.; Liang, C.; Wang, X.; Liu, Y.; Yang, Z.; Shen, M.; Han, C.; Ren, C. The prognostic value of circRNAs for gastric cancer: A systematic review and meta-analysis. Cancer Med. 2020, 9, 9096–9106.
- Xue, M.; Li, G.; Fang, X.; Wang, L.; Jin, Y.; Zhou, Q. hsa_circ_0081143 promotes cisplatin resistance in gastric cancer by targeting miR-646/CDK6 pathway. Cancer Cell Int. 2019, 19, 25.
- Wu, W.; Wei, N.; Shao, G.; Jiang, C.; Zhang, S.; Wang, L. circZNF609 promotes the proliferation and migration of gastric cancer by sponging miR-483-3p and regulating CDK6. OncoTargets Ther. 2019, 12, 8197–8205.
- Chen, B.; Ji, F.; Wen, X.; Jin, Z. Circular RNA circ_ASAP2 promotes cell viability, migration, and invasion of gastric cancer cells by regulating the miR-770-5p/CDK6 axis. Int. J. Clin. Exp. Pathol. 2020, 13, 2806–2819.
- Gao, H.; Yin, Y.; Qian, A.; Guo, R.; Qi, J. LncRNA LINC00974 Upregulates CDK6 to Promote Cell Cycle Progression in Gastric Carcinoma. Cancer Biother. Radiopharm. 2019, 34, 666–670.
- Bani-Hani, K.E.; Almasri, N.M.; Khader, Y.S.; Sheyab, F.M.; Karam, H.N. Combined Evaluation of Expressions of Cyclin E and p53 Proteins as Prognostic Factors for Patients with Gastric Cancer. Clin. Cancer Res. 2005, 11, 1447–1453.
- Akama, Y.; Yasui, W.; Yokozaki, H.; Kuniyasu, H.; Kitahara, K.; Ishikawa, T.; Tahara, E. Frequent Amplification of the Cyclin E Gene in Human Gastric Carcinomas. Jpn. J. Cancer Res. 1995, 86, 617–621.
- Alsina, M.; Landolfi, S.; Aura, C.; Caci, K.; Jimenez, J.; Prudkin, L.; Castro, S.; Moreno, D.; Navalpotro, B.; Tabernero, J.; et al. Cyclin E amplification/overexpression is associated with poor prognosis in gastric cancer. Ann. Oncol. 2015, 26, 438–439.
- Zhang, C.; Zhu, Q.; Gu, J.; Chen, S.; Li, Q.; Ying, L. Down-regulation of CCNE1 expression suppresses cell proliferation and sensitizes gastric carcinoma cells to Cisplatin. Biosci. Rep. 2019, 39, BSR20190381.
- Aoyagi, K.; Koufuji, K.; Yang, S.; Murakami, N.; Terasaki, Y.; Yamasaki, Y.; Takeda, J.; Tanaka, M.; Shirouzu, K. Immunohistochemical Study on the Expression of Cyclin D1 and E in Gastric Cancer. Kurume Med. J. 2000, 47, 199–203.
- Ahn, M.J.; Kim, B.H.; Jang, S.J.; Hong, E.K.; Lee, W.M.; Baik, H.K.; Park, H.K.; Lee, C.B.; Ki, M. Expression of cyclin D1 and cyclin E in human gastric carcinoma and its clinicopathologic significance. J. Korean Med. Sci. 1998, 13, 513–518.
- Zhang, G.-J.; Kou, Y.-W.; Wang, Q. Expression of P27 and cyclin D1 and E expression in gastric cancer. World Chin. J. Dig. 2007, 15, 3809–3814.
- Jiaqing, L.; Hokita, S.; Xiangming, C.; Natsugoe, S.; Tanabe, G.; Baba, M.; Takao, S.; Aikou, T. Role of cyclin E and p53 expression in progression of early gastric cancer. Gastric Cancer 1998, 1, 160–165.
- Jang, S.-J.; Park, Y.-W.; Park, M.-H.; Lee, J.-D.; Lee, Y.-Y.; Jung, T.-J.; Kim, I.-S.; Choi, I.-Y.; Ki, M.; Choi, B.-Y.; et al. Expression of cell-cycle regulators, cyclin E and p21WAF1/CIP1, potential prognostic markers for gastric cancer. Eur. J. Surg. Oncol. (EJSO) 1999, 25, 157–163.
- So, J.B.Y.; Samarasinge, K.; Raju, G.C.; Moochhala, S.M.; Ti, T.-K. Expression of Cell-Cycle Regulators p27 and Cyclin E Correlates with Survival in Gastric Carcinoma Patients. J. Surg. Res. 2000, 94, 56–60.
- Milne, A.N.A.; Carvalho, R.; Jansen, M.; Kranenbarg, E.M.-K.; Van De Velde, C.J.H.; Morsink, F.M.; Musler, A.R.; Weterman, M.A.J.; Offerhaus, G.J.A. Cyclin E low molecular weight isoforms occur commonly in early-onset gastric cancer and independently predict survival. J. Clin. Pathol. 2008, 61, 311–316.
- Kumari, S.; Puneet; Prasad, S.B.; Yadav, S.S.; Kumari, S.; Kumar, M.; Khanna, A.; Dixit, V.K.; Nath, G.; Singh, S.; et al. Cyclin D1 and cyclin E2 are differentially expressed in gastric cancer. Med. Oncol. 2016, 33, 40.
- Kouraklis, G.; Katsoulis, I.E.; Theocharis, S.; Tsourouflis, G.; Xipolitas, N.; Glinavou, A.; Sioka, C.; Kostakis, A. Does the Expression of Cyclin E, pRb, and p21 Correlate with Prognosis in Gastric Adenocarcinoma? Dig. Dis. Sci. 2009, 54, 1015–1020.
- Huang, L.; Ren, F.; Tang, R.; Feng, Z.; Chen, G. Prognostic Value of Expression of Cyclin E in Gastrointestinal Cancer: A Systematic Review and Meta-Analysis. Technol. Cancer Res. Treat. 2016, 15, 12–19.
- Kim, B.; Shin, H.C.; Heo, Y.J.; Ha, S.Y.; Jang, K.-T.; Kim, S.T.; Kang, W.K.; Lee, J.; Kim, K.-M. CCNE1 amplification is associated with liver metastasis in gastric carcinoma. Pathol. Res. Pract. 2019, 215, 152434.
- Yao, Y.-L.; Xu, B.; Song, Y.-G.; Zhang, W.-D. Overexpression of cyclin E in Mongolian gerbil with Helicobacter pylori-induced gastric precancerosis. World J. Gastroenterol. 2002, 8, 60–63.
- Min, A.; Kim, J.E.; Kim, Y.-J.; Lim, J.M.; Kim, S.; Kim, J.W.; Lee, K.-H.; Kim, T.-Y.; Oh, D.-Y.; Bang, Y.-J.; et al. Cyclin E overexpression confers resistance to the CDK4/6 specific inhibitor palbociclib in gastric cancer cells. Cancer Lett. 2018, 430, 123–132.
- Yeh, K.; Shen, Y.; Chiang, Y.; Liu, P.; Huang, C.; Cheng, A. Preferential chemosensitivity to gemcitabine by cyclin E overexpression in human gastric cancers. J. Clin. Oncol. 2007, 25, 21020.
- Fagundes, R.; Teixeira, L.K. Cyclin E/CDK2: DNA Replication, Replication Stress and Genomic Instability. Front. Cell Dev. Biol. 2021, 9, 774845.
- Zhang, Z.; Shen, M.; Zhou, G. Upregulation of CDCA5 promotes gastric cancer malignant progression via influencing cyclin E1. Biochem. Biophys. Res. Commun. 2018, 496, 482–489.
- Kim, D.; Lim, J.W.; Kim, H. β-carotene Inhibits Expression of c-Myc and Cyclin E in Helicobacter pylori-infected Gastric Epithelial Cells. J. Cancer Prev. 2019, 24, 192–196.
- Xia, Y.; Yan, Z.; Wan, Y.; Wei, S.; Bi, Y.; Zhao, J.; Liu, J.; Liao, D.J.; Huang, H. Knockdown of long noncoding RNA GHET1 inhibits cell-cycle progression and invasion of gastric cancer cells. Mol. Med. Rep. 2018, 18, 3375–3381.
- Gou, Y.; Shi, Y.; Zhang, Y.; Nie, Y.; Wang, J.; Song, J.; Jin, H.; He, L.; Gao, L.; Qiao, L.; et al. Ribosomal protein L6 promotes growth and cell cycle progression through upregulating cyclin E in gastric cancer cells. Biochem. Biophys. Res. Commun. 2010, 393, 788–793.
- Bie, L.; Li, D.; Mu, Y.; Wang, S.; Chen, B.; Lyu, H.; Han, L.; Nie, C.; Yang, C.; Wang, L.; et al. Analysis of cyclin E co-expression genes reveals nuclear transcription factor Y subunit alpha is an oncogene in gastric cancer. Chronic Dis. Transl. Med. 2019, 5, 44–52.
- Gong, X.; Yan, L.; Gu, H.; Mu, Y.; Tong, G.; Zhang, G. 14-3-3ɛ functions as an oncogene in SGC7901 gastric cancer cells through involvement of cyclin E and p27kip1. Mol. Med. Rep. 2014, 10, 3145–3150.
- Choi, J.H.; Song, Y.S.; Yoon, J.S.; Song, K.W.; Lee, Y.Y. Enhancer of zeste homolog 2 expression is associated with tumor cell proliferation and metastasis in gastric cancer. Apmis 2010, 118, 196–202.
- Li, L.; Dong, Z.; Shi, P.; Tan, L.; Xu, J.; Huang, P.; Wang, Z.; Cui, H.; Yang, L. Bruceine D inhibits Cell Proliferation Through Downregulating LINC01667/MicroRNA-138-5p/Cyclin E1 Axis in Gastric Cancer. Front. Pharmacol. 2020, 11, 584960.
- Yam, C.H.; Fung, T.K.; Poon, R.Y.C. Cyclin A in cell cycle control and cancer. Cell. Mol. Life Sci. 2002, 59, 1317–1326.
- Sanchez, I.; Dynlacht, B.D. New insights into cyclins, CDKs, and cell cycle control. Semin. Cell Dev. Biol. 2005, 16, 311–321.
- Mrena, J.; Wiksten, J.-P.; Kokkola, A.; Nordling, S.; Haglund, C.; Ristimäki, A. Prognostic significance of cyclin A in gastric cancer. Int. J. Cancer 2006, 119, 1897–1901.
- Tadesse, S.; Anshabo, A.T.; Portman, N.; Lim, E.; Tilley, W.; Caldon, C.E.; Wang, S. Targeting CDK2 in cancer: Challenges and opportunities for therapy. Drug Discov. Today 2020, 25, 406–413.
- Zheng, K.; He, Z.; Kitazato, K.; Wang, Y. Selective Autophagy Regulates Cell Cycle in Cancer Therapy. Theranostics 2019, 9, 104–125.
- Neganova, I.; Vilella, F.; Atkinson, S.P.; Lloret, M.; Passos, J.F.; von Zglinicki, T.; O’Connor, J.-E.; Burks, D.; Jones, R.; Armstrong, L.; et al. An Important Role for CDK2 in G1 to S Checkpoint Activation and DNA Damage Response in Human Embryonic Stem Cells. STEM CELLS 2011, 29, 651–659.
- Wang, Y.; Jiang, R.; Wang, Q.; Li, Y.; Sun, Z.; Zhao, H. Silencing LINC01021 inhibits gastric cancer through upregulation of KISS1 expression by blocking CDK2-dependent phosphorylation of CDX2. Mol. Ther.—Nucleic Acids 2021, 24, 832–844.
- Chen, C.; Lei, J.; Zheng, Q.; Tan, S.; Ding, K.; Yu, C. Poly(rC) binding protein 2 (PCBP2) promotes the viability of human gastric cancer cells by regulating CDK2. FEBS Open Bio 2018, 8, 764–773.
- Cao, J.; Wu, N.; Han, Y.; Hou, Q.; Zhao, Y.; Pan, Y.; Xie, X.; Chen, F. DDX21 promotes gastric cancer proliferation by regulating cell cycle. Biochem. Biophys. Res. Commun. 2018, 505, 1189–1194.
- Tang, Z.; Li, L.; Tang, Y.; Xie, D.; Wu, K.; Wei, W.; Xiao, Q. CDK2 positively regulates aerobic glycolysis by suppressing SIRT5 in gastric cancer. Cancer Sci. 2018, 109, 2590–2598.
- Liu, H.-T.; Liu, S.; Liu, L.; Ma, R.-R.; Gao, P. EGR1-Mediated Transcription of lncRNA-HNF1A-AS1 Promotes Cell-Cycle Progression in Gastric Cancer. Cancer Res 2018, 78, 5877–5890.
- Wang, Y.; Wu, H.; Dong, N.; Su, X.; Duan, M.; Wei, Y.; Wei, J.; Liu, G.; Peng, Q.; Zhao, Y. Sulforaphane induces S-phase arrest and apoptosis via p53-dependent manner in gastric cancer cells. Sci. Rep. 2021, 11, 2504.
- Kim, S.G.; Kim, S.N.; Jong, H.-S.; Kim, N.K.; Hong, S.H.; Kim, S.-J.; Bang, Y.-J. Caspase-mediated Cdk2 activation is a critical step to execute transforming growth factor-β1-induced apoptosis in human gastric cancer cells. Oncogene 2001, 20, 1254–1265.
- Li, S.-N.; Ma, J.-Q.; Zheng, Q.; Zhai, Y.; Zhou, J.; Liao, Z.-J.; Yao, Y. MICRORNA-638 INHIBITED CELL PROLIFERATION BY TARGETING CDK2. Acta Medica Mediterr. 2020, 36, 2839–2844.
- Liu, F.-Y.; Wang, L.-P.; Wang, Q.; Han, P.; Zhuang, W.-P.; Li, M.-J.; Yuan, H. miR-302b regulates cell cycles by targeting CDK 2 via ERK signaling pathway in gastric cancer. Cancer Med. 2016, 5, 2302–2313.
- Huang, V.; Place, R.F.; Portnoy, V.; Wang, J.; Qi, Z.; Jia, Z.; Yu, A.; Shuman, M.; Yu, J.; Li, L.-C. Upregulation of Cyclin B1 by miRNA and its implications in cancer. Nucleic Acids Res. 2012, 40, 1695–1707.
- Kim, D.-H. Prognostic Implications of Cyclin B1, p34cdc2, p27Kip1and p53 Expression in Gastric Cancer. Yonsei Med. J. 2007, 48, 694–700.
- Yasuda, M.; Takesue, F.; Inutsuka, S.; Honda, M.; Nozoe, T.; Korenaga, D. Overexpression of cyclin B1 in gastric cancer and its clinicopathological significance: An immunohistological study. J. Cancer Res. Clin. Oncol. 2002, 128, 412–416.
- Zhang, H.-P.; Li, S.-Y.; Wang, J.-P.; Jun, L. Clinical significance and biological roles of cyclins in gastric cancer. OncoTargets Ther. 2018, 11, 6673–6685.
- Begnami, M.D.; Fregnani, J.H.T.G.; Nonogaki, S.; Soares, F.A. Evaluation of cell cycle protein expression in gastric cancer: Cyclin B1 expression and its prognostic implication. Hum. Pathol. 2010, 41, 1120–1127.
- Xie, B.; Wang, S.; Jiang, N.; Li, J.J. Cyclin B1/CDK1-regulated mitochondrial bioenergetics in cell cycle progression and tumor resistance. Cancer Lett. 2019, 443, 56–66.
- Ding, L.; Yang, L.; He, Y.; Zhu, B.; Ren, F.; Fan, X.; Wang, Y.; Li, M.; Li, J.; Kuang, Y.; et al. CREPT/RPRD1B associates with Aurora B to regulate Cyclin B1 expression for accelerating the G2/M transition in gastric cancer. Cell Death Dis. 2018, 9, 1172.
- Liu, F.; Li, X.; Wang, C.; Cai, X.; Du, Z.; Xu, H.; Li, F. Downregulation of P21-Activated Kinase-1 Inhibits the Growth of Gastric Cancer Cells Involving Cyclin B1. Int. J. Cancer 2009, 125, 2511–2519.
- Pan, J.; Hu, H.; Zhou, Z.; Sun, L.; Peng, L.; Yu, L.; Sun, L.; Liu, J.; Yang, Z.; Ran, Y. Tumor-suppressive mir-663 gene induces mitotic catastrophe growth arrest in human gastric cancer cells. Oncol. Rep. 2010, 24, 105–112.
- Shi, Q.; Ni, X.; Lei, M.; Xia, Q.; Dong, Y.; Zhang, Q.; Wang, W. Phosphorylation of islet-1 serine 269 by CDK1 increases its transcriptional activity and promotes cell proliferation in gastric cancer. Mol. Med. 2021, 27, 47.
- Izadi, S.; Nikkhoo, A.; Hojat-Farsangi, M.; Namdar, A.; Azizi, G.; Mohammadi, H.; Yousefi, M.; Jadidi-Niaragh, F. CDK1 in Breast Cancer: Implications for Theranostic Potential. Anti-Cancer Agents Med. Chem. 2020, 20, 758–767.
- Zhang, X.; Ma, H.; Zou, Q.; Wu, J. Analysis of Cyclin-Dependent Kinase 1 as an Independent Prognostic Factor for Gastric Cancer Based on Statistical Methods. Front. Cell Dev. Biol. 2020, 8, 620164.
- Najar, M.A.; Aravind, A.; Dagamajalu, S.; Sidransky, D.; Ashktorab, H.; Smoot, D.T.; Gowda, H.; Prasad, T.S.K.; Modi, P.K.; Chatterjee, A. Hyperactivation of MEK/ERK pathway by Ca 2+/calmodulin-dependent protein kinase kinase 2 promotes cellular proliferation by activating cyclin-dependent kinases and minichromosome maintenance protein in gastric cancer cells. Mol. Carcinog. 2021, 60, 769–783.
- Lin, L.-L.; Huang, H.-C.; Juan, H.-F. Revealing the Molecular Mechanism of Gastric Cancer Marker Annexin A4 in Cancer Cell Proliferation Using Exon Arrays. PLoS ONE 2012, 7, e44615.
- Zhang, L.; Kang, W.; Lu, X.; Ma, S.; Dong, L.; Zou, B. LncRNA CASC11 promoted gastric cancer cell proliferation, migration and invasion in vitro by regulating cell cycle pathway. Cell Cycle 2018, 17, 1886–1900.
- Li, F.-N.; Zhang, Q.-Y.; Li, O.; Liu, S.-L.; Yang, Z.-Y.; Pan, L.-J.; Zhao, C.; Gong, W.; Shu, Y.-J.; Dong, P. ESRRA promotes gastric cancer development by regulating the CDC25C/CDK1/CyclinB1 pathway via DSN1. Int. J. Biol. Sci. 2021, 17, 1909–1924.
- Lakshmi Ch, N.P.; Sivagnanam, A.; Raja, S.; Mahalingam, S. Molecular basis for RASSF10/NPM/RNF2 feedback cascade–mediated regulation of gastric cancer cell proliferation. J. Biol. Chem. 2021, 297, 100935.
- Yuan, Y.; Zhang, X.; Du, K.; Zhu, X.; Chang, S.; Chen, Y.; Xu, Y.; Sun, J.; Luo, X.; Deng, S.; et al. Circ_CEA promotes the interaction between the p53 and cyclin-dependent kinases 1 as a scaffold to inhibit the apoptosis of gastric cancer. Cell Death Dis. 2022, 13, 827.
- Kim, S.-J.; Lee, H.-W.; Baek, J.-H.; Cho, Y.-H.; Kang, H.G.; Jeong, J.S.; Song, J.; Park, H.-S.; Chun, K.-H. Activation of nuclear PTEN by inhibition of Notch signaling induces G2/M cell cycle arrest in gastric cancer. Oncogene 2016, 35, 251–260.
- Huang, Z.; Zhang, S.; Du, J.; Zhang, X.; Zhang, W.; Huang, Z.; Ouyang, P. Cyclin-Dependent Kinase 1 (CDK1) is Co-Expressed with CDCA5: Their Functions in Gastric Cancer Cell Line MGC-803. Med. Sci. Monit. 2020, 26, e923664.
- Ma, Y.; Yue, Y.; Pan, M.; Sun, J.; Chu, J.; Lin, X.; Xu, W.; Feng, L.; Chen, Y.; Chen, D.; et al. Histone deacetylase 3 inhibits new tumor suppressor gene DTWD1 in gastric cancer. Am. J. Cancer Res. 2015, 5, 663–673.
- Javed, A.; Malagraba, G.; Yarmohammadi, M.; Perelló-Reus, C.M.; Barceló, C.; Rubio-Tomás, T. Therapeutic Potential of Mitotic Kinases’ Inhibitors in Cancers of the Gastrointestinal System. Futur. Pharmacol. 2022, 2, 214–237.
- Lee, D.-H.; Park, K.-I.; Park, H.-S.; Kang, S.-R.; Nagappan, A.; Kim, J.-A.; Kim, E.-H.; Lee, W.-S.; Hah, Y.-S.; Chung, H.-J.; et al. Flavonoids Isolated from Korea Citrus aurantium L. Induce G2/M Phase Arrest and Apoptosis in Human Gastric Cancer AGS Cells. Evid.-Based Complement. Altern. Med. 2012, 2012, 515901.
- Song, H.; Bao, J.; Wei, Y.; Chen, Y.; Mao, X.; Li, J.; Yang, Z.; Xue, Y. Kaempferol inhibits gastric cancer tumor growth: An in vitro and in vivo study. Oncol. Rep. 2015, 33, 868–874.
- Sankaranarayanan, R.; Valiveti, C.K.; Kumar, D.R.; Van Slambrouck, S.; Kesharwani, S.S.; Seefeldt, T.; Scaria, J.; Tummala, H.; Bhat, G.J. The Flavonoid Metabolite 2,4,6-Trihydroxybenzoic Acid Is a CDK Inhibitor and an Anti-Proliferative Agent: A Potential Role in Cancer Prevention. Cancers 2019, 11, 427.
- Lee, M.H.; Cho, Y.; Kim, D.H.; Woo, H.J.; Yang, J.Y.; Kwon, H.J.; Yeon, M.J.; Park, M.; Kim, S.H.; Moon, C.; et al. Menadione induces G2/M arrest in gastric cancer cells by down-regulation of CDC25C and proteasome mediated degradation of CDK1 and cyclin B1. Am. J. Transl. Res. 2016, 8, 5246–5255.
- Wu, B.; Zhang, Q.; Shen, W.; Zhu, J. Anti-proliferative and chemosensitizing effects of luteolin on human gastric cancer AGS cell line. Mol. Cell. Biochem. 2008, 313, 125–132.
- Xu, X.; Song, G.; Yu, Y.; Ma, H.; Ma, L.; Jin, Y.; Jiang, M. Apoptosis and G2/M arrest induced by Allium ursinum (ramson) watery extract in an AGS gastric cancer cell line. OncoTargets Ther. 2013, 6, 779–783.
- Gao, S.-Y.; Li, J.; Qu, X.-Y.; Zhu, N.; Ji, Y.-B. Downregulation of Cdk1 and CyclinB1 Expression Contributes to Oridonin-induced Cell Cycle Arrest at G2/M Phase and Growth Inhibition in SGC-7901 Gastric Cancer Cells. Asian Pac. J. Cancer Prev. 2014, 15, 6437–6441.
- Xiao, X.-Y.; Hao, M.; Yang, X.-Y.; Ba, Q.; Li, M.; Ni, S.-J.; Wang, L.-S.; Du, X. Licochalcone A inhibits growth of gastric cancer cells by arresting cell cycle progression and inducing apoptosis. Cancer Lett. 2011, 302, 69–75.
- Wang, Y.-X.; Cai, H.; Jiang, G.; Zhou, T.-B.; Wu, H. Silibinin Inhibits Proliferation, Induces Apoptosis and Causes Cell Cycle Arrest in Human Gastric Cancer MGC803 Cells Via STAT3 Pathway Inhibition. Asian Pac. J. Cancer Prev. 2014, 15, 6791–6798.
- Ren, M.; Zhou, X.; Gu, M.; Jiao, W.; Yu, M.; Wang, Y.; Liu, S.; Yang, J.; Ji, F. Resveratrol synergizes with cisplatin in antineoplastic effects against AGS gastric cancer cells by inducing endoplasmic reticulum stress-mediated apoptosis and G2/M phase arrest. Oncol. Rep. 2020, 44, 1605–1615.
- Ruan, T.; Liu, W.; Tao, K.; Wu, C. A Review of Research Progress in Multidrug-Resistance Mechanisms in Gastric Cancer. OncoTargets Ther. 2020, 13, 1797–1807.
- Jiang, H.; Chen, Y.; Xu, X.; Li, C.; Chen, Y.; Li, D.; Zeng, X.; Gao, H. Ubiquitylation of cyclin C by HACE1 regulates cisplatin-associated sensitivity in gastric cancer. Clin. Transl. Med. 2022, 12, e770.
- Fang, S.; Jin, X.; Zhou, C.; Gong, Z. Cyclin C: A new responser for chemosensitivity in cancer. Clin. Transl. Med. 2022, 12, e833.
- Choi, M.-G.; Noh, J.H.; An, J.Y.; Hong, S.K.; Park, S.B.; Baik, Y.H.; Kim, K.-M.; Sohn, T.S.; Kim, S. Expression Levels of Cyclin G2, But Not Cyclin E, Correlate with Gastric Cancer Progression. J. Surg. Res. 2009, 157, 168–174.
- Gao, J.; Zhao, C.; Liu, Q.; Hou, X.; Li, S.; Xing, X.; Yang, C.; Luo, Y. Cyclin G2 suppresses Wnt/β-catenin signaling and inhibits gastric cancer cell growth and migration through Dapper1. J. Exp. Clin. Cancer Res. 2018, 37, 317.
- Yin, G.; Zhou, H.; Xue, Y.; Yao, B.; Zhao, W. MicroRNA-340 promotes the tumor growth of human gastric cancer by inhibiting cyclin G2. Oncol. Rep. 2016, 36, 1111–1118.
- Gao, J.; Liu, Q.; Liu, X.; Zhang, Y.; Wang, S.; Luo, Y. Mutation analysis of the negative regulator cyclin G2 in gastric cancer. Afr. J. Biotechnol. 2011, 10, 14618–14624.
- Wang, Y.; Zhang, T.; Kwiatkowski, N.; Abraham, B.J.; Lee, T.I.; Xie, S.; Yuzugullu, H.; Von, T.; Li, H.; Lin, Z.; et al. CDK7-Dependent Transcriptional Addiction in Triple-Negative Breast Cancer. Cell 2015, 163, 174–186.
- Peissert, S.; Schlosser, A.; Kendel, R.; Kuper, J.; Kisker, C. Structural basis for CDK7 activation by MAT1 and Cyclin H. Proc. Natl. Acad. Sci. USA 2020, 117, 26739–26748.
- Huang, J.-R.; Qin, W.-M.; Wang, K.; Fu, D.-R.; Zhang, W.-J.; Jiang, Q.-W.; Yang, Y.; Yuan, M.-L.; Xing, Z.-H.; Wei, M.-N.; et al. Cyclin-dependent kinase 7 inhibitor THZ2 inhibits the growth of human gastric cancer in vitro and in vivo. Am. J. Transl. Res. 2018, 10, 3664–3676.
- Li, H.-L.; Huang, D.-Z.; Deng, T.; Zhou, L.-K.; Wang, X.; Bai, M.; Ba, Y. Overexpression of Cyclin L2 Inhibits Growth and Enhances Chemosensitivity in Human Gastric Cancer Cells. Asian Pac. J. Cancer Prev. 2012, 13, 1425–1430.
- Chen, X.; Zhang, L.; Song, Q.; Chen, Z. MicroRNA-216b regulates cell proliferation, invasion and cycle progression via interaction with cyclin T2 in gastric cancer. Anti-Cancer Drugs 2020, 31, 623–631.
- Bazzi, Z.A.; Tai, I.T. CDK10 in Gastrointestinal Cancers: Dual Roles as a Tumor Suppressor and Oncogene. Front. Oncol. 2021, 11, 655479.
- Kciuk, M.; Gielecińska, A.; Mujwar, S.; Mojzych, M.; Kontek, R. Cyclin-dependent kinases in DNA damage response. Biochim. Biophys. Acta (BBA) Rev. Cancer 2022, 1877, 188716.
- Sun, Y.-Q.; Xie, J.-W.; Xie, H.-T.; Chen, P.-C.; Zhang, X.-L.; Zheng, C.-H.; Li, P.; Wang, J.-B.; Lin, J.-X.; Cao, L.-L.; et al. Expression of CRM1 and CDK5 shows high prognostic accuracy for gastric cancer. World J. Gastroenterol. 2017, 23, 2012–2022.
- Cao, L.; Zhou, J.; Zhang, J.; Wu, S.; Yang, X.; Zhao, X.; Li, H.; Luo, M.; Yu, Q.; Lin, G.; et al. Cyclin-Dependent Kinase 5 Decreases in Gastric Cancer and Its Nuclear Accumulation Suppresses Gastric Tumorigenesis. Clin. Cancer Res. 2015, 21, 1419–1428.
- Lu, J.; Lin, J.-X.; Zhang, P.-Y.; Sun, Y.-Q.; Li, P.; Xie, J.-W.; Wang, J.-B.; Chen, Q.-Y.; Cao, L.-L.; Lin, Y.; et al. CDK5 suppresses the metastasis of gastric cancer cells by interacting with and regulating PP2A. Oncol. Rep. 2019, 41, 779–788.
- Wang, B.-Y.; Liu, Q.-Y.; Cao, J.; Chen, J.-W.; Liu, Z.-S. Selective CDK7 inhibition with BS-181 suppresses cell proliferation and induces cell cycle arrest and apoptosis in gastric cancer. Drug Des. Dev. Ther. 2016, 10, 1181–1189.
- Naseh, G.; Mohammadifard, M.; Mohammadifard, M. Upregulation of cyclin-dependent kinase 7 and matrix metalloproteinase-14 expression contribute to metastatic properties of gastric cancer. IUBMB Life 2016, 68, 799–805.
- Song, Y.-Q.; Ma, X.-H.; Ma, G.-L.; Lin, B.; Liu, C.; Deng, Q.-J.; Lv, W.-P. MicroRNA-107 promotes proliferation of gastric cancer cells by targeting cyclin dependent kinase 8. Diagn. Pathol. 2014, 9, 164.
- Kim, M.-Y.; Han, S.I.; Lim, S.-C. Roles of cyclin-dependent kinase 8 and?-catenin in the oncogenesis and progression of gastric adenocarcinoma. Int. J. Oncol. 2011, 38, 1375–1383.
- Lu, Y.; Tang, L.; Zhang, Q.; Zhang, Z.; Wei, W. MicroRNA-613 inhibits the progression of gastric cancer by targeting CDK9. Artif. Cells Nanomed. Biotechnol. 2018, 46, 980–984.
- Zhao, B.-W.; Chen, S.; Li, Y.-F.; Xiang, J.; Zhou, Z.-W.; Peng, J.-S.; Chen, Y.-B. Low Expression of CDK10 Correlates with Adverse Prognosis in Gastric Carcinoma. J. Cancer 2017, 8, 2907–2914.
- You, Y.; Bai, F.; Ye, Z.; Zhang, N.; Yao, L.; Tang, Y.; Li, X. Downregulated CDK10 expression in gastric cancer: Association with tumor progression and poor prognosis. Mol. Med. Rep. 2018, 17, 6812–6818.
- Liu, M.; Fan, H.; Li, T.; Sihong, L.; Qiao, S.; Bi, J. Low expression of CDK12 in gastric cancer is correlated with advanced stage and poor outcome. Pathol. Res. Pract. 2020, 216, 152962.
- Liu, H.; Shin, S.H.; Chen, H.; Liu, T.; Li, Z.; Hu, Y.; Liu, F.; Zhang, C.; Kim, D.J.; Liu, K.; et al. CDK12 and PAK2 as novel therapeutic targets for human gastric cancer. Theranostics 2020, 10, 6201–6215.
- Wu, Z.; Wang, M.; Li, F.; Wang, F.; Jia, J.; Feng, Z.; Huo, X.; Yang, J.; Jin, W.; Sa, R.; et al. CDK13-Mediated Cell Cycle Disorder Promotes Tumorigenesis of High HMGA2 Expression Gastric Cancer. Front. Mol. Biosci. 2021, 8, 707295.
- Yang, L.; Zhu, J.; Huang, H.; Yang, Q.; Cai, J.; Wang, Q.; Zhu, J.; Shao, M.; Xiao, J.; Cao, J.; et al. PFTK1 Promotes Gastric Cancer Progression by Regulating Proliferation, Migration and Invasion. PLoS ONE 2015, 10, e0140451.
- Li, P.; Ge, D.; Li, P.; Hu, F.; Chu, J.; Chen, X.; Song, W.; Wang, A.; Tian, G.; Gu, X. CXXC finger protein 4 inhibits the CDK18-ERK1/2 axis to suppress the immune escape of gastric cancer cells with involvement of ELK1/MIR100HG pathway. J. Cell. Mol. Med. 2020, 24, 10151–10165.
- Zhao, J.-Q.; Li, X.-N.; Fu, L.-P.; Zhang, N.; Cai, J.-H. ISOC1 promotes the proliferation of gastric cancer cells by positively regulating CDK19. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 11602–11609.
- Lee, Y.; Lee, C.E.; Oh, S.; Kim, H.; Lee, J.; Kim, S.B.; Kim, H.S. Pharmacogenomic Analysis Reveals CCNA2 as a Predictive Biomarker of Sensitivity to Polo-Like Kinase I Inhibitor in Gastric Cancer. Cancers 2020, 12, 1418.
- Ma, H.; He, Z.; Chen, J.; Zhang, X.; Song, P. Identifying of biomarkers associated with gastric cancer based on 11 topological analysis methods of CytoHubba. Sci. Rep. 2021, 11, 1331.
- Shan, Y.S.; Hsu, H.P.; Lai, M.D.; Hung, Y.H.; Wang, C.Y.; Yen, M.C.; Chen, Y.L. Cyclin D1 overexpression correlates with poor tumor differentiation and prognosis in gastric cancer. Oncol. Lett. 2017, 14, 4517–4526.
- Feakins, R.M.; Nickols, C.D.; Bidd, H.; Walton, S.-J. Abnormal expression of pRb, p16, and cyclin D1 in gastric adenocarcinoma and its lymph node metastases: Relationship with pathological features and survival. Hum. Pathol. 2003, 34, 1276–1282.
- Tenderenda, M. A study on the prognostic value of cyclins D1 and E expression levels in resectable gastric cancer and on some correlations between cyclins expression, histoclinical parameters and selected protein products of cell-cycle regulatory genes. J. Exp. Clin. Cancer Res. 2005, 24, 405–414.
- Takano, Y.; Kato, Y.; van Diest, P.J.; Masuda, M.; Mitomi, H.; Okayasu, I. Cyclin D2 Overexpression and Lack of p27 Correlate Positively and Cyclin E Inversely with a Poor Prognosis in Gastric Cancer Cases. Am. J. Pathol. 2000, 156, 585–594.
- Takano, Y.; Kato, Y.; Masuda, M.; Ohshima, Y.; Okayasu, I. Cyclin D2, but not cyclin D1, overexpression closely correlates with gastric cancer progression and prognosis. J. Pathol. 1999, 189, 194–200.
- Sakaguchi, T.; Watanabe, A.; Sawada, H.; Yamada, Y.; Yamashita, J.; Matsuda, M.; Nakajima, M.; Miwa, T.; Hirao, T.; Nakano, H. Prognostic value of cyclin E and p53 expression in gastric carcinoma. Cancer 1998, 82, 1238–1243.
- Xiangming, C.; Natsugoe, S.; Takao, S.; Hokita, S.; Tanabe, G.; Baba, M.; Kuroshima, K.; Aikou, T. The cooperative role of p27 with cyclin E in the prognosis of advanced gastric carcinoma. Cancer 2000, 89, 1214–1219.
- Masuda, T.-A.; Inoue, H.; Nishida, K.; Sonoda, H.; Yoshikawa, Y.; Kakeji, Y.; Utsunomiya, T.; Mori, M. Cyclin-dependent kinase 1 gene expression is associated with poor prognosis in gastric carcinoma. Clin. Cancer Res. 2003, 9, 5693–5698.
- Ihle, M.A.; Huss, S.; Jeske, W.; Hartmann, W.; Merkelbach-Bruse, S.; Schildhaus, H.-U.; Büttner, R.; Sihto, H.; Hall, K.S.; Eriksson, M.; et al. Expression of cell cycle regulators and frequency of TP53 mutations in high risk gastrointestinal stromal tumors prior to adjuvant imatinib treatment. PLoS ONE 2018, 13, e0193048.
- Yang, G.; Zhang, Y.; Yang, J. Identification of Potentially Functional CircRNA-miRNA-mRNA Regulatory Network in Gastric Carcinoma using Bioinformatics Analysis. Med. Sci. Monit. 2019, 25, 8777–8796.
- Wang, Q.; Li, M.; Zhang, X.; Huang, H.; Huang, J.; Ke, J.; Ding, H.; Xiao, J.; Shan, X.; Liu, Q.; et al. Upregulation of CDK7 in gastric cancer cell promotes tumor cell proliferation and predicts poor prognosis. Exp. Mol. Pathol. 2016, 100, 514–521.
- Ji, J.; Zhou, C.; Wu, J.; Cai, Q.; Shi, M.; Zhang, H.; Yu, Y.; Zhu, Z.; Zhang, J. Expression pattern of CDK12 protein in gastric cancer and its positive correlation with CD8+ cell density and CCL12 expression. Int. J. Med. Sci. 2019, 16, 1142–1148.
More
