Plant-microbe interactions are critical for ecosystem functioning and driving rhizosphere processes. To fully understand the communication pathways between plants and rhizosphere microbes, it is crucial to measure the numerous processes that occur in the plant and the rhizosphere. Plants can host a wide range of microbes, collectively known as the plant microbiome, in the rhizosphere (i.e., the region of soil in the vicinity of plant roots), endosphere (i.e., plant internal tissues), and phyllosphere (i.e., stem, leaves, or flowers). These microbiomes form long-lasting interactions with the host plant, leading to positive, neutral, or negative impacts on crop performance and microbe-mediated biogeochemical processes.
- biosensor
- localized surface plasmon resonance
- fluorescence
1. Introduction
2. The Interplay between Plants and Microbial Communities
Plants can host a wide range of microbes, collectively known as the plant microbiome, in the rhizosphere (i.e., the region of soil in the vicinity of plant roots), endosphere (i.e., plant internal tissues), and phyllosphere (i.e., stem, leaves, or flowers) as illustrated in Figure 1 [24]. These microbiomes form long-lasting interactions with the host plant, leading to positive, neutral, or negative impacts on crop performance and microbe-mediated biogeochemical processes [25]. The microbial community that is beneficial to the host plant’s health, function, and evolution, are termed microsymbionts for forming a symbiotic relationship with the plant. The other type of microbe functions as plant pathogens, causing damage to the host plant. In most cases, the beneficial effects of microorganisms on plants are not caused by a single microbe, but rather by a consortium of different microorganisms that induce systemic resistance and promote plant growth [26]. Berendsen et al., reported the prevalence of three bacterial genera, Microbacterium, Stenotrophomonas, and Xanthomonas, in the rhizosphere of Arabidopsis thaliana when the foliar defense was activated by the downy mildew pathogen Hyaloperonospora arabidopsidis [27]. This study revealed that the plant recruited these bacterial species in the root zone to induce systemic resistance against downy mildew. Moreover, the formation of this symbiotic relationship in the primary population of downy mildew-infected plants resulted in a higher chance of survival of the second population of plants grown in the same soil. Microbiomes found in the endosphere and rhizosphere regions have also been shown to suppress plant diseases caused by fungal pathogens Gaeumannomyces graminis and Rhizoctonia solani (a soil-born pathogen) [28,29][28][29]. Similarly, other studies suggested the impact of a consortium of endophytes, including the fungi Rhodotorula graminis, and the bacteria Burkholderia vietnamiensis, Rahnella sp., Burkholderia sp., Acinetobacter calcoaceticus, Sphingomonas yanoikuyae, Pseudomonas sp., Rhizobium tropici, and Curtobacterium sp. on the enhanced drought stress tolerance in poplar plants [30]. In another study, pepper plants inoculated with desert-adapted bacteria displayed higher tolerance to water shortage compared with control plants. The bacteria enhanced the root biomass and length of plants (by 40%), which in turn improved the plant’s ability to uptake water and survive under water stress conditions [31]. Furthermore, mutualism between plants and microbes increases nutrient availability for plants. Beneficial interactions of the host plant with the microbial community contribute to the co-existence of multiple plant species, thereby enhancing plant and microbial diversity. The heterospecific plant-soil feedback responses play an important role in the co-existence of species, ecological succession, and species invasiveness. A meta-analysis conducted by Kutakova et al., suggest that plants grew better in soil conditioned by their closely related species than in soil conditioned by less frequently co-occurring species [32].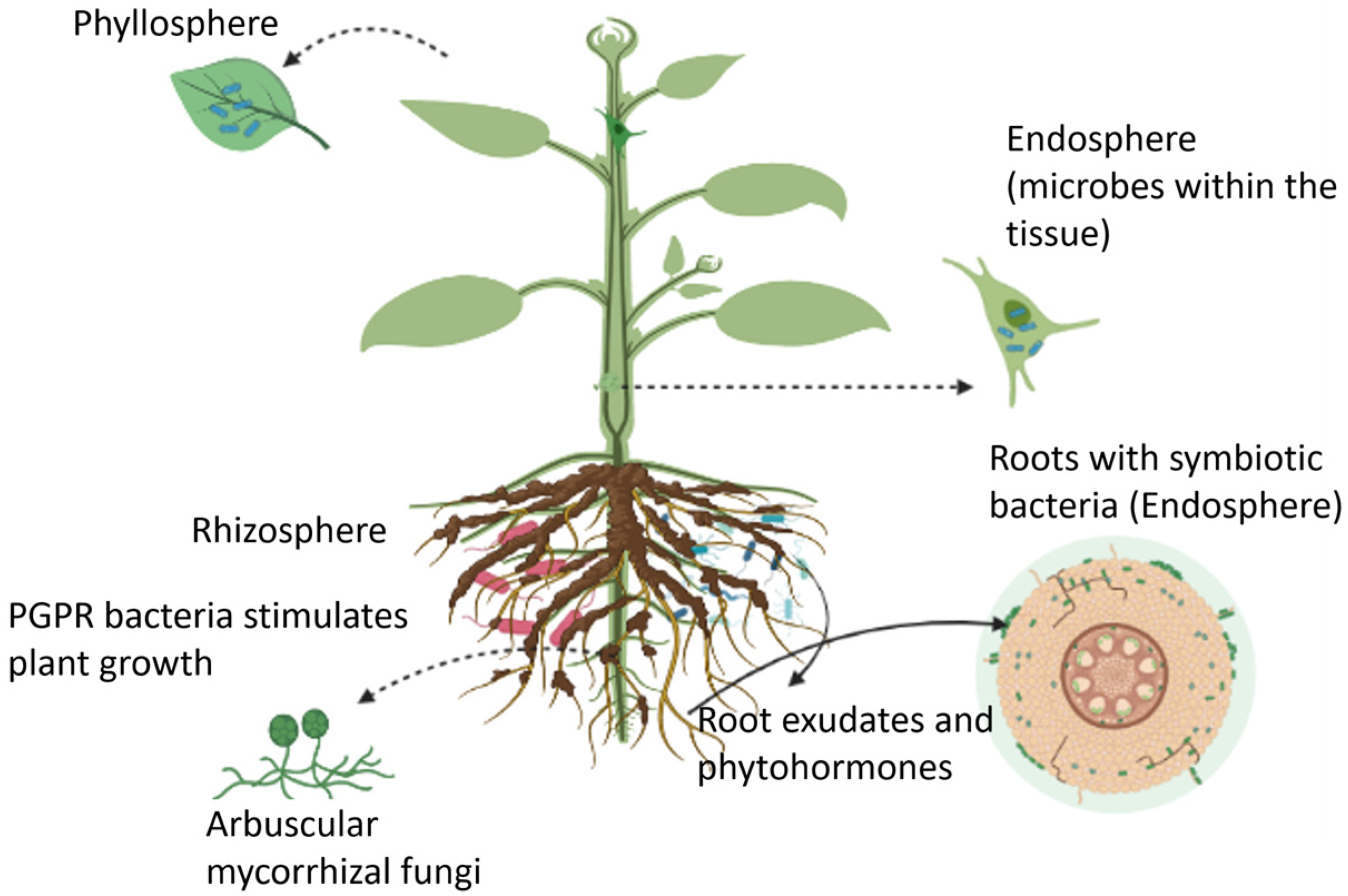
References
- Berendsen, R.L.; Pieterse, C.M.J.; Bakker, P.A.H.M. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012, 17, 478–486.
- Jacoby, R.; Peukert, M.; Succurro, A.; Koprivova, A.; Kopriva, S. The Role of Soil Microorganisms in Plant Mineral Nutrition—Current Knowledge and Future Directions. Front. Plant Sci. 2017, 8, 1617.
- Dazzo, F.B.; Ganter, S. Rhizosphere. In Encyclopedia of Microbiology, 3rd ed.; Schaechter, M., Ed.; Academic Press: Oxford, UK, 2009; pp. 335–349. ISBN 978-0-12-373944-5.
- Hayat, R.; Ali, S.; Amara, U.; Khalid, R.; Ahmed, I. Soil beneficial bacteria and their role in plant growth promotion: A review. Ann. Microbiol. 2010, 60, 579–598.
- Mantelin, S. Plant growth-promoting bacteria and nitrate availability: Impacts on root development and nitrate uptake. J. Exp. Bot. 2003, 55, 27–34.
- Saharan, B.; Nehra, V. Plant Growth Promoting Rhizobacteria: A Critical Review. Life Sci. Med. Res. 2011, 2011, 31.
- Joseph, B.; Ranjan Patra, R.; Lawrence, R. Characterization of plant growth promoting rhizobacteria associated with chickpea (Cicer arietinum L.). Int. J. Plant Prod. 2012, 1, 141–152.
- Wei, F.; Feng, H.; Zhang, D.; Feng, Z.; Zhao, L.; Zhang, Y.; Deakin, G.; Peng, J.; Zhu, H.; Xu, X. Composition of Rhizosphere Microbial Communities Associated With Healthy and Verticillium Wilt Diseased Cotton Plants. Front. Microbiol. 2021, 12, 618169.
- Zhang, X.; Zhao, C.; Yu, S.; Jiang, Z.; Liu, S.; Wu, Y.; Huang, X. Rhizosphere Microbial Community Structure Is Selected by Habitat but Not Plant Species in Two Tropical Seagrass Beds. Front. Microbiol. 2020, 11, 161.
- Lu, T.; Ke, M.; Lavoie, M.; Jin, Y.; Fan, X.; Zhang, Z.; Fu, Z.; Sun, L.; Gillings, M.; Peñuelas, J.; et al. Rhizosphere microorganisms can influence the timing of plant flowering. Microbiome 2018, 6, 231.
- Kashyap, B.; Kumar, R. Sensing Methodologies in Agriculture for Monitoring Biotic Stress in Plants Due to Pathogens and Pests. Inventions 2021, 6, 29.
- Bhar, A.; Chakraborty, A.; Roy, A. Plant Responses to Biotic Stress: Old Memories Matter. Plants Basel Switz. 2021, 11, 84.
- Rosenberg, E.; Zilber-Rosenberg, I. The hologenome concept of evolution after 10 years. Microbiome 2018, 6, 78.
- Knief, C. Analysis of plant microbe interactions in the era of next generation sequencing technologies. Front. Plant Sci. 2014, 5, 216.
- Shelake, R.M.; Pramanik, D.; Kim, J.-Y. Exploration of Plant-Microbe Interactions for Sustainable Agriculture in CRISPR Era. Microorganisms 2019, 7, 269.
- Lim, J.W.; Ha, D.; Lee, J.; Lee, S.K.; Kim, T. Review of Micro/Nanotechnologies for Microbial Biosensors. Front. Bioeng. Biotechnol. 2015, 3, 61.
- Levak, V.; Lukan, T.; Gruden, K.; Coll, A. Biosensors: A Sneak Peek into Plant Cell’s Immunity. Life 2021, 11, 209.
- Zolti, A.; Green, S.J.; Sela, N.; Hadar, Y.; Minz, D. The microbiome as a biosensor: Functional profiles elucidate hidden stress in hosts. Microbiome 2020, 8, 71.
- Galvan, C.; Montiel, R.; Lorenz, K.; Carter, J.; Hossain, N.I.; Tabassum, S. A microneedle-based Leaf Patch with IoT Integration for Real-time Monitoring of Salinity Stress in Plants. In Proceedings of the 2022 IEEE 15th Dallas Circuit And System Conference (DCAS), Dallas, TX, USA, 17–19 June 2022; pp. 1–2.
- Tabassum, S. Real-time quantification of salicylic acid with a fiber optic sensor functionalized by gold nanoparticles-copper metal organic conjugate coating. In Sensing for Agriculture and Food Quality and Safety XIV; SPIE Defense + Commercial Sensing: Orlando, FL, USA, 2022; Volume 12120, pp. 52–59.
- Ishtiaque Hossain, N.; Tabassum, S. Stem-FIT: A Microneedle-based Multi-parametric Sensor for In Situ Monitoring of Salicylic Acid and pH Levels in Live Plants. In Proceedings of the 2022 IEEE 17th International Conference on Nano/Micro Engineered and Molecular Systems (NEMS), Taoyuan, Taiwan, 14–17 April 2022; pp. 312–316.
- Hossain, N.I.; Noushin, T.; Tabassum, S. Leaf-FIT: A Wearable Leaf Sensor for In-Situ and Real-Time Monitoring of Plant Phytohormones. In Proceedings of the 2021 IEEE Sensors, Dallas, TX, USA, 30 October–2 November 2021; pp. 1–4.
- Hossain, N.I.; Tabassum, S. Fruit-FIT: Drone Interfaced Multiplexed Sensor Suite to Determine the Fruit Ripeness. In Proceedings of the 2022 IEEE Sensors, Dallas, TX, USA, 30 October–2 November 2022; pp. 1–4.
- Orozco-Mosqueda, M.d.C.; Rocha-Granados, M.d.C.; Glick, B.R.; Santoyo, G. Microbiome engineering to improve biocontrol and plant growth-promoting mechanisms. Microbiol. Res. 2018, 208, 25–31.
- Hodge, A.; Fitter, A.H. Microbial mediation of plant competition and community structure. Funct. Ecol. 2013, 27, 865–875.
- Song, C.; Zhu, F.; Carrión, V.J.; Cordovez, V. Beyond Plant Microbiome Composition: Exploiting Microbial Functions and Plant Traits via Integrated Approaches. Front. Bioeng. Biotechnol. 2020, 8, 896.
- Berendsen, R.L.; Vismans, G.; Yu, K.; Song, Y.; de Jonge, R.; Burgman, W.P.; Burmølle, M.; Herschend, J.; Bakker, P.A.H.M.; Pieterse, C.M.J. Disease-induced assemblage of a plant-beneficial bacterial consortium. ISME J. 2018, 12, 1496–1507.
- Durán, P.; Tortella, G.; Viscardi, S.; Barra, P.J.; Carrion, V.J.; de la Luz Mora, M.; Pozo, M.J. Microbial Community Composition in Take-All Suppressive Soils. Front. Microbiol. 2018, 9, 2198.
- Mendes, R.; Kruijt, M.; de Bruijn, I.; Dekkers, E.; van der Voort, M.; Schneider, J.H.M.; Piceno, Y.M.; DeSantis, T.Z.; Andersen, G.L.; Bakker, P.A.H.M.; et al. Deciphering the Rhizosphere Microbiome for Disease-Suppressive Bacteria. Science 2011, 332, 1097–1100.
- Khan, Z.; Rho, H.; Firrincieli, A.; Hung, S.H.; Luna, V.; Masciarelli, O.; Kim, S.-H.; Doty, S.L. Growth enhancement and drought tolerance of hybrid poplar upon inoculation with endophyte consortia. Curr. Plant Biol. 2016, 6, 38–47.
- Marasco, R.; Rolli, E.; Vigani, G.; Borin, S.; Sorlini, C.; Ouzari, H.; Zocchi, G.; Daffonchio, D. Are drought-resistance promoting bacteria cross-compatible with different plant models? Plant Signal. Behav. 2013, 8, e26741.
- Kuťáková, E.; Herben, T.; Münzbergová, Z. Heterospecific plant–soil feedback and its relationship to plant traits, species relatedness, and co-occurrence in natural communities. Oecologia 2018, 187, 679–688.
- Spoel, S.H.; Dong, X. How do plants achieve immunity? Defence without specialized immune cells. Nat. Rev. Immunol. 2012, 12, 89–100.
- Robert-Seilaniantz, A.; Grant, M.; Jones, J.D.G. Hormone crosstalk in plant disease and defense: More than just jasmonate-salicylate antagonism. Annu. Rev. Phytopathol. 2011, 49, 317–343.
- Yang, J.; Duan, G.; Li, C.; Liu, L.; Han, G.; Zhang, Y.; Wang, C. The Crosstalks Between Jasmonic Acid and Other Plant Hormone Signaling Highlight the Involvement of Jasmonic Acid as a Core Component in Plant Response to Biotic and Abiotic Stresses. Front. Plant Sci. 2019, 10, 1349.
- Fu, Z.Q.; Dong, X. Systemic acquired resistance: Turning local infection into global defense. Annu. Rev. Plant Biol. 2013, 64, 839–863.
- Huang, J.; Reichelt, M.; Chowdhury, S.; Hammerbacher, A.; Hartmann, H. Increasing carbon availability stimulates growth and secondary metabolites via modulation of phytohormones in winter wheat. J. Exp. Bot. 2017, 68, 1251–1263.
- Bari, R.; Jones, J.D.G. Role of plant hormones in plant defence responses. Plant Mol. Biol. 2009, 69, 473–488.
- Kunkel, B.N.; Harper, C.P. The roles of auxin during interactions between bacterial plant pathogens and their hosts. J. Exp. Bot. 2018, 69, 245–254.
- Mir, M.A.; John, R.; Alyemeni, M.N.; Alam, P.; Ahmad, P. Jasmonic acid ameliorates alkaline stress by improving growth performance, ascorbate glutathione cycle and glyoxylase system in maize seedlings. Sci. Rep. 2018, 8, 2831.
- Philippot, L.; Raaijmakers, J.M.; Lemanceau, P.; van der Putten, W.H. Going back to the roots: The microbial ecology of the rhizosphere. Nat. Rev. Microbiol. 2013, 11, 789–799.
- Vos, I.A.; Pieterse, C.M.J.; van Wees, S.C.M. Costs and benefits of hormone-regulated plant defences. Plant Pathol. 2013, 62, 43–55.
- Liu, J.; Qiu, G.; Liu, C.; Li, H.; Chen, X.; Fu, Q.; Lin, Y.; Guo, B. Salicylic Acid, a Multifaceted Hormone, Combats Abiotic Stresses in Plants. Life 2022, 12, 886.
- Pál, M.; Janda, T.; Szalai, G. Abscisic Acid May Alter the Salicylic Acid-Related Abiotic Stress Response in Maize: Abscisic Acid- and Salicylic Acid-Related Responses. J. Agron. Crop Sci. 2011, 197, 368–377.
- Sah, S.K.; Reddy, K.R.; Li, J. Abscisic Acid and Abiotic Stress Tolerance in Crop Plants. Front. Plant Sci. 2016, 7, 571.
- Wang, J.; Song, L.; Gong, X.; Xu, J.; Li, M. Functions of Jasmonic Acid in Plant Regulation and Response to Abiotic Stress. Int. J. Mol. Sci. 2020, 21, 1446.
- Li, N.; Han, X.; Feng, D.; Yuan, D.; Huang, L.-J. Signaling Crosstalk between Salicylic Acid and Ethylene/Jasmonate in Plant Defense: Do We Understand What They Are Whispering? Int. J. Mol. Sci. 2019, 20, 671.
- Mao, Y.-B.; Liu, Y.-Q.; Chen, D.-Y.; Chen, F.-Y.; Fang, X.; Hong, G.-J.; Wang, L.-J.; Wang, J.-W.; Chen, X.-Y. Jasmonate response decay and defense metabolite accumulation contributes to age-regulated dynamics of plant insect resistance. Nat. Commun. 2017, 8, 13925.
- Saruhan, N.; Saglam, A.; Kadioglu, A. Salicylic acid pretreatment induces drought tolerance and delays leaf rolling by inducing antioxidant systems in maize genotypes. Acta Physiol. Plant. 2012, 34, 97–106.
- Khan, M.I.R.; Fatma, M.; Per, T.S.; Anjum, N.A.; Khan, N.A. Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front. Plant Sci. 2015, 6, 462.
- Elhakem, A. Salicylic acid ameliorates salinity tolerance in maize by regulation of phytohormones and osmolytes. Plant Soil Environ. 2020, 66, 533–541.
- Abdel Latef, A.A.H.; Akter, A.; Tahjib-Ul-Arif, M. Foliar Application of Auxin or Cytokinin Can Confer Salinity Stress Tolerance in Vicia faba L. Agronomy 2021, 11, 790.
- Sharma, M.; Laxmi, A. Jasmonates: Emerging Players in Controlling Temperature Stress Tolerance. Front. Plant Sci. 2016, 6, 1129.
- Li, G.; Zhang, C.; Zhang, G.; Fu, W.; Feng, B.; Chen, T.; Peng, S.; Tao, L.; Fu, G. Abscisic Acid Negatively Modulates Heat Tolerance in Rolled Leaf Rice by Increasing Leaf Temperature and Regulating Energy Homeostasis. Rice 2020, 13, 18.
- Bielach, A.; Hrtyan, M.; Tognetti, V.B. Plants under Stress: Involvement of Auxin and Cytokinin. Int. J. Mol. Sci. 2017, 18, 1427.
- Yarzábal, L.A.; Chica, E.J. Chapter 3—Role of Rhizobacterial Secondary Metabolites in Crop Protection Against Agricultural Pests and Diseases. In New and Future Developments in Microbial Biotechnology and Bioengineering; Gupta, V.K., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 31–53. ISBN 978-0-444-63504-4.
- Turner, T.R.; James, E.K.; Poole, P.S. The plant microbiome. Genome Biol. 2013, 14, 209.
- Galbally, I.E.; Kirstine, W. The Production of Methanol by Flowering Plants and the Global Cycle of Methanol. J. Atmos. Chem. 2002, 43, 195–229.
- Wang, K.-Y.; Shallcross, D.E. Modelling terrestrial biogenic isoprene fluxes and their potential impact on global chemical species using a coupled LSM–CTM model. Atmos. Environ. 2000, 34, 2909–2925.
