Oil-contaminated soil is one of the most concerning problems due to its potential damage to humans, animals, and the environment. In recent years, surfactant foam and nanoparticles have shown high removal of oil pollutants from contaminated soil. Researchers provide an overview on the remediation of oil pollutants in soil using nanoparticles, surfactant foams, and nanoparticle-stabilized surfactant foams. In particular, the fate and transport of oil compounds in the soil, the interaction of nanoparticles and surfactant foam, the removal mechanisms of nanoparticles and various surfactant foams, the effect of some factors (e.g., soil characteristics and amount, nanoparticle properties, surfactant concentration) on remediation efficiency, and some advantages and disadvantages of these methods are evaluated.
Oil-contaminated soil is one of the most concerning problems due to its potential damage to humans, animals, and the environment. In recent years, surfactant foam and nanoparticles have shown high removal of oil pollutants from contaminated soil. This review provides an overview on the remediation of oil pollutants in soil using nanoparticles, surfactant foams, and nanoparticle-stabilized surfactant foams. In particular, the fate and transport of oil compounds in the soil, the interaction of nanoparticles and surfactant foam, the removal mechanisms of nanoparticles and various surfactant foams, the effect of some factors (e.g., soil characteristics and amount, nanoparticle properties, surfactant concentration) on remediation efficiency, and some advantages and disadvantages of these methods are evaluated.
- soil remediation
- oil pollutants
- surfactant
- biosurfactant
- surfactant foam
- nanoparticles
- contaminated soil
1. Introduction
2. Remediation Methods of Oil Pollutants in Soil
Different approaches have been utilized to remediate oil pollutants in soil. Some common techniques are physicochemical (e.g., surface capping, pump and treat, soil washing, soil vapor extraction, soil extraction) [13][14][15][16][13,14,15,16], chemical (e.g., stabilization, oxidation–reduction, adsorption, supercritical fluid extraction and oxidation, encapsulation) [15][17][18][15,17,18], biological (bioremediation, bioattenuation, biodegradation, bioventing, biosparging, biotransformation, composting) [19][20][21][22][19,20,21,22], thermal (e.g., incineration, pyrolysis) [23][24][23,24], and phytoremediation (phytostabilization, phytovolatilization, phytotransformation) methods [15][25][15,25]. Many criteria should be considered to select the optimal treatment method, such as site characteristics, oil pollutant features, soil composition and properties, remediation time and cost [15]. Generally, these common methods have many disadvantages that limit their wide application, e.g., they are not effective for removing oil pollutants adsorbed on clay-size particles (soil washing) [26][27][26,27] or high oil content (soil vapor extraction) [25]. There is also the possibility of the formation of by-products (chemical oxidation–reduction) [17]. They are not effective for clay soils and have the potential to generate more toxic by-products (biodegradation) [22]. In addition, they have high operation costs, further treatment demand for off-gases and combustion residuals (thermal treatment) [24], and long treatment time (phytoremediation) [15]. Thus, it is critical to research and develop new oil-contaminated soil remediation approaches.2.1. Application of Nanoparticles for Remediating Oil Pollutants in Soil
Nanoparticles are particles with a size of less than 100 nm (or 10−9 m). Due to their unique characteristics, for example, small size or high specific surface area, they can be transported to complex target zones at contaminated sites [28]. Together with their simple and uniform operating conditions [29], they have been widely used for soil remediation. In contrast, long treatment times and possible formation of toxic by-products are some disadvantages of using nanoparticles for soil remediation [30].2.1.1. Effect of Nanoparticles on Soil Properties
The presence of nanoparticles decreases the soil pH, organic carbon, activity of dehydrogenase enzyme, microbial biomass transformation rate, soil bacteria, and amount of fungal colonies in the soil, reducing the soil microbial diversity [31]. Due to their melectrostagnetic attraction, nanoparticles tend to aggregate to form larger particles, lowering soil mobility and reactivity [32]. Meanwhile, the addition of nanoparticles enhances the available phosphorus in the soil. In another study, adding ZnO nanoparticles (10 mg/kg soil) reduced the soil pH after seven days and decreased the eqCO2 value in soil or the conversion rate of carbon sources into biomass. However, the presence of ZnO nanoparticles also enhanced the development of some bacteria in the soil, which improved the soil microbial diversity [33].2.1.2. Removal Mechanisms
Nanoparticles have been used to remediate contaminated soil under different conditions for a long time. Due to their high solvent affinity and large specific surface area, nanoparticles can easily contact oil compounds and improve their solubility, leading to a high removal rate [34][35][34,35]. The interaction of nanoparticles and other counterparts strongly depends on their types, amount, and properties [36]. Their main treatment mechanisms are adsorption (e.g., nZVI, carbon nanotubes), oxidation (e.g., manganese nanoparticles, cobalt nanoparticles), and photocatalysis (e.g., bismuth nanocomposite, BiPO4-based photocatalysts) applications [37][38][37,38]. Oil pollutants can be removed from contaminated soil by adsorption on the nanoparticle’s surface via π–π and van der Waals interactions [18][39][40][18,39,40]. Nonetheless, the potential aggregation of nanoparticles, which can decrease the surface area and active sites of nanoparticles and reduce the treatment efficiency, is one of the most significant disadvantages of this method. In the oxidation method, the oil pollutants can be reduced into less toxic or non-toxic compounds, such as CO2 and H2O, by Fenton-like reactions [35][41][42][35,41,42]. This method involves the degradation of oil pollutants by reactive oxygen species (ROS), which are formed via the reaction of iron oxides with H2O2, UV light, or under ultrasound [43]. In particular, the generation of ROS such as hydroxyl radicals (HO.) or hydroperoxyl (HO2.), may degrade oil pollutants to form final products, such as CO2 and H2O, as follows [44]: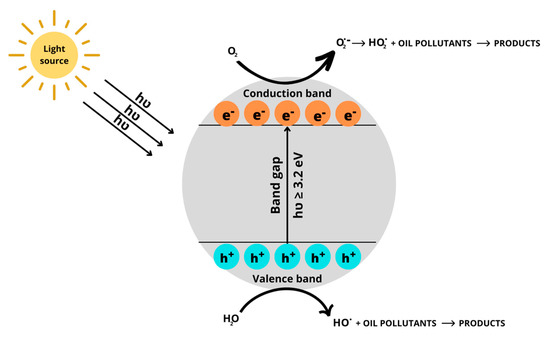
2.1.3. Treatment of Oil Pollutants in Soil by Nanoparticles
Various nanoparticle types have been successfully utilized to remove different oil pollutants from contaminated soil. Carbon nanotubes (CNTs) were effectively utilized for adsorbing PAHs [47] or dichloro-diphenyl-trichloroethane (DDT) in the natural soil, whereas 56% of DDT was degraded by nZVI after 7-day treatment [48]. Bentonite clay combined with nZVI removed PCBs from soil-sorbed PCBs 10 times more than only nZVI [49]. Furthermore, the addition of ethanol increased PCB desorption and enhanced the contact between PCBs and nZVI, leading to 50% higher treatment efficiency. Iron nano-oxide particles removed 99% pyrene in contaminated soils via a Fenton oxidation reaction with hydrogen peroxide (H2O2) [50]. Karam et al. [51] showed a high degradation rate of anthracene using nano-TiO2-photocatalysts. Furthermore, PAHs were productively treated by different nanoparticles, such as gold nanoparticles [52], iron hexacyanoferrate nanoparticles [53], ZrO2 nanoparticles [54], nano Fe3+-montmorillonite [55], nano anatase TiO2, [56], ZnO nanoparticles [57], Ti/ZnO-Cr2O3 nanocomposite [58], Fe3O4 nanoparticles [59], TiO2-graphene nanocomposites [45], Fe/Cu bimetallic nanoparticles [38][60][38,60]. More oil-contaminated soil treatment methods using nanoparticles are shown in Table 1.| Nanoparticle Name | Pollutant Name | Treatment Time, Day | Treatment Efficiency, % | Reference | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MWCNTs | 1 | Phenanthrene | 21 | 54.2 | [61] | |||||||
| MWCNTs | PAHs | 2 | 5 | 79 | [62] | |||||||
| nZVI | 3 | Phenol | 12 h | 9 | [63] | |||||||
| nZVI/BFN | 4 | Phenol | 7 h | 98.5 | [63] | |||||||
| Iron nanoparticles | PCBs | 5 | 6 h | 95 | [64] | |||||||
| nZVI | PAHs | 1 h | 70 | [65] | ||||||||
| APU nanoparticles | 6 | PAHs | 5 | 67 | [66] | |||||||
| nZVI/biosurfactant | Oil compounds | 1 h | 83 | [40] | ||||||||
| nZVI/biosurfactant foam | Oil compounds | 30 min | 67 | [34] | ||||||||
| nZVI | PCBs | 15 | 42 | [67] | ||||||||
| nZVI-Pd | PCBs | 15 | 64 | [67] | ||||||||
| nFe | 3 | O | 4 | PCBs | 15 | 68 | [67] | |||||
| Fe-Cu/biochar/geopolymer | Naphthalene | 2 h | 68 | [68] | ||||||||
| nZVI/bioattenuation | Diesel fuel | 75 | 41.0 | [21] | ||||||||
| nZVI/biostimulation | Diesel fuel | 45 | 64.6 | [21] | ||||||||
| nZVI/bioaugmentation | Diesel fuel | 15–30 | 85.3 | [21] | ||||||||
| nZVI/biostimulation + bioaugmentation | Diesel fuel | 30–60 | 89.5 | [21] | ||||||||
| Iron oxide nanoparticles | Crude oil | 1 | N/A | [42] | ||||||||
| Nano rutile TiO | 2 | Pyrene | 25 h | 52.2 | [69] | |||||||
| Nano rutile TiO | 2 | Phenanthrene | 25 h | 38.9 | [69] | |||||||
| Iron oxide nanoparticles | PAHs | 5 | 70 | [70] | ||||||||
| Akaganeite nano-rods | PAHs | 1 | 65 | [71] | ||||||||
| Iron oxide nanoparticles | Anthracene | 10 | 99 | [41] | ||||||||
| Graphene oxide | PAHs | 7 min | ~100 | [72] | ||||||||
| Fe-doped TiO | 2 | nanocatalyst | PAHs | 35 min | 80 | [73] | ||||||
| TiO | 2 | -based ZnHCF nanocomposite | PAHs | 1 | 86 | [74] | ||||||
| C | 3 | N | 4 | /Fe | 3 | O | 4 | nanocomposite | Phenanthrene | 2 h | 92.3 | [75] |
| Cu | 2 | OPLA composite nanofiber | Fluoranthene | 8 h | 67.6 | [76] |
3. Remediation of Oil Pollutants in Soil by Surfactant Foam/Nanoparticle Mixture
3.1. Interaction of Surfactant Foam and Nanoparticles
The existence of contaminants may decrease the spreading velocity of aqueous foam in soil layers [80]. In this case, the foam interaction with soil contaminants can be represented by the dimensionless Lamela number [81]. Hence, nanoparticles can stabilize the surfactant foam (Figure 52), enhancing their movement in the unsaturated soil zone [82]. The foam stabilization by nanoparticles involves the agglomeration of nanoparticles at the oil–water interface to create a thick layer that may hinder foam aggregation [83]. In particular, the generation of nanoparticle monolayers or adjacent nanoparticle bilayers may cause the stabilization of liquid films in the foam [84][85][84,85]. The attachment of colloidal nanoparticles at the gas–liquid or liquid–liquid interfaces of foam may decrease bubble breakage, contributing to foam stabilization [86][87][86,87]. For example, the foam formation by silica nanoparticles and SDS surfactant was 10 times more stable than that by only SDS surfactant due to the attachment of SDS molecules, which lowered the silica nanoparticle surface charge [87]. Li and Prigiobbe [88] showed a similar result, where high foam quality was formed by cationic surfactant and silica nanoparticles under N2-gas. The generation mechanism of anionic surfactant foam in porous media with or without nanoparticles is similar [89]. In another study, the mixture of hydrophobic fine particles and surfactant was also proven to improve the bubble combination efficiency and reduce the foam stability [90]. The reduction in nanoparticle retention due to the decrease of surface tension at the liquid–gas interfaces may influence foam stability [91][92][91,92]. The gas used for foam generation may also influence the transport of surfactant foam–nanoparticle mixture through the soil, affecting the remediation efficiency of oil pollutants [93].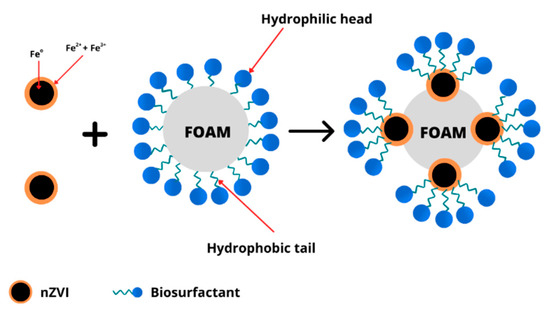
3.2. Use of Surfactant Foam–Nanoparticle Mixture in Soil Remediation
The effect of colloidal particles on foam formation, stability, and prevention has been studied for a long time [94]. Due to their ability to enhance foam stability, nanoparticles have been effectively used for oil recovery [95] or soil remediation [38][40][96][38,40,96]. The viscoelastic layer was enhanced by attaching 50% silica nanoparticles to the interface, which hindered the collapse of the bubble and improved the foam stability up to 23 h [97]. In another study, the half-life of SDBS surfactant foam after adding silica nanoparticles was double that of only SDBS surfactant foam due to the development of foam stability [98]. The presence of silica nanoparticles in a sand column also decreased the hydraulic conductivity of CTAB surfactant foam, which enhanced the foam stability after 17 days and led to higher isolation efficiency of the contaminant in soil [99]. The transport of nanoparticles in soil was improved by combining them with surfactant foam. Surfactant foam may stabilize the nanoparticle suspension and prevent them from aggregating in an aqueous solution, which decreases the nanoparticle retention on the soil surface and enhances the movement of nanoparticles in the soil. For example, 1% SLES surfactant foam delivered 100% nZVI in the soil vadose zone, leading to higher removal efficiency of the soil contaminants [100]. Shen et al. [101] reported that the transport of nZVI in the soil subsurface was significantly improved with foam generated by different surfactants, such as SDS, TW20, TW80, TX100, leading to higher remediation efficiency in the vadose zone. The film breakage of foam was reduced with the addition of nZVI, which enhanced the microsphere transport in soil and led to better treatment effectiveness. The same results were reported in other papers, where the remediation rate of oil contaminants was 78–99% by foam-stabilized nanoparticles under various environmental conditions [102][103][102,103]. Using a surfactant foam–nanoparticle combination for soil remediation is less common than surfactant solutions only or surfactant foams. However, due to the synergistic effect of nanoparticles and surfactant foam, the soil remediation efficiency by this mixture is surpassed by only surfactant or surfactant foam at the same concentration [103[103]2]]. With other advantages, such as simplicity and effectiveness for various soil contaminants, applying a surfactant foam–nanoparticle mixture can become a productive method for soil remediation in the future. A surfactant foam–nanoparticle mixture has been employed for soil remediation in the field. According to Quinn et al. [104], the treatment performance of TCE from contaminated soil was remarkably enhanced (up to 100%) after five months. In another article, Zhao et al. [105] pointed out that the degradation rate of chlorinated volatile organic compounds (CVOCs) and perchloroethylene (PCE) in field sites using a surfactant–corn oil–nZVI mixture after 2.5 years was 86% and 93%, respectively. He et al. [106] showed that 88% TCE was removed using carboxymethyl cellulose and Fe/Pd nanoparticle mixture after 596 days. Moreover, the presence of hydrogen improved the remediation performance. Bennett et al. [107] pointed out that the chlorinated ethenes at an aerospace facility were rapidly degraded by applying a carboxymethyl cellulose and Fe/Pd nanoparticle combination. The reduction in oil concentration from contaminated soil using biosurfactant foam–nanoparticle mixture has been shown by gas chromatography (Figure 3).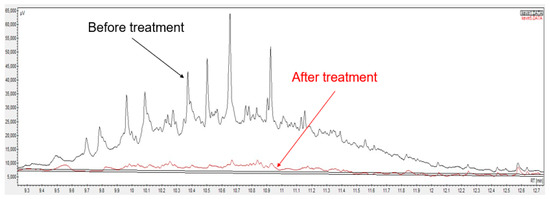
3.3. Effect of Some Factors on Soil Remediation Performance by Surfactant Foam–Nanoparticles
3.3.1. Effect of Environmental Conditions
The environmental conditions (or weather) are one of the most vital factors affecting the remediation efficiency of surfactant foam–nanoparticles. The weather may change the toxicity and biodegradability of surfactant foam, which alters the properties of the surfactant foam–nanoparticle mixture [108]. Toxicity and biodegradability are possible adverse effects of surfactant foam on the soil and the potential influence of soil microorganisms on the surfactant foam, respectively [109]. Due to the low biodegradability of most chemical surfactant foams used for soil remediation, combining these surfactant foams with nanoparticles may adsorb on the soil surface and harm the soil properties and soil microorganisms [110]. The adsorption of these surfactant foams, especially nonionic surfactant foam, on the soil surface can generate aggregation, which will alter the soil hydrophobicity, reduce soil retention, and cause toxicity to the soil [111]. The toxicity to the soil is more serious due to the potential absorption of surfactant foam into the plant roots, which can decrease crop growth and yield. In addition, the surfactant foam can break the cellular membrane, interact with lipids and proteins, and harm the soil microorganisms. The potential toxicity of some surfactants on soil microbes has been indicated in previous studies [112][113][112,113]. Therefore, biosurfactant foam, biologically produced from the microbial population, is suggested for soil remediation [34][114][34,114].3.3.2. Effect of Soil Characteristics
Soil type and particle size can affect the remediation rate. The smaller the soil particle size, the higher porosity and stronger bonds with oil pollutants, which will decrease the soil wettability and lead to lower treatment effectiveness. The remediation of motor oil from clay soil (soil porosity of 68.7%) was found to be lower than from desert soil and coastal soil (soil porosity of 42.5% and 37.5%) [115]. In another study, the removal performance of PCBs in clay soil was lower than in sandy soil due to the smaller desorption of PCBs in clay soil [17]. The presence of organic matter in soil components creates more competitive factors with oil pollutants in the mixture, which will inhibit and limit the removal efficiency of oil pollutants [116]. The bond of organic matter molecules to the nanoparticle surface may generate a film that prevents the mass and electron transfer rate, resulting in a lower remediation percentage [16]. Soil type also changes the activation energy connecting oil pollutants and soil surface, thus influencing the oil remediation efficiency [117]. In particular, the binding of adsorbed oil compounds and soil particles may cause clogging and block the available pores, limiting the transport of flow through the pores and decreasing the removal effectiveness [118]. A reduction in treatment efficiency was also observed by adding some salts representing the ionic strength in soil components [40]. Consequently, treatment of oil compounds from contaminated soil greatly relies on the soil characteristics. Soil pH also plays a critical role in the treatment efficiency of oil pollutants. Mańko et al. showed a change in CMC value and micelle generation because of the pH effect on the surface and interfacial tension of surfactant molecules, which will alter the oil treatment performance [98][119][120][121][98,119,120,121]. At low pH, more H+ ions are present, which makes the soil surface more positively charged, leading to a higher reduction of oil pollutants from the soil [122].3.3.3. Effect of Nanoparticle Properties
The surface area of nanoparticles plays a vital role in the treatment effectiveness of surfactant foam–nanoparticle mixtures. The higher the surface area of nanoparticles, the greater the remediation rate. The larger surface area will lead to more interaction between nanoparticles and oil pollutants. In other words, more oil compounds may be adsorbed, complexed, or reduced on the nanoparticle surface, resulting in higher treatment efficiency [123]. The high specific area also increases the agglomeration of nanoparticles due to their magnetic attraction, which may reduce their reactivity and mobility in soil, subsequently leading to lower remediation efficiency. However, the presence of surfactant foam may act as a stabilizer and inhibit nanoparticle aggregation [34]. The use of 20 nm Fe/Cu nanoparticles showed higher treatment efficiency of oil pollutants in soil than 200 nm Fe/Cu nanoparticles [40]. The interaction of nanoparticles and hydrophilic components of surfactant foam may prevent surfactant foam collapse and enhance foam stability and quality [10][40][124][10,40,124]. The repulsive electrostatic force between nanoparticles and surrounding liquid is improved due to the adsorption of surfactant molecules on the solid–liquid interface, which will lower the surface tension of the mixture, resulting in a change in remediation rate [125]. If the number of nanoparticles exceeds the threshold value, more surfactant molecules will be attracted to the solid–liquid interface. Therefore, fewer surfactant molecules appear at the gas–liquid interface, reducing the cohesive force between surfactant molecules. Consequently, the surfactant foam will collapse, and treatment efficiency will decrease [124]. The increase in nanoparticle quantity may also improve the attractive van der Waals force, decreasing the interfacial tension of surfactant and oil pollutants and affecting the soil remediation rate [126]. The role of biosurfactant foam and nanoparticles on the remediation of oil pollutants in soil is shown in Figure 4, where the oil treatment efficiency by biosurfactant foam/nanoparticle mixture is higher than only biosurfactant foam and only nanoparticles.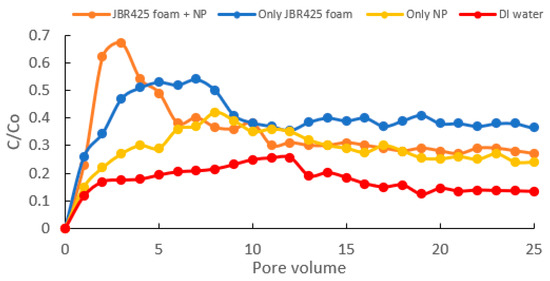
3.3.4. Effect of Surfactant Concentration
The presence of surfactant foam may prevent nanoparticle aggregation, which increases the delivery and transport of nanoparticles in soil, leading to higher treatment efficiency [34][127][34,127]. Surfactant concentration changes the CMC value of the mixture, which may alter and surface and interfacial tension of oil pollutants, resulting in a change in oil treatment efficiency. Moreover, foam quality and stability greatly depend on the surfactant concentration. At pH 7, the combination of 2 vol% rhamnolipid and 2 wt% Fe/Cu nanoparticles displayed high foam stability and quality, leading to a better remediation rate of oil pollutants in soil [34]. Therefore, a suitable amount of surfactant can generate high foam quality, improving the aggregation of nanoparticles at the interface and increasing the interaction of a surfactant foam–nanoparticle mixture with oil pollutants, resulting in high effectiveness.3.4. Limitations
Nanoparticles can penetrate organisms through ingestion or inhalation and cause some negative effects. The toxicity of nanoparticles to humans, animals, and soil microorganisms has raised some public concerns [128][129][128,129]. Some adverse effects are cell membrane damage, respiration interference, and DNA oxidative damage [130][131][130,131]. When nanoparticles enter the cell, they may concentrate at the cell membrane and increase their concentration on the cell surface. Some nanoparticles, such as nZVI or nano-iron oxide, can react with hydrogen peroxide on the cell surface to generate ROS, damaging the cell membrane [132]. In addition, nanoparticles may precipitate on the cell surface through the interaction with lipoteichoic acids in the cell wall, which will block the pores on the outer cell membrane, prevent nutrient transformation, and lead to the death of the cell [133]. However, the potential toxicity of nanoparticles is still controversial and needs more research. Vanzetto and Thome [134] found that nZVI caused no negative effect on the development of bacteria (Bacillus and P. aeruginosa) in pentachlorophenol-contaminated soil during the nanoremediation process. No major change in temperature, electrical conductivity, pH, and humidity of soil was observed after 90 days. Fajardo et al. [135] found no cytotoxicity on Klebsiella planticola bacteria in soil by the high concentration of nZVI. Nanoparticles have little or no adverse influence on the growth of different fungi, such as Trametes versicolor and Aspergillus versicolor. In other studies, nanoparticles caused no significant toxicity on different bacteria, such as P. stutzeri, Klebsiella oxytoca, P. putida, or Escherichia coli under various incubation conditions [132][136][137][138][132,136,137,138]. The resistance mechanisms of bacteria or fungi are mainly due to the limitation of nanoparticle adsorption into the cell by some cell wall components, such as intracellular antioxidants, which decreases the adverse effects of nanoparticles [125][126][125,126]. Chitin cell walls also play a critical role in the low adsorption of nanoparticles, leading to their high resistance to nanoparticles [121][131][121,131].References
- Fate and Transport of Petroleum Hydrocarbons in Soil and Ground Water at Big South Fork National River and Recreation Area, Tennessee and Kentucky. 2002–2003. Available online: https://pubs.er.usgs.gov/publication/sir20055104 (accessed on 11 November 2022).Vu, K.A. Synthesis and Application of Iron/Copper Nanoparticles and Biosurfactants for Remediation of Oil-Contaminated Soil. Ph.D. Dissertation, Concordia University, Montreal, QC, Canada, 27 October 2022.
- Varjani, S.J. Microbial degradation of petroleum hydrocarbons. Bioresour. Technol. 2017, 223, 277–286. Yang, G.; Wang, B.; Vu, K.; Tawfiq, K.; Chen, G. Role of bacterial adhesion in their subsurface deposition and transport: A critical review. Rev. Adhes. Adhes. 2015, 3, 216–252.
- Vu, K.A.; Tawfiq, K.; Chen, G. Rhamnolipid transport in biochar-amended agricultural soil. Water Air Soil Pollut. 2015, 226, 256–264. El-Temsah, Y.S.; Sevcu, A.; Bobcikova, K.; Cernik, M.; Joner, E.J. DDT degradation efficiency and ecotoxicological effects of two types of nano-sized zero-valent iron (nZVI) in water and soil. Chemosphere 2016, 144, 2221–2228.
- Vu, K.A. Rhamnolipid Biosurfactant Adsorption and Transport in Biochar Amended Agricultural Soil. Mater’s Thesis, Florida State University, Tallahassee, FL, USA, 1 November 2013. Ortega-Calvo, J.; Jimenez-Sanchez, C.; Pratarolo, P.; Pullin, H.; Scott, T.B.; Thompson, I.P. Tactic response of bacteria to zero-valent iron nanoparticles. Environ. Pollut. 2016, 213, 438–445.
- Vandana; Priyadarshanee, M.; Mahto, U.; Das, S. Mechanism of toxicity and adverse health effects of environmental pollutants. In Microbial Biodegradation and Bioremediation, 2nd ed.; Das, S., Dash, H.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; Volume 1, pp. 33–53. Vu, K.; Yang, G.; Wang, B.; Tawfiq, K.; Chen, G. Bacterial interactions and transport in geological formation of alumino-silica clays. Colloids Surf. B Biointerfaces 2015, 125, 45–50.
- BP Statistical Review of World Energy. Available online: www.bp.com/statisticalreview (accessed on 16 November 2022).Xu, Q.; Huang, Z.; Ji, S.; Zhou, J.; Shi, R.; Shi, W. Cu2O nanoparticles grafting onto PLA fibers via electron beam irradiation: Bifunctional composite fibers with enhanced photocatalytic of organic pollutants in aqueous and soil systems. J. Radioanal. Nucl. Chem. 2020, 323, 253–261.
- Ogunneye, A.L.; Omoboyowa, D.A.; Sonibare, A.L.; Adebusuyi, A.J.; Faniran, T.P. Hepatotoxic and nephrotoxic effects of petroleum fumes on petrol attendants in Ibadan, Nigeria. Niger. J. Basic Appl. Sci. 2014, 22, 57–62. Xia, S.; Zhang, L.; Zhou, X.; Shao, M.; Pan, G.; Ni, Z. Fabrication of highly dispersed Ti/ZnO-Cr2O3 composite as highly efficient photocatalyst for naphthalene degradation. Appl. Catal. B Environ. 2015, 176, 266–277.
- Treatment Technologies for Site Cleanup: Annual Status Report. Available online: https://www.epa.gov/remedytech/treatment-technologies-site-cleanup-annual-status-report-twelfth-edition (accessed on 16 November 2022).Liao, W.; Ma, Y.; Chen, A.; Yang, Y. Preparation of fatty acids coated Fe3O4 nanoparticles for adsorption and determination of benzo (a) pyrene in environmental water samples. Chem. Eng. J. 2015, 271, 232–239.
- Vu, K.A.; Mulligan, C.N. Synthesis of carbon-based nanomaterials and their use in nanoremediation. In Bio and Nanoremediation of Hazardous Environmental Pollutants, 1st ed.; Taylor & Francis Group: Boca Raton, FL, USA, 2023; accepted. Vu, K.A.; Mulligan, C.N. Synthesis and application of nanoparticles and biosurfactant for oil-contaminated soil removal. In Proceedings of the 73rd Canadian Geotechnical Conference, Calgary, AB, Canada, 14–16 September 2020.
- Zheng, M.; Ahuja, M.; Bhattacharya, D.; Clement, T.P.; Hayworth, J.S.; Dhanasekaran, M. Evaluation of differential cytotoxic effects of the oil spill dispersant Corexit 9500. Life Sci. 2014, 95, 108–117. Zheng, M.; Ahuja, M.; Bhattacharya, D.; Clement, T.P.; Hayworth, J.S.; Dhanasekaran, M. Evaluation of differential cytotoxic effects of the oil spill dispersant Corexit 9500. Life Sci. 2014, 95, 108–117.
- Vázquez-Luna, D. Chronic toxicity of weathered oil-contaminated soil. In Environmental Risk Assessment of Soil Contamination, 1st ed.; Hernamdez-Soriano, C., Ed.; IntechOpen: London, UK, 2014; Volume 1, pp. 8–103. Vázquez-Luna, D. Chronic toxicity of weathered oil-contaminated soil. In Environmental Risk Assessment of Soil Contamination, 1st ed.; Hernamdez-Soriano, C., Ed.; IntechOpen: London, UK, 2014; Volume 1, pp. 8–103.
- Yuanyuan, W.; Qixing, Z.; Shengwei, P.; Lena, Q.M.; Xiaowei, N. Toxic effects of crude-oil-contaminated soil in aquatic environment on Carassius auratus and their hepatic antioxidant defense system. J. Environ. Sci. 2009, 21, 612–617.
- Li, X.; Karakashev, S.I.; Evans, G.M.; Stevenson, P. Effect of environmental humidity on static foam stability. Langmuir 2012, 28, 4060–4068. Li, X.; Karakashev, S.I.; Evans, G.M.; Stevenson, P. Effect of environmental humidity on static foam stability. Langmuir 2012, 28, 4060–4068.
- Mulligan, C.N. Sustainable remediation of contaminated soil using biosurfactants. Front. Bioeng. Biotechnol. 2021, 9, 635196. Mulligan, C.N. Sustainable remediation of contaminated soil using biosurfactants. Front. Bioeng. Biotechnol. 2021, 9, 635196.
- Ossai, I.C.; Ahmed, A.; Hassan, A.; Hamid, F.S. Remediation of soil and water contaminated with petroleum hydrocarbon: A review. Environ. Technol. Innov. 2020, 17, 100526. Ossai, I.C.; Ahmed, A.; Hassan, A.; Hamid, F.S. Remediation of soil and water contaminated with petroleum hydrocarbon: A review. Environ. Technol. Innov. 2020, 17, 100526.
- Zhang, M.; He, F.; Zhao, D.; Hao, X. Degradation of soil-sorbed trichloroethylene by stabilized zero valent iron nanoparticles: Effects of sorption, surfactants, and natural organic matter. Water Res. 2011, 45, 2401–2414. Zhang, M.; He, F.; Zhao, D.; Hao, X. Degradation of soil-sorbed trichloroethylene by stabilized zero valent iron nanoparticles: Effects of sorption, surfactants, and natural organic matter. Water Res. 2011, 45, 2401–2414.
- Chen, X.; Yao, X.; Yu, C.; Su, X.; Shen, C.; Chen, C.; Huang, R.; Xu, X. Hydrodechlorination of polychlorinated biphenyls in contaminated soil from an e-waste recycling area, using nanoscale zerovalent iron and Pd/Fe bimetallic nanoparticles. Environ. Sci. Pollut. Res. 2014, 21, 5201–5210. Chen, X.; Yao, X.; Yu, C.; Su, X.; Shen, C.; Chen, C.; Huang, R.; Xu, X. Hydrodechlorination of polychlorinated biphenyls in contaminated soil from an e-waste recycling area, using nanoscale zerovalent iron and Pd/Fe bimetallic nanoparticles. Environ. Sci. Pollut. Res. 2014, 21, 5201–5210.
- Wang, J.; Chen, Z.; Chen, B. Adsorption of polycyclic aromatic hydrocarbons by graphene and graphene oxide nanosheets. Environ. Sci. Technol. 2014, 48, 4817–4825. Wang, J.; Chen, Z.; Chen, B. Adsorption of polycyclic aromatic hydrocarbons by graphene and graphene oxide nanosheets. Environ. Sci. Technol. 2014, 48, 4817–4825.
- Chaillan, F.; Chaineau, C.H.; Point, V.; Saliot, A.; Oudot, J. Factors inhibiting bioremediation of soil contaminated with weathered oils and drill cuttings. Environ. Pollut. 2006, 144, 255–265. Chaillan, F.; Chaineau, C.H.; Point, V.; Saliot, A.; Oudot, J. Factors inhibiting bioremediation of soil contaminated with weathered oils and drill cuttings. Environ. Pollut. 2006, 144, 255–265.
- Jeong, S.W.; Jeong, J.; Kim, J. Simple surface foam application enhances bioremediation of oil-contaminated soil in cold conditions. J. Hazard. Mater. 2015, 286, 164–170. Jeong, S.W.; Jeong, J.; Kim, J. Simple surface foam application enhances bioremediation of oil-contaminated soil in cold conditions. J. Hazard. Mater. 2015, 286, 164–170.
- Kahraman, B.F.; Altin, A.; Ozdogan, N. Remediation of Pb-diesel fuel co-contaminated soil using nano/bio process: Subsequent use of nanoscale zero-valent iron and bioremediation approaches. Environ. Sci. Pollut. Res. 2022, 29, 41110–41124. Kahraman, B.F.; Altin, A.; Ozdogan, N. Remediation of Pb-diesel fuel co-contaminated soil using nano/bio process: Subsequent use of nanoscale zero-valent iron and bioremediation approaches. Environ. Sci. Pollut. Res. 2022, 29, 41110–41124.
- Ren, H.; Zhou, S.; Wang, B.; Peng, L.; Li, X. Treatment mechanism of sludge containing highly viscous heavy oil using biosurfactant. Colloids Surf. A Physicochem. Eng. Asp. 2020, 585, 124117. Ren, H.; Zhou, S.; Wang, B.; Peng, L.; Li, X. Treatment mechanism of sludge containing highly viscous heavy oil using biosurfactant. Colloids Surf. A Physicochem. Eng. Asp. 2020, 585, 124117.
- Perfumo, A.; Banat, I.M.; Marchant, R.; Vezzulli, L. Thermally enhanced approaches for bioremediation of hydrocarbon-contaminated soils. Chemosphere 2007, 66, 179–184. Perfumo, A.; Banat, I.M.; Marchant, R.; Vezzulli, L. Thermally enhanced approaches for bioremediation of hydrocarbon-contaminated soils. Chemosphere 2007, 66, 179–184.
- Wang, L.; Peng, L.; Xie, L.; Deng, P.; Deng, D. Compatibility of surfactants and thermally activated persulfate for enhanced subsurface remediation. Environ. Sci. Technol. 2017, 51, 7055–7064. Wang, L.; Peng, L.; Xie, L.; Deng, P.; Deng, D. Compatibility of surfactants and thermally activated persulfate for enhanced subsurface remediation. Environ. Sci. Technol. 2017, 51, 7055–7064.
- Zhang, X.; Zhang, X.; Wang, S.; Zhao, S. Improved remediation of co-contaminated soils by heavy metals and PAHs with biosurfactant-enhanced soil washing. Sci. Rep. 2022, 12, 3801. Zhang, X.; Zhang, X.; Wang, S.; Zhao, S. Improved remediation of co-contaminated soils by heavy metals and PAHs with biosurfactant-enhanced soil washing. Sci. Rep. 2022, 12, 3801.
- Huguenot, D.; Mousset, E.; van Hullebusch, E.D.; Oturan, M.A. Combination of surfactant enhanced soil washing and electro-Fenton process for the treatment of soils contaminated by petroleum hydrocarbons. J. Environ. Manag. 2015, 153, 40–47. Huguenot, D.; Mousset, E.; van Hullebusch, E.D.; Oturan, M.A. Combination of surfactant enhanced soil washing and electro-Fenton process for the treatment of soils contaminated by petroleum hydrocarbons. J. Environ. Manag. 2015, 153, 40–47.
- Rongsayamanont, W.; Tongcumpou, C.; Phasukarratchai, N. Diesel-contaminated soil washing by mixed nonionic surfactant emulsion and seed germination test. Water Air Soil Pollut. 2020, 231, 267. Rongsayamanont, W.; Tongcumpou, C.; Phasukarratchai, N. Diesel-contaminated soil washing by mixed nonionic surfactant emulsion and seed germination test. Water Air Soil Pollut. 2020, 231, 267.
- Souza, L.R.R.; Pomarolli, L.C.; da Veiga, M.A.M.S. From classic methodologies to application of nanomaterials for soil remediation: An integrated view of methods for decontamination of toxic metal(oid)s. Environ. Sci. Pollut. Res. 2020, 27, 10205–10227. Souza, L.R.R.; Pomarolli, L.C.; da Veiga, M.A.M.S. From classic methodologies to application of nanomaterials for soil remediation: An integrated view of methods for decontamination of toxic metal(oid)s. Environ. Sci. Pollut. Res. 2020, 27, 10205–10227.
- Pan, B.; Xing, B. Applications and implications of manufactured nanoparticles in soils: A review. Eur. J. Soil Sci. 2012, 63, 437–456. Pan, B.; Xing, B. Applications and implications of manufactured nanoparticles in soils: A review. Eur. J. Soil Sci. 2012, 63, 437–456.
- Nador, F.; Moglie, Y.; Vitale, C.; Yus, M.; Alonso, F.; Radivoy, G. Reduction of polycyclic aromatic hydrocarbons promoted by cobalt or manganese nanoparticles. Tetrahedron 2010, 66, 4318–4325. Nador, F.; Moglie, Y.; Vitale, C.; Yus, M.; Alonso, F.; Radivoy, G. Reduction of polycyclic aromatic hydrocarbons promoted by cobalt or manganese nanoparticles. Tetrahedron 2010, 66, 4318–4325.
- Verma, Y.; Singh, S.K.; Jatav, H.S.; Rajput, V.D.; Minkina, T. Interaction of zinc oxide nanoparticles with soil: Insights into the chemical and biological properties. Environ. Geochem. Health 2022, 44, 221–234. Verma, Y.; Singh, S.K.; Jatav, H.S.; Rajput, V.D.; Minkina, T. Interaction of zinc oxide nanoparticles with soil: Insights into the chemical and biological properties. Environ. Geochem. Health 2022, 44, 221–234.
- Xue, W.; Huang, D.; Zeng, G.; Wan, J.; Zhang, C.; Xu, R.; Cheng, M.; Deng, R. Nanoscale zero-valent iron coated with rhamnolipid as an effective stabilizer for immobilization of Cd and Pb in river sediments. J. Hazard. Mater. 2018, 341, 381–389. Xue, W.; Huang, D.; Zeng, G.; Wan, J.; Zhang, C.; Xu, R.; Cheng, M.; Deng, R. Nanoscale zero-valent iron coated with rhamnolipid as an effective stabilizer for immobilization of Cd and Pb in river sediments. J. Hazard. Mater. 2018, 341, 381–389.
- Huang, H.; Chen, J.; Liu, S.; Pu, S. Impact of ZnO nanoparticles on soil lead bioavailability and microbial properties. Sci. Total Environ. 2022, 806, 150299. Huang, H.; Chen, J.; Liu, S.; Pu, S. Impact of ZnO nanoparticles on soil lead bioavailability and microbial properties. Sci. Total Environ. 2022, 806, 150299.
- Vu, K.A.; Mulligan, C.N. Utilization of a biosurfactant foam/nanoparticle mixture for treatment of oil pollutants in soil. Environ. Sci. Pollut. Res. 2022, 29, 88618–88629. Vu, K.A.; Mulligan, C.N. Utilization of a biosurfactant foam/nanoparticle mixture for treatment of oil pollutants in soil. Environ. Sci. Pollut. Res. 2022, 29, 88618–88629.
- Vu, K.A.; Mulligan, C.N. Oil removal from contaminated soil by biosurfactants and Fe/Cu nanoparticles. In Proceedings of the Canadian Society for Civil Engineering Annual Conference, Whistler, BC, Canada, 25–28 May 2022.
- Vu, K.A.; Mulligan, C.N. Preparation of a biosurfactant foam/nanoparticle mixture for removing the oil from soil. In Proceedings of the 44th AMOP Technical Seminar on Environmental Contamination and Response, Edmonton, AB, Canada, 7–9 June 2022.
- Kumar, R.; Raizada, P.; Khan, A.A.P.; Nguyen, V.; Van Le, Q.; Ghotekar, S.; Selvasembian, R.; Gandhi, V.; Singh, A.; Singh, P. Recent progress in emerging BiPO4-based photocatalysts: Synthesis, properties, modification strategies, and photocatalytic applications. J. Mater. Sci. Technol. 2022, 108, 208–225. Kumar, R.; Raizada, P.; Khan, A.A.P.; Nguyen, V.; Van Le, Q.; Ghotekar, S.; Selvasembian, R.; Gandhi, V.; Singh, A.; Singh, P. Recent progress in emerging BiPO4-based photocatalysts: Synthesis, properties, modification strategies, and photocatalytic applications. J. Mater. Sci. Technol. 2022, 108, 208–225.
- Vu, K.A.; Mulligan, C.N. Remediation of Organic Contaminated Soil by Fe-Based Nanoparticles and Surfactants: A review. Environ. Technol. Rev. 2022, accepted.
- Chen, X.; Chen, B. Macroscopic and spectroscopic investigations of the adsorption of nitroaromatic compounds on graphene oxide, reduced graphene oxide, and graphene nanosheets. Environ. Sci. Technol. 2015, 49, 6181–6189. Chen, X.; Chen, B. Macroscopic and spectroscopic investigations of the adsorption of nitroaromatic compounds on graphene oxide, reduced graphene oxide, and graphene nanosheets. Environ. Sci. Technol. 2015, 49, 6181–6189.
- Vu, K.A.; Mulligan, C.N. Remediation of oil-contaminated soil using Fe/Cu nanoparticles and biosurfactants. Environ. Technol. 2022, 44, 1–13. Vu, K.A.; Mulligan, C.N. Remediation of oil-contaminated soil using Fe/Cu nanoparticles and biosurfactants. Environ. Technol. 2022, 44, 1–13.
- Gupta, H.; Kumar, R.; Park, H.; Jeon, B. Photocatalytic efficiency of iron oxide nanoparticles for the degradation of priority pollutant anthracene. Geosyst. Eng. 2017, 20, 21–27. Gupta, H.; Kumar, R.; Park, H.; Jeon, B. Photocatalytic efficiency of iron oxide nanoparticles for the degradation of priority pollutant anthracene. Geosyst. Eng. 2017, 20, 21–27.
- Konnova, S.A.; Lvov, Y.M.; Fakhrullin, R.F. Nanoshell assembly for magnet-responsive oil-degrading bacteria. Langmuir 2016, 32, 12552–12558. Konnova, S.A.; Lvov, Y.M.; Fakhrullin, R.F. Nanoshell assembly for magnet-responsive oil-degrading bacteria. Langmuir 2016, 32, 12552–12558.
- Barzegar, G.; Jorfi, S.; Soltani, R.D.C.; Ahmadi, M.; Saeedi, R.; Abtahi, M.; Ramavandi, B.; Baboli, Z. Enhanced Sono-Fenton-like oxidation of PAH-contaminated soil using Nano-sized magnetite as catalyst: Optimization with response surface methodology. Soil Sediment Contam. Int. J. 2017, 26, 538–557. Barzegar, G.; Jorfi, S.; Soltani, R.D.C.; Ahmadi, M.; Saeedi, R.; Abtahi, M.; Ramavandi, B.; Baboli, Z. Enhanced Sono-Fenton-like oxidation of PAH-contaminated soil using Nano-sized magnetite as catalyst: Optimization with response surface methodology. Soil Sediment Contam. Int. J. 2017, 26, 538–557.
- Hou, L.; Wang, L.; Royer, S.; Zhang, H. Ultrasound-assisted heterogeneous Fenton-like degradation of tetracycline over a magnetite catalyst. J. Hazard. Mater. 2016, 302, 458–467. Hou, L.; Wang, L.; Royer, S.; Zhang, H. Ultrasound-assisted heterogeneous Fenton-like degradation of tetracycline over a magnetite catalyst. J. Hazard. Mater. 2016, 302, 458–467.
- Bai, H.; Zhou, J.; Zhang, H.; Tang, G. Enhanced adsorbability and photocatalytic activity of TiO2-graphene composite for polycyclic aromatic hydrocarbons removal in aqueous phase. Colloids Surf. B Biointerfaces 2017, 150, 68–77. Bai, H.; Zhou, J.; Zhang, H.; Tang, G. Enhanced adsorbability and photocatalytic activity of TiO2-graphene composite for polycyclic aromatic hydrocarbons removal in aqueous phase. Colloids Surf. B Biointerfaces 2017, 150, 68–77.
- Marquès, M.; Mari, M.; Audí-Miró, C.; Sierra, J.; Soler, A.; Nadal, M.; Domingo, J.L. Photodegradation of polycyclic aromatic hydrocarbons in soils under a climate change base scenario. Chemosphere 2016, 148, 495–503. Marquès, M.; Mari, M.; Audí-Miró, C.; Sierra, J.; Soler, A.; Nadal, M.; Domingo, J.L. Photodegradation of polycyclic aromatic hydrocarbons in soils under a climate change base scenario. Chemosphere 2016, 148, 495–503.
- Li, S.; Anderson, T.A.; Green, M.J.; Maul, J.D.; Cañas-Carrell, J.E. Polyaromatic hydrocarbons (PAHs) sorption behavior unaffected by the presence of multi-walled carbon nanotubes (MWNTs) in a natural soil system. Environ. Sci. Process. Impacts 2013, 15, 1130–1136. Li, S.; Anderson, T.A.; Green, M.J.; Maul, J.D.; Cañas-Carrell, J.E. Polyaromatic hydrocarbons (PAHs) sorption behavior unaffected by the presence of multi-walled carbon nanotubes (MWNTs) in a natural soil system. Environ. Sci. Process. Impacts 2013, 15, 1130–1136.
- El-Temsah, Y.S.; Joner, E.J. Effects of nano-sized zero-valent iron (nZVI) on DDT degradation in soil and its toxicity to collembola and ostracods. Chemosphere 2013, 92, 131–137. El-Temsah, Y.S.; Joner, E.J. Effects of nano-sized zero-valent iron (nZVI) on DDT degradation in soil and its toxicity to collembola and ostracods. Chemosphere 2013, 92, 131–137.
- Yu, K.; Sheng, G.D.; McCall, W. Cosolvent effects on dechlorination of soil-sorbed polychlorinated biphenyls using bentonite clay-templated nanoscale zero valent iron. Environ. Sci. Technol. 2016, 50, 12949–12956. Yu, K.; Sheng, G.D.; McCall, W. Cosolvent effects on dechlorination of soil-sorbed polychlorinated biphenyls using bentonite clay-templated nanoscale zero valent iron. Environ. Sci. Technol. 2016, 50, 12949–12956.
- Jorfi, S.; Rezaee, A.; Moheb-Ali, G. Pyrene removal from contaminated soils by modified Fenton oxidation using iron nano particles. J. Environ. Health Sci. Eng. 2013, 11, 17. Jorfi, S.; Rezaee, A.; Moheb-Ali, G. Pyrene removal from contaminated soils by modified Fenton oxidation using iron nano particles. J. Environ. Health Sci. Eng. 2013, 11, 17.
- Karam, F.F.; Hussein, F.H.; Baqir, S.J.; Halbus, A.F.; Dillert, R.; Bahnemann, D. Photocatalytic degradation of anthracene in closed system reactor. Int. J. Photoenergy 2014, 2014, 503825. Karam, F.F.; Hussein, F.H.; Baqir, S.J.; Halbus, A.F.; Dillert, R.; Bahnemann, D. Photocatalytic degradation of anthracene in closed system reactor. Int. J. Photoenergy 2014, 2014, 503825.
- Gu, H.; Hu, K.; Li, D.; Long, Y. SERS detection of polycyclic aromatic hydrocarbons using a bare gold nanoparticles coupled film system. Analyst 2016, 141, 4359–4365. Gu, H.; Hu, K.; Li, D.; Long, Y. SERS detection of polycyclic aromatic hydrocarbons using a bare gold nanoparticles coupled film system. Analyst 2016, 141, 4359–4365.
- Shanker, U.; Jassal, V.; Rani, M. Green synthesis of iron hexacyanoferrate nanoparticles: Potential candidate for the degradation of toxic PAHs. J. Environ. Chem. Eng. 2017, 5, 4108–4120. Shanker, U.; Jassal, V.; Rani, M. Green synthesis of iron hexacyanoferrate nanoparticles: Potential candidate for the degradation of toxic PAHs. J. Environ. Chem. Eng. 2017, 5, 4108–4120.
- Sannino, F.; Pirozzi, D.; Vitiello, G.; D’errico, G.; Aronne, A.; Fanelli, E.; Pernice, P. Oxidative degradation of phenanthrene in the absence of light irradiatiion by hybrid ZrO2-acetylacetonate gel-derived catalyst. Appl. Catal. B Environ. 2014, 156, 101–107. Sannino, F.; Pirozzi, D.; Vitiello, G.; D’errico, G.; Aronne, A.; Fanelli, E.; Pernice, P. Oxidative degradation of phenanthrene in the absence of light irradiatiion by hybrid ZrO2-acetylacetonate gel-derived catalyst. Appl. Catal. B Environ. 2014, 156, 101–107.
- Zhao, S.; Jia, H.; Nulaji, G.; Gao, H.; Wang, F.; Wang, C. Photolysis of polycyclic aromatic hydrocarbons (PAHs) on Fe3+-montmorillonite surface under visible light: Degradation kinetics, mechanism, and toxicity assessments. Chemosphere 2017, 184, 1346–1354. Zhao, S.; Jia, H.; Nulaji, G.; Gao, H.; Wang, F.; Wang, C. Photolysis of polycyclic aromatic hydrocarbons (PAHs) on Fe3+-montmorillonite surface under visible light: Degradation kinetics, mechanism, and toxicity assessments. Chemosphere 2017, 184, 1346–1354.
- Dong, D.; Li, P.; Li, X.; Zhao, Q.; Zhang, Y.; Jia, C.; Li, P. Investigation on the photocatalytic degradation of pyrene on soil surfaces using nanometer anatase TiO2 under UV irradiation. J. Hazard. Mater. 2010, 174, 859–863. Dong, D.; Li, P.; Li, X.; Zhao, Q.; Zhang, Y.; Jia, C.; Li, P. Investigation on the photocatalytic degradation of pyrene on soil surfaces using nanometer anatase TiO2 under UV irradiation. J. Hazard. Mater. 2010, 174, 859–863.
- Moussawi, R.N.; Patra, D. Nanoparticle self-assembled grain like curcumin conjugated ZnO: Curcumin conjugation enhances removal of perylene, fluoranthene and chrysene by ZnO. Sci. Rep. 2016, 6, 24565. Moussawi, R.N.; Patra, D. Nanoparticle self-assembled grain like curcumin conjugated ZnO: Curcumin conjugation enhances removal of perylene, fluoranthene and chrysene by ZnO. Sci. Rep. 2016, 6, 24565.
- Xia, S.; Zhang, L.; Zhou, X.; Shao, M.; Pan, G.; Ni, Z. Fabrication of highly dispersed Ti/ZnO-Cr2O3 composite as highly efficient photocatalyst for naphthalene degradation. Appl. Catal. B Environ. 2015, 176, 266–277. Xia, S.; Zhang, L.; Zhou, X.; Shao, M.; Pan, G.; Ni, Z. Fabrication of highly dispersed Ti/ZnO-Cr2O3 composite as highly efficient photocatalyst for naphthalene degradation. Appl. Catal. B Environ. 2015, 176, 266–277.
- Liao, W.; Ma, Y.; Chen, A.; Yang, Y. Preparation of fatty acids coated Fe3O4 nanoparticles for adsorption and determination of benzo (a) pyrene in environmental water samples. Chem. Eng. J. 2015, 271, 232–239. Liao, W.; Ma, Y.; Chen, A.; Yang, Y. Preparation of fatty acids coated Fe3O4 nanoparticles for adsorption and determination of benzo (a) pyrene in environmental water samples. Chem. Eng. J. 2015, 271, 232–239.
- Vu, K.A.; Mulligan, C.N. Synthesis and application of nanoparticles and biosurfactant for oil-contaminated soil removal. In Proceedings of the 73rd Canadian Geotechnical Conference, Calgary, AB, Canada, 14–16 September 2020. Vu, K.A.; Mulligan, C.N. Synthesis and application of nanoparticles and biosurfactant for oil-contaminated soil removal. In Proceedings of the 73rd Canadian Geotechnical Conference, Calgary, AB, Canada, 14–16 September 2020.
- Xia, X.; Li, Y.; Zhou, Z.; Feng, C. Bioavailability of adsorbed phenanthrene by black carbon and multi-walled carbon nanotubes to Agrobacterium. Chemosphere 2010, 78, 1329–1336. Xia, X.; Li, Y.; Zhou, Z.; Feng, C. Bioavailability of adsorbed phenanthrene by black carbon and multi-walled carbon nanotubes to Agrobacterium. Chemosphere 2010, 78, 1329–1336.
- Wang, X.; Tao, S.; Xing, B. Sorption and competition of aromatic compounds and humic acid on multiwalled carbon nanotubes. Environ. Sci. Technol. 2009, 43, 6214–6219. Wang, X.; Tao, S.; Xing, B. Sorption and competition of aromatic compounds and humic acid on multiwalled carbon nanotubes. Environ. Sci. Technol. 2009, 43, 6214–6219.
- Kuang, Y.; Zhou, Y.; Chen, Z.; Megharaj, M.; Naidu, R. Impact of Fe and Ni/Fe nanoparticles on biodegradation of phenol by the strain Bacillus fusiformis (BFN) at various pH values. Bioresour. Technol. 2013, 136, 588–594. Kuang, Y.; Zhou, Y.; Chen, Z.; Megharaj, M.; Naidu, R. Impact of Fe and Ni/Fe nanoparticles on biodegradation of phenol by the strain Bacillus fusiformis (BFN) at various pH values. Bioresour. Technol. 2013, 136, 588–594.
- Varanasi, P.; Fullana, A.; Sidhu, S. Remediation of PCB contaminated soils using iron nano-particles. Chemosphere 2007, 66, 1031–1038. Varanasi, P.; Fullana, A.; Sidhu, S. Remediation of PCB contaminated soils using iron nano-particles. Chemosphere 2007, 66, 1031–1038.
- Chang, M.; Shu, H.; Hsieh, W.; Wang, M. Remediation of soil contaminated with pyrene using ground nanoscale zero-valent iron. J. Air Waste Manag. Assoc. 2007, 57, 221–227. Chang, M.; Shu, H.; Hsieh, W.; Wang, M. Remediation of soil contaminated with pyrene using ground nanoscale zero-valent iron. J. Air Waste Manag. Assoc. 2007, 57, 221–227.
- Tungittiplakorn, W.; Lion, L.W.; Cohen, C.; Kim, J. Engineered polymeric nanoparticles for soil remediation. Environ. Sci. Technol. 2004, 38, 1605–1610. Tungittiplakorn, W.; Lion, L.W.; Cohen, C.; Kim, J. Engineered polymeric nanoparticles for soil remediation. Environ. Sci. Technol. 2004, 38, 1605–1610.
- Gil-Díaz, M.; Pérez, R.A.; Alonso, J.; Miguel, E.; Diez-Pascual, S.; Lobo, M.C. Iron nanoparticles to recover a co-contaminated soil with Cr and PCBs. Sci. Rep. 2022, 12, 3541. Gil-Díaz, M.; Pérez, R.A.; Alonso, J.; Miguel, E.; Diez-Pascual, S.; Lobo, M.C. Iron nanoparticles to recover a co-contaminated soil with Cr and PCBs. Sci. Rep. 2022, 12, 3541.
- Zhu, Y.; Ji, S.; Liang, W.; Li, C.; Nie, Y.; Dong, J.; Shi, W.; Ai, S. A low-cost and eco-friendly powder catalyst: Iron and copper nanoparticles supported on biochar/geopolymer for activating potassium peroxymonosulfate to degrade naphthalene in water and soil. Chemosphere 2022, 303, 135185. Zhu, Y.; Ji, S.; Liang, W.; Li, C.; Nie, Y.; Dong, J.; Shi, W.; Ai, S. A low-cost and eco-friendly powder catalyst: Iron and copper nanoparticles supported on biochar/geopolymer for activating potassium peroxymonosulfate to degrade naphthalene in water and soil. Chemosphere 2022, 303, 135185.
- Dong, D.; Li, P.; Li, X.; Xu, C.; Gong, D.; Zhang, Y.; Zhao, Q.; Li, P. Photocatalytic degradation of phenanthrene and pyrene on soil surfaces in the presence of nanometer rutile TiO2 under UV-irradiation. Chem. Eng. J. 2010, 158, 378–383. Dong, D.; Li, P.; Li, X.; Xu, C.; Gong, D.; Zhang, Y.; Zhao, Q.; Li, P. Photocatalytic degradation of phenanthrene and pyrene on soil surfaces in the presence of nanometer rutile TiO2 under UV-irradiation. Chem. Eng. J. 2010, 158, 378–383.
- Gupta, H.; Gupta, B. Photocatalytic degradation of polycyclic aromatic hydrocarbon benzo pyrene by iron oxides and identification of degradation products. Chemosphere 2015, 138, 924–931. Gupta, H.; Gupta, B. Photocatalytic degradation of polycyclic aromatic hydrocarbon benzo [a] pyrene by iron oxides and identification of degradation products. Chemosphere 2015, 138, 924–931.
- Gupta, H. Photocatalytic degradation of phenanthrene in the presence of akaganeite nano-rods and the identification of degradation products. RSC Adv. 2016, 6, 112721–112727. Gupta, H. Photocatalytic degradation of phenanthrene in the presence of akaganeite nano-rods and the identification of degradation products. RSC Adv. 2016, 6, 112721–112727.
- Yang, X.; Cai, H.; Bao, M.; Yu, J.; Lu, J.; Li, Y. Insight into the highly efficient degradation of PAHs in water over graphene oxide/Ag3PO4 composites under visible light irradiation. Chem. Eng. J. 2018, 334, 355–376. Yang, X.; Cai, H.; Bao, M.; Yu, J.; Lu, J.; Li, Y. Insight into the highly efficient degradation of PAHs in water over graphene oxide/Ag3PO4 composites under visible light irradiation. Chem. Eng. J. 2018, 334, 355–376.
- Theerakarunwong, C.D.; Phanichphant, S. Visible-light-induced photocatalytic degradation of PAH-contaminated soil and their pathways by Fe-doped TiO2 nanocatalyst. Water Air Soil Pollut. 2018, 229, 291. Theerakarunwong, C.D.; Phanichphant, S. Visible-light-induced photocatalytic degradation of PAH-contaminated soil and their pathways by Fe-doped TiO2 nanocatalyst. Water Air Soil Pollut. 2018, 229, 291.
- Rachna; Rani, M.; Shanker, U. Degradation of tricyclic polyaromatic hydrocarbons in water, soil and river sediment with a novel TiO2 based heterogeneous nanocomposite. J. Environ. Manag. 2019, 248, 109340. Rachna; Rani, M.; Shanker, U. Degradation of tricyclic polyaromatic hydrocarbons in water, soil and river sediment with a novel TiO2 based heterogeneous nanocomposite. J. Environ. Manag. 2019, 248, 109340.
- Wang, J.; Luo, Z.; Song, Y.; Zheng, X.; Qu, L.; Qian, J.; Wu, Y.; Wu, X.; Wu, Z. Remediation of phenanthrene contaminated soil by g-C3N4/Fe3O4 composites and its phytotoxicity evaluation. Chemosphere 2019, 221, 554–562. Wang, J.; Luo, Z.; Song, Y.; Zheng, X.; Qu, L.; Qian, J.; Wu, Y.; Wu, X.; Wu, Z. Remediation of phenanthrene contaminated soil by g-C3N4/Fe3O4 composites and its phytotoxicity evaluation. Chemosphere 2019, 221, 554–562.
- Xu, Q.; Huang, Z.; Ji, S.; Zhou, J.; Shi, R.; Shi, W. Cu2O nanoparticles grafting onto PLA fibers via electron beam irradiation: Bifunctional composite fibers with enhanced photocatalytic of organic pollutants in aqueous and soil systems. J. Radioanal. Nucl. Chem. 2020, 323, 253–261. Xu, Q.; Huang, Z.; Ji, S.; Zhou, J.; Shi, R.; Shi, W. Cu2O nanoparticles grafting onto PLA fibers via electron beam irradiation: Bifunctional composite fibers with enhanced photocatalytic of organic pollutants in aqueous and soil systems. J. Radioanal. Nucl. Chem. 2020, 323, 253–261.
- Kumari, B.; Singh, D.P. A review on multifaceted application of nanoparticles in the field of bioremediation of petroleum hydrocarbons. Ecol. Eng. 2016, 97, 98–105. Kumari, B.; Singh, D.P. A review on multifaceted application of nanoparticles in the field of bioremediation of petroleum hydrocarbons. Ecol. Eng. 2016, 97, 98–105.
- Khatoon, H.; Rai, J.P.N. Optimization studies on biodegradation of atrazine by Bacillus badius ABP6 strain using response surface methodology. Biotechnol. Rep. 2020, 26, e00459. Khatoon, H.; Rai, J.P.N. Optimization studies on biodegradation of atrazine by Bacillus badius ABP6 strain using response surface methodology. Biotechnol. Rep. 2020, 26, e00459.
- Bebić, J.; Banjanac, K.; Ćorović, M.; Milivojević, A.; Simović, M.; Marinković, A.; Bezbradica, D. Immobilization of laccase from Myceliophthora thermophila on functionalized silica nanoparticles: Optimization and application in lindane degradation. Chin. J. Chem. Eng. 2020, 28, 1136–1144. Bebić, J.; Banjanac, K.; Ćorović, M.; Milivojević, A.; Simović, M.; Marinković, A.; Bezbradica, D. Immobilization of laccase from Myceliophthora thermophila on functionalized silica nanoparticles: Optimization and application in lindane degradation. Chin. J. Chem. Eng. 2020, 28, 1136–1144.
- Simjoo, M.; Rezaei, T.; Andrianov, A.; Zitha, P.L.J. Foam stability in the presence of oil: Effect of surfactant concentration and oil type. Colloid. Surf. A Physicochem. Eng. Asp. 2013, 438, 148–158. Vu, K.A. Synthesis and Application of Iron/Copper Nanoparticles and Biosurfactants for Remediation of Oil-Contaminated Soil. Ph.D. Dissertation, Concordia University, Montreal, QC, Canada, 27 October 2022.
- Schramm, L.L.; Novosad, J.J. Micro-visualization of foam interactions with a crude oil. Colloids Surf. 1990, 46, 21–43. Schramm, L.L.; Novosad, J.J. Micro-visualization of foam interactions with a crude oil. Colloids Surf. 1990, 46, 21–43.
- Hurtado, Y.; Beltrán, C.; Zabala, R.D.; Lopera, S.H.; Franco, C.A.; Nassar, N.N.; Cortés, F.B. Effects of surface acidity and polarity of SiO2 nanoparticles on the foam stabilization applied to natural gas flooding in tight gas-condensate reservoirs. Energy Fuels 2018, 32, 5824–5833. Hurtado, Y.; Beltrán, C.; Zabala, R.D.; Lopera, S.H.; Franco, C.A.; Nassar, N.N.; Cortés, F.B. Effects of surface acidity and polarity of SiO2 nanoparticles on the foam stabilization applied to natural gas flooding in tight gas-condensate reservoirs. Energy Fuels 2018, 32, 5824–5833.
- Binks, B.P. Colloidal particles at a range of fluid–fluid interfaces. Langmuir 2017, 33, 6947–6963. Binks, B.P. Colloidal particles at a range of fluid–fluid interfaces. Langmuir 2017, 33, 6947–6963.
- Horozov, T.S.; Aveyard, R.; Clint, J.H.; Neumann, B. Particle zips: Vertical emulsion films with particle monolayers at their surfaces. Langmuir 2005, 21, 2330–2341. Horozov, T.S.; Aveyard, R.; Clint, J.H.; Neumann, B. Particle zips: Vertical emulsion films with particle monolayers at their surfaces. Langmuir 2005, 21, 2330–2341.
- Xie, W.; Vu, K.; Yang, G.; Tawfiq, K.; Chen, G. Escherichia coli growth and transport in the presence of nanosilver under variable growth conditions. Environ. Technol. 2014, 35, 2306–2313. Xie, W.; Vu, K.; Yang, G.; Tawfiq, K.; Chen, G. Escherichia coli growth and transport in the presence of nanosilver under variable growth conditions. Environ. Technol. 2014, 35, 2306–2313.
- Gonzenbach, U.T.; Studart, A.R.; Tervoort, E.; Gauckler, L.J. Stabilization of foams with inorganic colloidal particles. Langmuir 2006, 22, 10983–10988. Gonzenbach, U.T.; Studart, A.R.; Tervoort, E.; Gauckler, L.J. Stabilization of foams with inorganic colloidal particles. Langmuir 2006, 22, 10983–10988.
- Sun, Q.; Li, Z.; Wang, J.; Li, S.; Li, B.; Jiang, L.; Wang, H.; Lü, Q.; Zhang, C.; Liu, W. Aqueous foam stabilized by partially hydrophobic nanoparticles in the presence of surfactant. Colloids Surf. A Physicochem. Eng. Asp. 2015, 471, 54–64. Sun, Q.; Li, Z.; Wang, J.; Li, S.; Li, B.; Jiang, L.; Wang, H.; Lü, Q.; Zhang, C.; Liu, W. Aqueous foam stabilized by partially hydrophobic nanoparticles in the presence of surfactant. Colloids Surf. A Physicochem. Eng. Asp. 2015, 471, 54–64.
- Li, Q.; Prigiobbe, V. Measuring and modeling nanoparticle transport by foam in porous media. J. Contam. Hydrol. 2021, 243, 103881. Li, Q.; Prigiobbe, V. Measuring and modeling nanoparticle transport by foam in porous media. J. Contam. Hydrol. 2021, 243, 103881.
- Li, Q.; Prigiobbe, V. Studying the generation of foam in the presence of nanoparticles using a microfluidic system. Chem. Eng. Sci. 2020, 215, 115427. Li, Q.; Prigiobbe, V. Studying the generation of foam in the presence of nanoparticles using a microfluidic system. Chem. Eng. Sci. 2020, 215, 115427.
- Oliveira, R.C.; Oliveira, J.F.; Moudgil, B.M. The effect of hydrophobic fine particles on the foam flushing remediation process. Surf. Colloid Sci. 2004, 128, 293–297. Oliveira, R.C.; Oliveira, J.F.; Moudgil, B.M. The effect of hydrophobic fine particles on the foam flushing remediation process. Surf. Colloid Sci. 2004, 128, 293–297.
- Garbin, V.; Jenkins, I.; Sinno, T.; Crocker, J.C.; Stebe, K.J. Interactions and stress relaxation in monolayers of soft nanoparticles at fluid-fluid interfaces. Phys. Rev. Lett. 2015, 114, 108301. Garbin, V.; Jenkins, I.; Sinno, T.; Crocker, J.C.; Stebe, K.J. Interactions and stress relaxation in monolayers of soft nanoparticles at fluid-fluid interfaces. Phys. Rev. Lett. 2015, 114, 108301.
- Zargartalebi, M.; Kharrat, R.; Barati, N. Enhancement of surfactant flooding performance by the use of silica nanoparticles. Fuel 2015, 143, 21–27. Zargartalebi, M.; Kharrat, R.; Barati, N. Enhancement of surfactant flooding performance by the use of silica nanoparticles. Fuel 2015, 143, 21–27.
- Yu, H.; He, Y.; Li, P.; Li, S.; Zhang, T.; Rodriguez-Pin, E.; Du, S.; Wang, C.; Cheng, S.; Bielawski, C.W. Flow enhancement of water-based nanoparticle dispersion through microscale sedimentary rocks. Sci. Rep. 2015, 5, 8702. Yu, H.; He, Y.; Li, P.; Li, S.; Zhang, T.; Rodriguez-Pin, E.; Du, S.; Wang, C.; Cheng, S.; Bielawski, C.W. Flow enhancement of water-based nanoparticle dispersion through microscale sedimentary rocks. Sci. Rep. 2015, 5, 8702.
- Shearer, L.T.; Akers, W.W. Foam stability. J. Phys. Chem. 1958, 62, 1264–1268. Shearer, L.T.; Akers, W.W. Foam stability. J. Phys. Chem. 1958, 62, 1264–1268.
- Ju, B.; Fan, T.; Ma, M. Enhanced oil recovery by flooding with hydrophilic nanoparticles. China Particuol. 2006, 4, 41–46. Ju, B.; Fan, T.; Ma, M. Enhanced oil recovery by flooding with hydrophilic nanoparticles. China Particuol. 2006, 4, 41–46.
- Otto, M.; Floyd, M.; Bajpai, S. Nanotechnology for site remediation. Remediat. J. Environ. Cleanup Costs Technol. Tech. 2008, 19, 99–108. Otto, M.; Floyd, M.; Bajpai, S. Nanotechnology for site remediation. Remediat. J. Environ. Cleanup Costs Technol. Tech. 2008, 19, 99–108.
- Worthen, A.J.; Bagaria, H.G.; Chen, Y.; Bryant, S.L.; Huh, C.; Johnston, K.P. Nanoparticle-stabilized carbon dioxide-in-water foams with fine texture. J. Colloid Interface Sci. 2013, 391, 142–151. Worthen, A.J.; Bagaria, H.G.; Chen, Y.; Bryant, S.L.; Huh, C.; Johnston, K.P. Nanoparticle-stabilized carbon dioxide-in-water foams with fine texture. J. Colloid Interface Sci. 2013, 391, 142–151.
- Lv, Q.; Li, Z.; Li, B.; Li, S.; Sun, Q. Study of nanoparticle-surfactant-stabilized foam as a fracturing fluid. Ind. Eng. Chem. Res. 2015, 54, 9468–9477. Lv, Q.; Li, Z.; Li, B.; Li, S.; Sun, Q. Study of nanoparticle-surfactant-stabilized foam as a fracturing fluid. Ind. Eng. Chem. Res. 2015, 54, 9468–9477.
- Zheng, X.; Jang, J. Hydraulic properties of porous media saturated with nanoparticle-stabilized air-water foam. Sustainability 2016, 8, 1317. Zheng, X.; Jang, J. Hydraulic properties of porous media saturated with nanoparticle-stabilized air-water foam. Sustainability 2016, 8, 1317.
- Ding, Y.; Liu, B.; Shen, X.; Zhong, L.; Li, X. Foam-assisted delivery of nanoscale zero valent iron in porous media. J. Environ. Eng. 2013, 139, 1206–1212. Ding, Y.; Liu, B.; Shen, X.; Zhong, L.; Li, X. Foam-assisted delivery of nanoscale zero valent iron in porous media. J. Environ. Eng. 2013, 139, 1206–1212.
- Shen, X.; Zhao, L.; Ding, Y.; Liu, B.; Zeng, H.; Zhong, L.; Li, X. Foam, a promising vehicle to deliver nanoparticles for vadose zone remediation. J. Hazard. Mater. 2011, 186, 1773–1780. Shen, X.; Zhao, L.; Ding, Y.; Liu, B.; Zeng, H.; Zhong, L.; Li, X. Foam, a promising vehicle to deliver nanoparticles for vadose zone remediation. J. Hazard. Mater. 2011, 186, 1773–1780.
- Chattopadhyay, P.; Karthick, R.A. Characterization and Application of Surfactant Foams Produced from Ethanol-Sodium Lauryl Sulfate-Silica Nanoparticle Mixture for Soil Remediation. Macromol. Symp. 2017, 376, 1600182. Chattopadhyay, P.; Karthick, R.A. Characterization and Application of Surfactant Foams Produced from Ethanol-Sodium Lauryl Sulfate-Silica Nanoparticle Mixture for Soil Remediation. Macromol. Symp. 2017, 376, 1600182.
- Karthick, R.A.; Chattopadhyay, P. Remediation of diesel contaminated soil by tween-20 foam stabilized by silica nanoparticles. Int. J. Chem. Eng. Appl. 2017, 8, 194–198. Karthick, R.A.; Chattopadhyay, P. Remediation of diesel contaminated soil by tween-20 foam stabilized by silica nanoparticles. Int. J. Chem. Eng. Appl. 2017, 8, 194–198.
- Quinn, J.; Geiger, C.; Clausen, C.; Brooks, K.; Coon, C.; O’Hara, S.; Krug, T.; Major, D.; Yoon, W.; Gavaskar, A. Field demonstration of DNAPL dehalogenation using emulsified zero-valent iron. Environ. Sci. Technol. 2005, 39, 1309–1318. Quinn, J.; Geiger, C.; Clausen, C.; Brooks, K.; Coon, C.; O’Hara, S.; Krug, T.; Major, D.; Yoon, W.; Gavaskar, A. Field demonstration of DNAPL dehalogenation using emulsified zero-valent iron. Environ. Sci. Technol. 2005, 39, 1309–1318.
- Zhao, X.; Liu, W.; Cai, Z.; Han, B.; Qian, T.; Zhao, D. An overview of preparation and applications of stabilized zero-valent iron nanoparticles for soil and groundwater remediation. Water Res. 2016, 100, 245–266. Zhao, X.; Liu, W.; Cai, Z.; Han, B.; Qian, T.; Zhao, D. An overview of preparation and applications of stabilized zero-valent iron nanoparticles for soil and groundwater remediation. Water Res. 2016, 100, 245–266.
- He, F.; Zhao, D.; Paul, C. Field assessment of carboxymethyl cellulose stabilized iron nanoparticles for in situ destruction of chlorinated solvents in source zones. Water Res. 2010, 44, 2360–2370. He, F.; Zhao, D.; Paul, C. Field assessment of carboxymethyl cellulose stabilized iron nanoparticles for in situ destruction of chlorinated solvents in source zones. Water Res. 2010, 44, 2360–2370.
- Bennett, P.; He, F.; Zhao, D.; Aiken, B.; Feldman, L. In situ testing of metallic iron nanoparticle mobility and reactivity in a shallow granular aquifer. J. Contam. Hydrol. 2010, 116, 35–46. Bennett, P.; He, F.; Zhao, D.; Aiken, B.; Feldman, L. In situ testing of metallic iron nanoparticle mobility and reactivity in a shallow granular aquifer. J. Contam. Hydrol. 2010, 116, 35–46.
- Scott, M.J.; Jones, M.N. The biodegradation of surfactants in the environment. Biochim. Biophys. Acta BBA Biomembr. 2000, 1508, 235–251. Scott, M.J.; Jones, M.N. The biodegradation of surfactants in the environment. Biochim. Biophys. Acta BBA Biomembr. 2000, 1508, 235–251.
- Jahan, K.; Balzer, S.; Mosto, P. Toxicity of nonionic surfactants. WIT Trans. Ecol. Environ. 2008, 110, 281–290. Jahan, K.; Balzer, S.; Mosto, P. Toxicity of nonionic surfactants. WIT Trans. Ecol. Environ. 2008, 110, 281–290
- Ishiguro, M.; Koopal, L.K. Surfactant adsorption to soil components and soils. Adv. Colloid Interface Sci. 2016, 231, 59–102. Ishiguro, M.; Koopal, L.K. Surfactant adsorption to soil components and soils. Adv. Colloid Interface Sci. 2016, 231, 59–102.
- Ramprasad, C.; Philip, L. Sorption of surfactants and personal care products in Indian soils. Int. J. Environ. Sci. Technol. 2017, 14, 853–866. Ramprasad, C.; Philip, L. Sorption of surfactants and personal care products in Indian soils. Int. J. Environ. Sci. Technol. 2017, 14, 853–866.
- Brandt, K.K.; Hesselsoe, M.; Roslev, P.; Henriksen, K.; Sorensen, J. Toxic effects of linear alkylbenzene sulfonate on metabolic activity, growth rate, and microcolony formation of Nitrosomonas and Nitrosospira strains. Appl. Environ. Microbiol. 2001, 67, 2489–2498. Brandt, K.K.; Hesselsoe, M.; Roslev, P.; Henriksen, K.; Sorensen, J. Toxic effects of linear alkylbenzene sulfonate on metabolic activity, growth rate, and microcolony formation of Nitrosomonas and Nitrosospira strains. Appl. Environ. Microbiol. 2001, 67, 2489–2498.
- Volkering, F.; Breure, A.M.; Rulkens, W.H. Microbiological aspects of surfactant use for biological soil remediation. Biodegradation 1997, 8, 401–417. Volkering, F.; Breure, A.M.; Rulkens, W.H. Microbiological aspects of surfactant use for biological soil remediation. Biodegradation 1997, 8, 401–417.
- Mnif, I.; Sahnoun, R.; Ellouz-Chaabouni, S.; Ghribi, D. Application of bacterial biosurfactants for enhanced removal and biodegradation of diesel oil in soil using a newly isolated consortium. Process Saf. Environ. Prot. 2017, 109, 72–81. Mnif, I.; Sahnoun, R.; Ellouz-Chaabouni, S.; Ghribi, D. Application of bacterial biosurfactants for enhanced removal and biodegradation of diesel oil in soil using a newly isolated consortium. Process Saf. Environ. Prot. 2017, 109, 72–81.
- Karthick, A.; Roy, B.; Chattopadhyay, P. Comparison of zero-valent iron and iron oxide nanoparticle stabilized alkyl polyglucoside phosphate foams for remediation of diesel-contaminated soils. J. Environ. Manag. 2019, 240, 93–107. Karthick, A.; Roy, B.; Chattopadhyay, P. Comparison of zero-valent iron and iron oxide nanoparticle stabilized alkyl polyglucoside phosphate foams for remediation of diesel-contaminated soils. J. Environ. Manag. 2019, 240, 93–107.
- Yen, C.; Chen, K.; Kao, C.; Liang, S.; Chen, T. Application of persulfate to remediate petroleum hydrocarbon-contaminated soil: Feasibility and comparison with common oxidants. J. Hazard. Mater. 2011, 186, 2097–2102. Yen, C.; Chen, K.; Kao, C.; Liang, S.; Chen, T. Application of persulfate to remediate petroleum hydrocarbon-contaminated soil: Feasibility and comparison with common oxidants. J. Hazard. Mater. 2011, 186, 2097–2102.
- Li, J.; He, N.; Wei, X.; Gao, Y.; Zuo, Y. Changes in temperature sensitivity and activation energy of soil organic matter decomposition in different Qinghai-Tibet Plateau grasslands. PLoS ONE 2015, 10, e0132795. Li, J.; He, N.; Wei, X.; Gao, Y.; Zuo, Y. Changes in temperature sensitivity and activation energy of soil organic matter decomposition in different Qinghai-Tibet Plateau grasslands. PLoS ONE 2015, 10, e0132795.
- Li, G.; Guo, S.; Hu, J. The influence of clay minerals and surfactants on hydrocarbon removal during the washing of petroleum-contaminated soil. Chem. Eng. J. 2016, 286, 191–197. Li, G.; Guo, S.; Hu, J. The influence of clay minerals and surfactants on hydrocarbon removal during the washing of petroleum-contaminated soil. Chem. Eng. J. 2016, 286, 191–197.
- Vu, K.A.; Mulligan, C.N. Treatment of oil pollutants in soil using a biosurfactant/nanoparticle suspension. In Proceedings of the 20th Global Joint Seminar on Geo-Environmental Engineering, Osaka, Japan, 19–20 May 2022. Vu, K.A.; Mulligan, C.N. Treatment of oil pollutants in soil using a biosurfactant/nanoparticle suspension. In Proceedings of the 20th Global Joint Seminar on Geo-Environmental Engineering, Osaka, Japan, 19–20 May 2022.
- Xinhong, G.; Ying, T.; Wenjie, R.; Jun, M.A.; Christie, P.; Yongming, L. Optimization of ex-situ washing removal of polycyclic aromatic hydrocarbons from a contaminated soil using nano-sulfonated graphene. Pedosphere 2017, 27, 527–536. Xinhong, G.; Ying, T.; Wenjie, R.; Jun, M.A.; Christie, P.; Yongming, L. Optimization of ex-situ washing removal of polycyclic aromatic hydrocarbons from a contaminated soil using nano-sulfonated graphene. Pedosphere 2017, 27, 527–536.
- Mańko, D.; Zdziennicka, A.; Jańczuk, B. Thermodynamic properties of rhamnolipid micellization and adsorption. Colloids Surf. B Biointerfaces 2014, 119, 22–29. Mańko, D.; Zdziennicka, A.; Jańczuk, B. Thermodynamic properties of rhamnolipid micellization and adsorption. Colloids Surf. B Biointerfaces 2014, 119, 22–29.
- Wang, Y.; Zhou, D.; Wang, Y.; Wang, L.; Cang, L. Automatic pH control system enhances the dechlorination of 2,4,4′-trichlorobiphenyl and extracted PCBs from contaminated soil by nanoscale Fe0 and Pd/Fe0. Environ. Sci. Pollut. Res. 2012, 19, 448–457. Wang, Y.; Zhou, D.; Wang, Y.; Wang, L.; Cang, L. Automatic pH control system enhances the dechlorination of 2,4,4′-trichlorobiphenyl and extracted PCBs from contaminated soil by nanoscale Fe0 and Pd/Fe0. Environ. Sci. Pollut. Res. 2012, 19, 448–457.
- Gil-Díaz, M.; Alonso, J.; Rodríguez-Valdés, E.; Gallego, J.R.; Lobo, M.C. Comparing different commercial zero valent iron nanoparticles to immobilize As and Hg in brownfield soil. Sci. Total Environ. 2017, 584, 1324–1332. Gil-Díaz, M.; Alonso, J.; Rodríguez-Valdés, E.; Gallego, J.R.; Lobo, M.C. Comparing different commercial zero valent iron nanoparticles to immobilize As and Hg in brownfield soil. Sci. Total Environ. 2017, 584, 1324–1332.
- Blanco, E.; Lam, S.; Smoukov, S.K.; Velikov, K.P.; Khan, S.A.; Velev, O.D. Stability and viscoelasticity of magneto-pickering foams. Langmuir 2013, 29, 10019–10027. Blanco, E.; Lam, S.; Smoukov, S.K.; Velikov, K.P.; Khan, S.A.; Velev, O.D. Stability and viscoelasticity of magneto-pickering foams. Langmuir 2013, 29, 10019–10027.
- Harikrishnan, A.R.; Dhar, P.; Agnihotri, P.K.; Gedupudi, S.; Das, S.K. Effects of interplay of nanoparticles, surfactants and base fluid on the surface tension of nanocolloids. Eur. Phys. J. E 2017, 40, 53. Harikrishnan, A.R.; Dhar, P.; Agnihotri, P.K.; Gedupudi, S.; Das, S.K. Effects of interplay of nanoparticles, surfactants and base fluid on the surface tension of nanocolloids. Eur. Phys. J. E 2017, 40, 53.
- Bhuiyan, M.; Saidur, R.; Amalina, M.A.; Mostafizur, R.M.; Islam, A. Effect of nanoparticles concentration and their sizes on surface tension of nanofluids. Procedia Eng. 2015, 105, 431–437. Bhuiyan, M.; Saidur, R.; Amalina, M.A.; Mostafizur, R.M.; Islam, A. Effect of nanoparticles concentration and their sizes on surface tension of nanofluids. Procedia Eng. 2015, 105, 431–437.
- Sharma, N.; Ghosh, A.; Fortner, J.D.; Giammar, D.E. Modeling performance of rhamnolipid-coated engineered magnetite nanoparticles for U (vi) sorption and separation. Environ. Sci. Nano 2020, 7, 2010–2020. Sharma, N.; Ghosh, A.; Fortner, J.D.; Giammar, D.E. Modeling performance of rhamnolipid-coated engineered magnetite nanoparticles for U (vi) sorption and separation. Environ. Sci. Nano 2020, 7, 2010–2020.
- Gong, X.; Huang, D.; Liu, Y.; Zeng, G.; Wang, R.; Wan, J.; Zhang, C.; Cheng, M.; Qin, X.; Xue, W. Stabilized nanoscale zerovalent iron mediated cadmium accumulation and oxidative damage of Boehmeria nivea (L.) Gaudich cultivated in cadmium contaminated sediments. Environ. Sci. Technol. 2017, 51, 11308–11316. Gong, X.; Huang, D.; Liu, Y.; Zeng, G.; Wang, R.; Wan, J.; Zhang, C.; Cheng, M.; Qin, X.; Xue, W. Stabilized nanoscale zerovalent iron mediated cadmium accumulation and oxidative damage of Boehmeria nivea (L.) Gaudich cultivated in cadmium contaminated sediments. Environ. Sci. Technol. 2017, 51, 11308–11316.
- Liang, J.; Xia, X.; Yuan, L.; Zhang, W.; Lin, K.; Zhou, B.; Hu, S. The reproductive responses of earthworms (Eisenia fetida) exposed to nanoscale zero-valent iron (nZVI) in the presence of decabromodiphenyl ether (BDE209). Environ. Pollut. 2018, 237, 784–791. Liang, J.; Xia, X.; Yuan, L.; Zhang, W.; Lin, K.; Zhou, B.; Hu, S. The reproductive responses of earthworms (Eisenia fetida) exposed to nanoscale zero-valent iron (nZVI) in the presence of decabromodiphenyl ether (BDE209). Environ. Pollut. 2018, 237, 784–791.
- Burketová, L.; Martinec, J.; Siegel, J.; Macůrková, A.; Maryška, L.; Valentová, O. Noble metal nanoparticles in agriculture: Impacts on plants, associated microorganisms, and biotechnological practices. Biotechnol. Adv. 2022, 58, 107929. Burketová, L.; Martinec, J.; Siegel, J.; Macůrková, A.; Maryška, L.; Valentová, O. Noble metal nanoparticles in agriculture: Impacts on plants, associated microorganisms, and biotechnological practices. Biotechnol. Adv. 2022, 58, 107929.
- Pawlett, M.; Ritz, K.; Dorey, R.A.; Rocks, S.; Ramsden, J.; Harris, J.A. The impact of zero-valent iron nanoparticles upon soil microbial communities is context dependent. Environ. Sci. Pollut. Res. 2013, 20, 1041–1049. Pawlett, M.; Ritz, K.; Dorey, R.A.; Rocks, S.; Ramsden, J.; Harris, J.A. The impact of zero-valent iron nanoparticles upon soil microbial communities is context dependent. Environ. Sci. Pollut. Res. 2013, 20, 1041–1049.
- Saccà, M.L.; Fajardo, C.; Nande, M.; Martín, M. Effects of nano zero-valent iron on Klebsiella oxytoca and stress response. Microb. Ecol. 2013, 66, 806–812. Saccà, M.L.; Fajardo, C.; Nande, M.; Martín, M. Effects of nano zero-valent iron on Klebsiella oxytoca and stress response. Microb. Ecol. 2013, 66, 806–812.
- Diao, M.; Yao, M. Use of zero-valent iron nanoparticles in inactivating microbes. Water Res. 2009, 43, 5243–5251. Diao, M.; Yao, M. Use of zero-valent iron nanoparticles in inactivating microbes. Water Res. 2009, 43, 5243–5251.
- Vanzetto, G.V.; Thomé, A. Toxicity of nZVI in the growth of bacteria present in contaminated soil. Chemosphere 2022, 303, 135002. Vanzetto, G.V.; Thomé, A. Toxicity of nZVI in the growth of bacteria present in contaminated soil. Chemosphere 2022, 303, 135002.
- Fajardo, C.; Ortíz, L.T.; Rodríguez-Membibre, M.L.; Nande, M.; Lobo, M.C.; Martin, M. Assessing the impact of zero-valent iron (ZVI) nanotechnology on soil microbial structure and functionality: A molecular approach. Chemosphere 2012, 86, 802–808. Fajardo, C.; Ortíz, L.T.; Rodríguez-Membibre, M.L.; Nande, M.; Lobo, M.C.; Martin, M. Assessing the impact of zero-valent iron (ZVI) nanotechnology on soil microbial structure and functionality: A molecular approach. Chemosphere 2012, 86, 802–808.
- El-Temsah, Y.S.; Sevcu, A.; Bobcikova, K.; Cernik, M.; Joner, E.J. DDT degradation efficiency and ecotoxicological effects of two types of nano-sized zero-valent iron (nZVI) in water and soil. Chemosphere 2016, 144, 2221–2228. El-Temsah, Y.S.; Sevcu, A.; Bobcikova, K.; Cernik, M.; Joner, E.J. DDT degradation efficiency and ecotoxicological effects of two types of nano-sized zero-valent iron (nZVI) in water and soil. Chemosphere 2016, 144, 2221–2228.
- Ortega-Calvo, J.; Jimenez-Sanchez, C.; Pratarolo, P.; Pullin, H.; Scott, T.B.; Thompson, I.P. Tactic response of bacteria to zero-valent iron nanoparticles. Environ. Pollut. 2016, 213, 438–445. Ortega-Calvo, J.; Jimenez-Sanchez, C.; Pratarolo, P.; Pullin, H.; Scott, T.B.; Thompson, I.P. Tactic response of bacteria to zero-valent iron nanoparticles. Environ. Pollut. 2016, 213, 438–445.
- Vu, K.; Yang, G.; Wang, B.; Tawfiq, K.; Chen, G. Bacterial interactions and transport in geological formation of alumino-silica clays. Colloids Surf. B Biointerfaces 2015, 125, 45–50. Vu, K.; Yang, G.; Wang, B.; Tawfiq, K.; Chen, G. Bacterial interactions and transport in geological formation of alumino-silica clays. Colloids Surf. B Biointerfaces 2015, 125, 45–50.
