Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Beata Kasztelan-Szczerbinska and Version 2 by Lindsay Dong.
Bile acids (BAs) represent heterogeneous amphipathic molecules that have both polar (water-soluble or hydrophilic) and apolar (water-insoluble or hydrophobic) parts, and therefore they can dissolve in water as well as in fat. The regulation of cholesterol homeostasis remains their main role in the host. The better water solubility in comparison with their precursor promotes cholesterol removal from the human body. The proper bile ratio of BAs and cholesterol prevents cholesterol precipitation and further formation of gallstones; therefore, the loss of BAs increases the risk of cholesterol stone development.
- bile acids
- BAs
- liver
- gut microbiota
1. Bile Acid Synthesis and Circulation
Bile acids (BAs) are synthesized in the human liver through the cholesterol oxidation process mediated by cytochrome P450. Seventeen enzymes are involved in their biosynthesis and about 500 mg of cholesterol is biotransformed to BAs within 24 h in adults [1][2][55,56]. There are four main BAs in human bile:
-
primary BAs, which are synthesized in the liver and secreted with bile into the small intestine: cholic acid (CA) and chenodeoxycholic acid (CDCA);
-
secondary BAs, which are formed from primary BAs in the large intestine under the influence of bacterial enzymes: deoxycholic acid (DCA) originating from cholic acid, and lithocholic acid (LCA) originating from chenodeoxycholic acid;
The BA synthesis and circulation are presented in Figure 12.
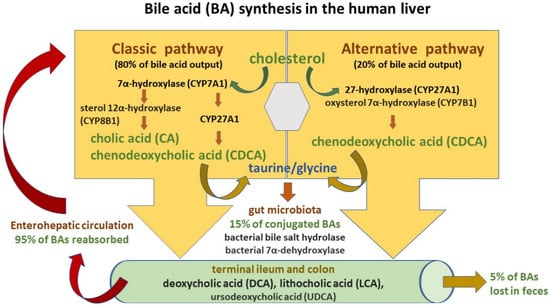
Figure 12.
Bile acid (BA) synthesis and circulation.
Two pathways are engaged in this process: the classic and alternative [4][5][48,50]. The classic pathway of BA synthesis accounts for about 75% of BA output and is catalyzed by cholesterol 7α-hydroxylase (CYP7A1), which via a rate-limiting manner produces the primary cholic acid (CA) and chenodeoxycholic acid (CDCA) [3][6][49,53]. On the other hand, the alternative pathway is associated with the activity of mitochondrial sterol 27-hydroxylase (CYP27A1) and results in CDCA output. Primary BAs are then conjugated predominantly with glycine and to a lesser extent with taurine (at a ratio of 3:1) before their active transport from hepatocytes into the bile via the bile salt export pump (BSEP) [3][7][53,57]. Conjugation increases the water solubility of BAs before their hepatic secretion [3][53]. Moreover, conjugated BAs are nonabsorbable and indigestible in the proximal small intestine where lipid absorption takes place [6][8][49,58]. BSEP mutations may induce the development of cholestatic liver diseases such as progressive familial intrahepatic cholestasis type 2 (PFIC-2) and benign recurrent intrahepatic cholestasis type 2 (BRIC-2). Moreover, BSEP genetic polymorphisms are associated with intrahepatic cholestasis of pregnancy (ICP) as well as drug-induced liver injury (DILI) [9][59]. In the intestinal lumen, BAs facilitate fat and fat-soluble vitamin uptake and undergo further modification by microbiota into secondary BAs—deoxycholic acid (DCA), lithocholic acid (LCA)—and tertiary BAs such as ursodeoxycholic acid (UDCA) [3][6][49,53]. High-fat diets stimulate BA release to the gut and result in their increased concentrations in the lumen of the large bowel with their negative impact on intestinal mucosa homeostasis and increased risk of colorectal cancer development [2][10][56,60]. BAs, present in the gut lumen, regulate hepatic BA synthesis through the farnesoid X receptor (FXR), which gives rise to the transcription of intestinal hormone fibroblast growth factor 19 (FGF19). Since FGF19 inhibits cholesterol 7α-monooxygenase in liver cells, it decreases hepatic BA synthesis (a negative-feedback loop) [11][61]. Moreover, the interaction of BAs with FXR causes a discharge of antimicrobial peptides (AMPs), which diminish intestinal bacterial overgrowth and accordingly prevent dysbiosis and further gut barrier dysfunction [12][62]. In turn, microbiota may change BA composition by favoring secondary BA production. Secondary BAs exert poorer antimicrobial effects due to their weaker FXR affinity. Therefore, intestinal BA imbalance present in the course of cholestatic liver disease may promote bacterial overgrowth [2][3][11][53,56,61].
Since the vast majority of conjugated BAs are reabsorbed in both the terminal ileum by the apical sodium-dependent bile acid transporter (ASBT), as well as in the colon by passive absorption and return to the liver with portal blood, more than 95% of the BA pool is preserved this way [4][48]. In the portal blood, conjugated bile salts binding to albumin flow back to the liver and are taken up by active transporters (sodium taurocholate co-transporting polypeptide (NTCP) and organic anion transporting polypeptide (OATP) transporter) located on the sinusoidal membrane of liver cells and then resecreted into the bile [3][53]. In humans, so-called enterohepatic circulation occurs about six times a day [2][56]. Pharmacological inhibition of the ileal apical sodium-dependent bile acid transporter (ASBT/SLC10A2) followed by a decline in intestinal BA uptake was reported to ameliorate cholestatic liver and bile duct injury in a mouse model of sclerosing cholangitis [13][63]. Approximately 5% of the BA pool is eliminated in feces and the small quantity of conjugated secondary BAs is absorbed in the lumen of the large bowel via passive diffusion.
2. Effects of BAs in the Human Body
Accumulating evidence reveals that BAs exert pleiotropic effects in the human body and secure various metabolic and inflammatory routes in a large number of cells, tissue types, and organs through an active interplay with host receptors and intestinal microbiota [14][15][64,65]. They not only participate in the digestion and absorption of lipids and fat-soluble vitamins but also are engaged in the feedback regulatory loop of their own hepatic synthesis [1][55]. BAs were reported to modify gallbladder motor function [16][66]. They are also involved in the gut–liver axis and related inflammatory response activation [4][17][48,52]. BAs act as signaling mediators regulating metabolic homeostasis, mainly through the nuclear farnesoid X receptor (FXR) and membrane-associated receptors such as G protein-coupled bile acid receptor 1 (GPBAR-1) and sphingosine 1 phosphate receptor 2 (S1PR2) [15][18][65,67]. Several reports indicate that BAs have an impact on epithelial cell proliferation and carcinogenesis [19][20][68,69]. They also may directly interact with gut microbiota and modify gene expression through epigenetic mechanisms [20][21][22][69,70,71].
As previously mentioned, BAs are produced in the liver, but they are further metabolized by intestinal microbiota. Currently, BA interactions with intestinal microbiota are increasingly recognized. Some studies indicate that the gut microbiome may influence BA uptake by modulation of ASBT action [21][70]. The same group of researchers reported that microbiota regulated the expression of several enzymes engaged in BA syntheses, including CYP7A1, CYP7B1, and CYP27A1. Only the 12a-hydroxylase (CYP8B1) necessary for effective cholic acid synthesis remains outside microbiota modulation [2][56]. It becomes clear that the gut microbiota may change the host BA pool through the complex regulations of BA synthesis and absorption. In turn, BAs may impact microbiota composition, so their interaction is bidirectional. Studies have shown that the human microbiome is shaped by BAs that support the growth of bacteria that can metabolize them and hamper the development of the other ones. Bile acids are considered powerful antimicrobials and guard the host against pathogens [22][71]. No wonder biliary obstruction usually results in bacterial overgrowth, intestinal barrier disruption, and bacterial translocation. The above negative effects of the disorder can be inhibited by the administration of BAs and their signaling via the farnesoid-X-receptor (FXR) [23][72]. Therefore, the antimicrobial impact of BAs is based not only on their direct detergent action on pathogen membranes but also on an indirect receptor-mediated release of antimicrobial factors and immune system activation [2][12][56,62]. Bile acids are agonists of so-called bile acid-activated receptors (BARs) and act as signaling molecules. Two main BARs include the farnesoid-X-receptor (FXR) and G protein-coupled bile acid receptor 1 (GPBAR1 or G-protein receptor 5), which are solely activated by BAs. The other receptors, such as pregnane X receptor (PXR), vitamin D receptor (VDR), constitutive androstane receptor (CAR), liver X receptors (LXRs), sphingosine-1-phosphate receptor 2 (S1PR2), and retinoic acid-related orphan receptor (ROR)-γt (ROR-γt), are activated by BAs together with other endogenous molecules [24][73]. BAs are also involved in cell signaling pathways such as c-Jun N-terminal kinase (JNK) and extracellular signal-regulated kinase (ERK) [3][25][53,74]. They can interact with cell receptors located in the gut and liver, contributing to both organs’ mutual communication and a bidirectional transfer of signals. These receptors are key players in the host’s innate immune responses. Nevertheless, nuclear FXR expression is found not only in the liver and intestine but also in other cells including those of adrenal glands, and the immune system (e.g., macrophages, the Kupffer cells, natural killer cells, and dendritic cells) [26][75].
However, the interplay of BAs, microbiota, and the adaptive immune response remains undefined. Recently, Song et al. reported that BA composition in the gut might be modified by both nutritional and microbial factors and have an impact on colonic FOXP3+ regulatory T (Treg) cells, which express the transcription factor RORγ [27][76]. The aforementioned lymphocyte subsets adjust immune response, prevent autoimmunity, and control inflammation. The researchers confirmed that restoration of the intestinal BA pool, increased colonic RORγ+ Treg counts and modulated host predisposition to colitis via BA nuclear receptors. Other reports also indicate that BAs can regulate host immune responses by modulating the Th17–Treg lymphocyte inflammatory balance [14][28][64,77]. BA gut content modifications may change BA-mediated signaling as well as the microbiome composition and induce further metabolic consequences, such as impairment of host lipid, glucose, and energy balance. Accordingly, it is strongly suggested that alterations in BA enterohepatic circulation and/or their metabolism are relevant contributors to the pathogenesis of cholestatic liver diseases, metabolic syndrome, inflammatory bowel diseases, and colorectal cancer. Therefore, BAs and their cellular receptors create interesting therapeutic targets and an important scientific field for future drug discovery.
