Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Guanqiong Na and Version 4 by Conner Chen.
Diet plays an important role in health. A high intake of plant chemicals such as glucosinolates/isothiocyanates can promote optimal health and decrease the risk of cancer. Some research has discovered more novel mechanisms of action for the effects of isothiocyanates including the modulation of tumor microenvironment, the inhibition of the self-renewal of stem cells, the rearrangement of multiple pathways of energy metabolism, the modulation of microbiota, and protection against Helicobacter pylori.
- isothiocyanates
- sulforaphane
- cancer
- microbiota
- stem cells
- tumor microenvironment
- rearrangement of energy metabolism
1. Microbiota and Anti-Cancer Effects of ITCs
Microbiota: A Mediator Transforming Glucosinolate Precursors to the Active Isothiocyanates
Isothiocyanates (ITCs) mainly exist in cruciferous vegetables in the form of glucosinolate (GLS) precursors. Myrosinase (thioglucoside glucohydrolase EC3.2.1.147, formerly EC 3.2.3.1) [1][16], normally found in plant cells but not in the human body, is the only known endogenous enzyme that degrades the GLS precursors into their corresponding active forms-ITCs [2][17], mainly including sulforaphane (SFN), allyl isothiocyanate (AITC), benzyl isothiocyanate (BITC) and phenethyl isothiocyanate (PEITC). The dietary source and chemical structures of ITCs are summarized in Figure 12. However, endogenous myrosinase can be largely inactivated during cooking, which hampers the degradation of GLSs to ITCs and, thus, their beneficial effects. The gut microflora may provide an outstanding substitute for endogenous myrosinase following the consumption of cooked cruciferous vegetables. Therefore, for people who often consume cooked cruciferous vegetables, the acquisition of ITCs in their bodies mainly depends on the intestinal microecology.
Figure 12.
The dietary source and basic structure of ITCs.
The microflora of the gastrointestinal tract is thought to be primarily responsible for ITCs production in the human body. ITCs were shown to appear in the mesenteric plasma 120 min following the injection of glucoraphanin (GRP) into the cecum (150 μmol/L), further confirming the capabilities of the gut microflora to hydrolyze GLSs [3][18]. Peptostreptococcus spp. and Bifidobacterium spp. have been proven to be able to metabolize GLSs [4][5][19,20]. Two beneficial bacteria, Lactobacillus plantarum KW30 and Lactococcuslactissubsp. lactis KF147, were shown to be capable of transforming 30–33% of GRP and/or glucoerucin into SFN nitrile, erucin nitrile and some unknown metabolites [6][21]. Another lactic acid bacterial strain, Lactobacillusagilis R16, possessed an outstanding ability to degrade sinigrin into allyl isothiocyanate (AITC) [4][19]. Some other microorganisms, such as Bacillus cereus and Aspergillus niger, have also presented myrosinase activity [4][5][6][7][19,20,21,22].
2. Activity of ITCs on Helicobacter pylori Strains
Helicobacter pylori infections affect more than 50% of the world’s human population and are associated with gastritis, peptic ulcer disease and gastric cancer [8][23]. Gastric H. pylori infections express high urease activity, which promotes inflammation. ITCs have potent antibacterial effects against gastric H. pylori, and the MIC90 values of these ITCs range between 4 and 32 µg/mL [9][15]. SFN is a potent bacteriostatic agent that is effective against three reference strains and 45 clinical isolates of H. pylori (the MICs for 90% of the strains are <4 µg/mL). Furthermore, SFN prevented the formation of benzo[a]pyrene-induced stomach tumors in ICR mice [10][24]. In human gastric xenografts that were implanted in nude mice, SFN significantly inhibited the proliferation of H. pylori, with an observed eradication rate of 73%, which depended on the Nrf2-dependent induction of phase II detoxication and antioxidant enzymes. Moreover, broccoli sprouts (0.125 mg/mL, w/v) reduced H. pylori-associated inflammation by inhibiting IL-8 release in response to TNFα exposure [11][25]. Some population intervention studies have also suggested that ITCs can reduce Helicobacter pylori infections. Among nine patients who consumed broccoli sprout preparations (14, 28 or 56 g fresh weight) twice daily for 7 days, the eradication of H. pylori infection was observed in three [12][26]. Forty-eight H. pylori–infected patients were randomly assigned to the feeding of broccoli sprouts (70 g/d; containing 420 μmol of SFN precursor) for 8 weeks or to the consumption of an equal weight of alfalfa sprouts (not containing SFN) as a placebo. The intervention with broccoli sprouts (BS) decreased the levels of urease measured and serum pepsinogens I and II [13][27]. In another trial involving 25 H. pylori–positive volunteers, eight subjects had Helicobacter pylori stool antigen (HpSA) values below the cutoff (0.100) at the end of the 8 week BS treatment (70 g/day) period. In six of these subjects, the HpSA values became positive again at 8 weeks after the cessation of BS consumption, indicating that BS treatment reduced H. pylori colonization but did not result in complete eradication [14][28]. SFN protected against small intestinal injury induced by nonsteroidal anti-inflammatory drugs, through inhibiting the invasion of anaerobic bacteria into the mucosa [15][29].3. Rearrangement of Energy Metabolism Phenotype by Sulforaphane
Malignant cells depend on glycolysis to provide energy, which produces lactate and forms an acidic tumor microenvironment, which finally favors tumor metastasis and chemoresistance. SFN prevents the propagation of bladder cancer cells by the inhibition of glycolysis, mediated by the downregulation of hypoxia inducible factor 1 and its nuclear translocation [16][13]. Furthermore, SFN prevented the increase in glycolysis, hexokinase and pyruvate kinase activity, and reduced the HIF-1α stabilization induced by an androgen and Tip60 in LNCaP cells [17][31]. SFN weakens the glycolytic flux by suppressing multiple metabolic enzymes, including hexokinase 2 (HK2) and pyruvate dehydrogenase (PDH). Moreover, SFN significantly downregulated glycolysis and mitochondrial OXPHOS via blocking the AKT1/HK2 axis and PDH expression [18][32]. SFN significantly downregulated the expression of hexokinase II (HKII), pyruvate kinase M2 and/or lactate dehydrogenase A (LDHA) in vitro and in vivo in neoplastic lesions in the prostates of transgenic adenocarcinoma of mouse prostate (TRAMP) and Hi-Myc mice, and significantly suppressed glycolysis in the prostates of Hi-Myc mice [19][33]. Another metabolic hallmark of malignant cells is the dysregulation of lipid metabolism, such as the enhancement of de novo fatty acid synthesis, lipid absorption and steatolysis, which provide energy for the rapidly proliferating malignant cells. The treatment of prostate cancer cells with SFN (5 and 10 μM) resulted in the downregulation of acetyl-CoA carboxylase 1 (ACC1) and fatty acid synthase (FASN), inhibiting fatty acid synthesis. Similarly, SFN administration to TRAMP mice resulted in a significant decrease in the plasma and/or prostate adenocarcinoma levels of total free fatty acids, total phospholipids, acetyl-CoA and ATP. Additionally, SFN prevented fatty acid absorption by decreasing carnitine palmitoyl-transferase 1A (CPT1A), followed by the β-oxidation of fatty acids [20][14]. SFN downregulated FASN, acetyl CoA carboxylase (ACACA), and ATP citrate lyase (ACLY) via activating proteasomes and downregulating the transcriptional factor SREBP1. SFN also decreased the amount of intracellular fatty acid and inhibited microtubule-mediated mitophagy, leading to apoptosis in human non-small-cell lung cancer (NSCLC) cells [21][34].4. Inhibition of Cancer Stem Cells by Sulforaphane
Cancer stem cells (CSCs) are a small group of cancer cells present in malignant tumors that are characterized by high drug resistance, potent metastatic and invasive ability, and infinite self-renewal. Mounting evidence confirms that CSCs are the root cause of the initiation and relapse of malignant tumors [22][35]. SFN inhibited CSC self-renewal via the blockade of Wnt/β-catenin, Hedgehog and Notch signaling. SFN reduced aldehyde dehydrogenase (ALDH)-positive CSCs by 65–80% and mammosphere formation, suggesting that it is effective in eliminating breast CSCs. Such inhibitory effects were due to the attenuation of β-catenin-mediated transcription [23][12]. SFN significantly inhibited spheroids derived from human pancreatic CSCs by inhibiting sonic hedgehog (Shh) pathway components and Gli transcriptional activity (PDGFRα and Cyclin D1). Thus, the preventative effects of SFN on pancreatic cancer may result from the inhibition of the Shh pathway [24][36]. In addition, the stemness of pancreatic CSCs was repressed by SFN in vitro and in vivo, which is characterized by the inhibition of tumor-sphere formation and of the expression of pluripotency maintaining factors (Oct4 and Nanog) [25][37]. Recent studies have also found that YAP1, the Hippo signaling transcription adaptor protein, and ∆Np63α, a key epidermal stem cell survival protein, form a complex to drive epidermal cancer stem cell survival. However, sulforaphane suppresses epidermal squamous carcinoma cell formation and this is associated with reduced levels of YAP1 and ∆Np63α [26][38]. The modulation of microRNAs contributes to the anti-CSC effect of SFN [27][39]. SFN exhibited inhibitory effects on lung CSCs through suppressing miR-19 and the Wnt/β-catenin pathway [28][40]. SFN increased exosomal miR-140 expression and decreased both miR-21 and miR-29, which led to reduced ALDH1 levels and decreased mammosphere formation [29][41]. In addition, SFN restored miR-140 expression, inhibited the tumorigenicity of MCF10DCIS (a model cell line of poorly differentiated basal-like ductal carcinoma in situ) stem-like cells in immune-deficient nude female mice, and decreased the percentage of CSCs (CD44high/CD24low) in MDA-MB-231 cells [30][42]. SFN downregulated Bmi1 via increasing miR-200c expression, thereby blocking the expression of CSC markers and, subsequently, the migration and invasion of oral squamous CSCs [31][43].5. Remodeling of the Tumor Microenvironment by Sulforaphane
In the tumor microenvironment, cancer cells bound to the T cell co-inhibitory CD28 family receptors including programmed cell death receptor 1 (PD-1) and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), which decreased immune cell activity and allowed cancer cells to escape from immune surveillance [32][44]. The activities of PD-1 and its ligand PD-L1 are responsible for reducing activation, proliferation, and cytokine secretion of T cells in TME, resulting in decreased anti-tumor immune responses [33][34][45,46]. SFN inhibited the transformation of normal monocytes to myeloid-derived suppressor cells (MDSCs) by glioma-conditioned media in vitro, and promoted the proliferation of T cells. SFN also suppressed PD-L1 expression in a dose-dependent manner [35][47]. By inducing miR-194-5p (targeting the B7-H1 gene) and inhibiting miR-155-5p expression, SFN attenuates the phosphorylation of STAT3 in dendritic cells. The inhibition of JAK/STAT3 leads to the downregulation of B7-H1 expression, thereby promoting the activation of T cells [36][48]. Epithelial–mesenchymal transition (EMT) is a highly conserved program in which epithelial cells alter their morphology and gain the properties of mesenchymal cells. This process is critical for initiation, invasion and metastasis in a variety of cancers [37][49]. The inhibition of anchorage-independent growth, invasion, and migration of the endometrial cancer cell lines by SFN was associated with the increased E-cadherin and the decreased N-cadherin and vimentin expression [38][50]. Other results showed that SFN prevented EMT via inducing miR-200c [39][51], and dose-dependently induced the EMT hallmark E-cadherin, and downregulated vimentin and the transcriptional repressor ZEB1, which depended on the regulation of miR-200c [40][52]. In addition, SFN reversed mesenchymal-like changes induced by TGF-β1 and restored cells to their epithelial-like morphology. SFN increased the expression of E-cadherin, while decreased the expression of the mesenchymal markers including N-cadherin, vimentin, and α-SMA in A549 cells. SFN inhibited TGF-β1-induced mRNA expression of the EMT-related transcription factors, Slug, Snail, and Twist [41]. Conclusions: Here, Figure 2 present more mechanistic evidence that dietary ITCs target tumorigenic processes, with effects on the tumor microenvironment, stem cells, mitochondrial function, energy metabolism and the microbiota.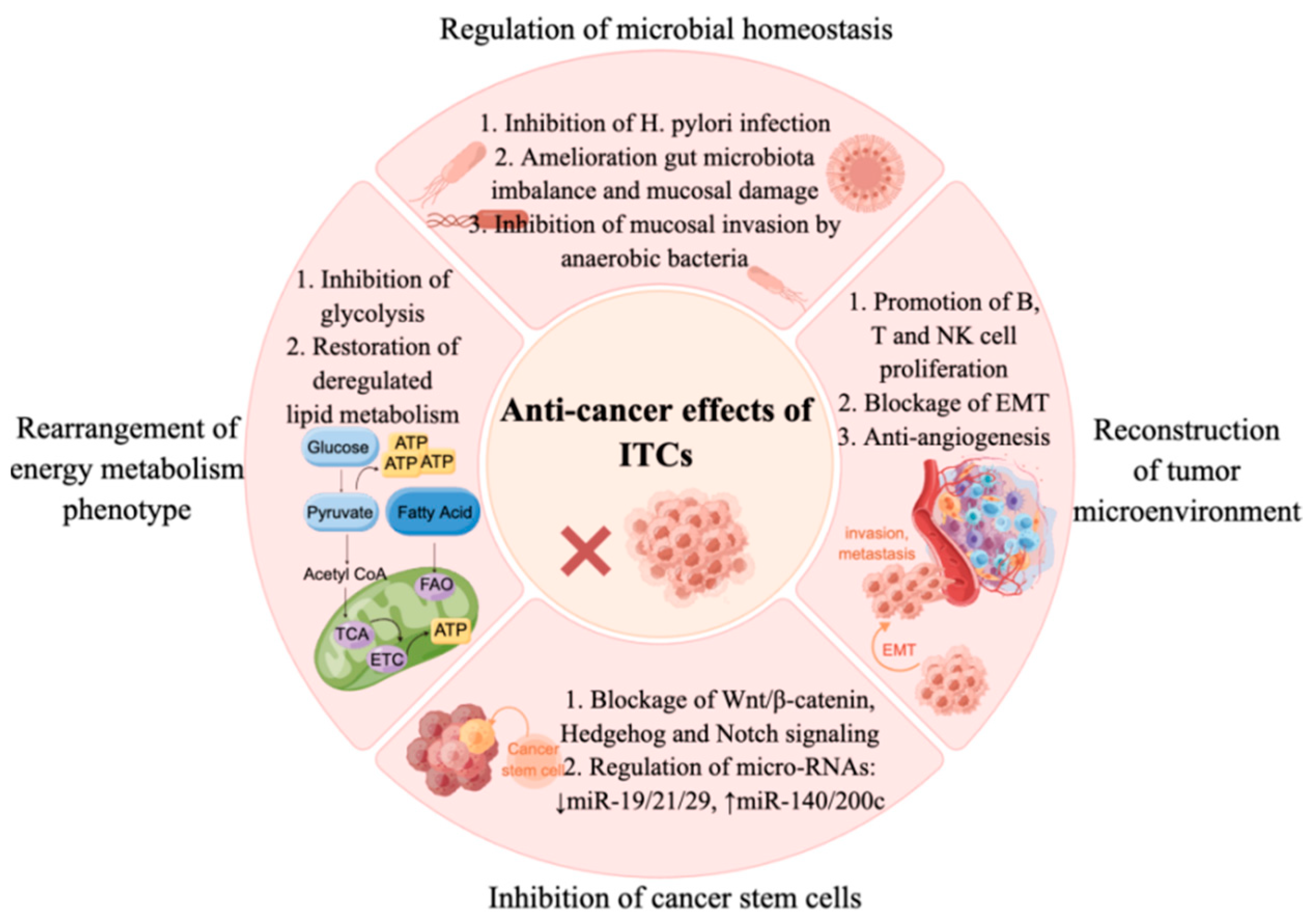 Figure 2. Multiple mechanisms of isothiocyanates in cancer prevention and therapy. ITCs inhibit cancers through regulating microbial homeostasis, such as inhibiting H. pylori infection, ameliorating gut microbiota imbalances and mucosal damage, and inhibiting mucosal invasion by anaerobic bacteria. ITCs inhibit cancer cell proliferation via suppressing glycolysis, restoring deregulated lipid metabolism, and inhibiting CSCs via regulating self-renewal signaling (Wnt/β-catenin, Hedgehog and Notch signaling) and miRNA pathways. Furthermore, ITCs exert anti-cancer effects via remodeling the tumor microenvironment, such as promoting B, T and NK cells’ proliferation, blocking EMT and inhibiting angiogenesis[53].
Figure 2. Multiple mechanisms of isothiocyanates in cancer prevention and therapy. ITCs inhibit cancers through regulating microbial homeostasis, such as inhibiting H. pylori infection, ameliorating gut microbiota imbalances and mucosal damage, and inhibiting mucosal invasion by anaerobic bacteria. ITCs inhibit cancer cell proliferation via suppressing glycolysis, restoring deregulated lipid metabolism, and inhibiting CSCs via regulating self-renewal signaling (Wnt/β-catenin, Hedgehog and Notch signaling) and miRNA pathways. Furthermore, ITCs exert anti-cancer effects via remodeling the tumor microenvironment, such as promoting B, T and NK cells’ proliferation, blocking EMT and inhibiting angiogenesis[53].
