You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 6 by Jessie Wu and Version 5 by Jessie Wu.
Listeria monocytogenes can cause severe foodborne infections in humans and invasive diseases in different animal species, especially in small ruminants. Infection of sheep and goats can occur via contaminated feed or through the teat canal. Both infection pathways result in direct (e.g., raw milk from an infected udder or fresh cheese produced from such milk) or indirect exposure of consumers.
- Listeria monocytogenes
- mastitis
- small ruminant
- dairy products
- contamination routes
- farm sales
1. Transmission Scenario 1: Impact of Ovine and Caprine Listerial Mastitis
Listeria monocytogenes (L. monocytogenes) can colonize the mammary complex of ruminants. Although L. monocytogenes is common in the faeces of ruminants and widespread in the environment [1], only a few cases of bovine [2][3][4][5][6][7][8][9] and ovine [10][11][12][13][14][15][16] listerial mastitis have been reported. Researchers could retrieve merely a single study on caprine mastitis [17]. Interestingly, listerial mastitis has not yet been covered in any review article (Figure 1).
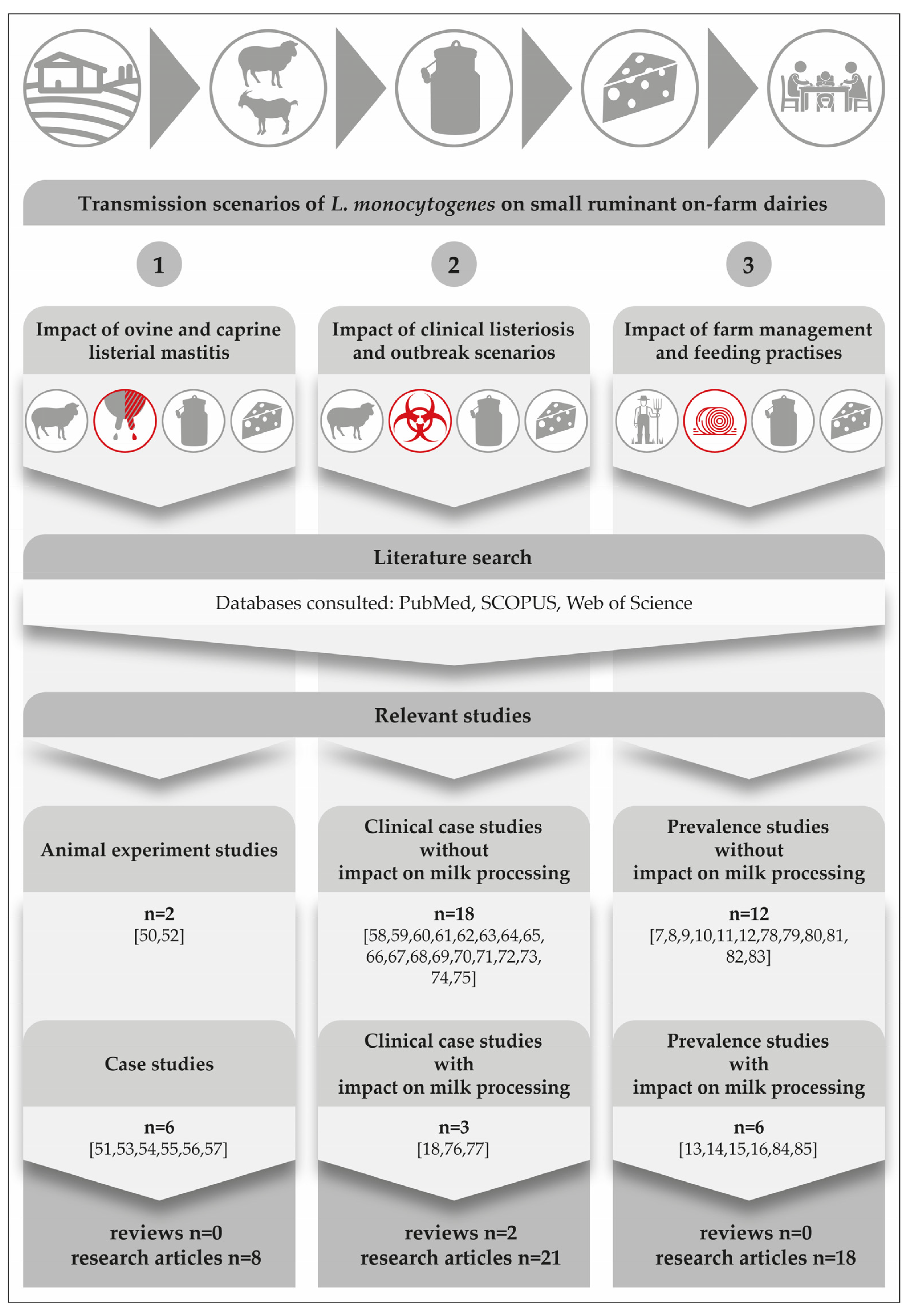
Figure 1. Results from a comprehensive literature search focusing on three main transmission scenarios on small ruminant on-farm dairies. The icons on the top of the figure depict the major steps in the field-to-table continuum [10][11][12][13][14][15][16][17][18][19][20][21][22][23][24][25][26][27][28][29][30][31][32][33][34][35][36][37][38][39][40][41][42][43][44][45][46][47][48][49][50][51][52][53][54][55][56].
Another surprising detail is that there is not a single study from America, Asia, Australia, or Africa; all listerial mastitis studies were performed in Europe. This fact is quite remarkable, if one considers that there was a dramatic increase especially in dairy goat production during the past decade, with Asia seeing the largest growth of 22%, followed by Africa (13%), Oceania (9%), and America (5%) [57].
A comprehensive search of the literature revealed two experimental studies on the course of listerial mastitis in small ruminants (Figure 1). They have shown that inoculations of 300 CFU to 1000 CFU of L. monocytogenes into the udder of ewes are sufficient to result in mastitis [5][12]. All inoculated ewes became infected and developed chronic subclinical mastitis, regardless of the serotype or origin of the strains used. According to Tzora et al. [12], only one single ewe out of 34 animals showed typical signs of acute clinical mastitis immediately after the inoculation. The gland was larger and hotter and its secretion contained clots. There was also an increase in the internal body temperature.
The somatic cell count of all infected sheep was always greater than 1.0 × 106 cells per ml and L. monocytogenes could be consistently isolated from the milk over a period of 88 days. L. monocytogenes was also detected from the mammary lymph nodes, but not from any internal organ of any inoculated ewe. Histologically, in the early stage of the infection, extra-alveolar neutrophilic infiltration and interstitial oedema were predominant. Subsequently, 25 days after inoculation, chronic inflammatory signs predominated, such as destruction of alveoli and fibrous tissue proliferation, with lymphocytes as the main cell type [12]. The findings of both studies provide clear evidence that L. monocytogenes is pathogenic for the ovine and caprine mammary gland.
With regard to naturally occurring cases of ovine mastitis (Figure 1), it is worthwhile to compare a Greek study from Fthenakis et al. [11] with the findings of an Austrian research team [13][14][15]. Fthenakis et al. [11] monitored the udder health, somatic cell count and the shedding of L. monocytogenes in 98 ewes. Half udder milk samples were collected at three separate time points during the lactation period, including: (i) 15 ± 30 days post- lambing; (ii) 6 ± 7 weeks after initial sampling; and (iii) 6 ± 7 weeks on from collection of the second sample. There were diagnoses of clinical mastitis in any of the ewes, though the prevalence of subclinical L. monocytogenes mammary infections was 3.1% during collection of the first and the second samples and this had increased to 6.2% by the third time point. Examination of the milk of ewes with mammary infection revealed somatic cell counts ranging from 1.8 × 106 to 3.0 × 106 cells/mL. Furthermore, L. monocytogenes could also be detected in the faeces of 19.4% of the animals. The authors concluded that infection of the mammary gland with L. monocytogenes had occurred via the bloodstream. Firstly, there was an 83% higher prevalence of bilateral mammary infection and, secondly, the pathogen was isolated from the liver of two of the four infected ewes. These findings are only partly in accordance with other case studies, which consider intramammary infection to be the most likely and emphasize that L. monocytogenes has to contaminate the teat before penetration into the udder [5][13][14][15][16][17].
The histopathological and immunohistochemical findings revealed that chronic inflammatory features predominated [14][17]. There was a diffuse infiltration with lymphocytes, plasma cells and macrophages. Additionally, alveolar destruction and proliferation of fibrous tissue were recorded with a very strong immunoreactivity for CD5 cells.
Listeria could be cultivated from the mammary parenchyma of the infected halves and from the lymph nodes [12][14][17]. In contrast to the Greek study, all other internal organs showed no abnormalities, and no single Listeria could be isolated [14][17]).
Research findings suggest that caprine and ovine mastitis are very much comparable. Furthermore, the typical listerial mastitis in small ruminants is defined: (i) by its subclinical nature; (ii) a high somatic cell count (≥106 SCC per ml); (iii) persistent shedding of the pathogen bacteria; (iv) by induration and atrophy of the mammary parenchyma in progredient stages of the infection and, finally; (v) the local invasion via the teat canal seems to be the most likely route of infection. Figure 2 illustrates the main clinical and pathological findings of listerial mastitis in small ruminants. A risk scenario was designed to highlight the dimension of the consumers’ exposure.
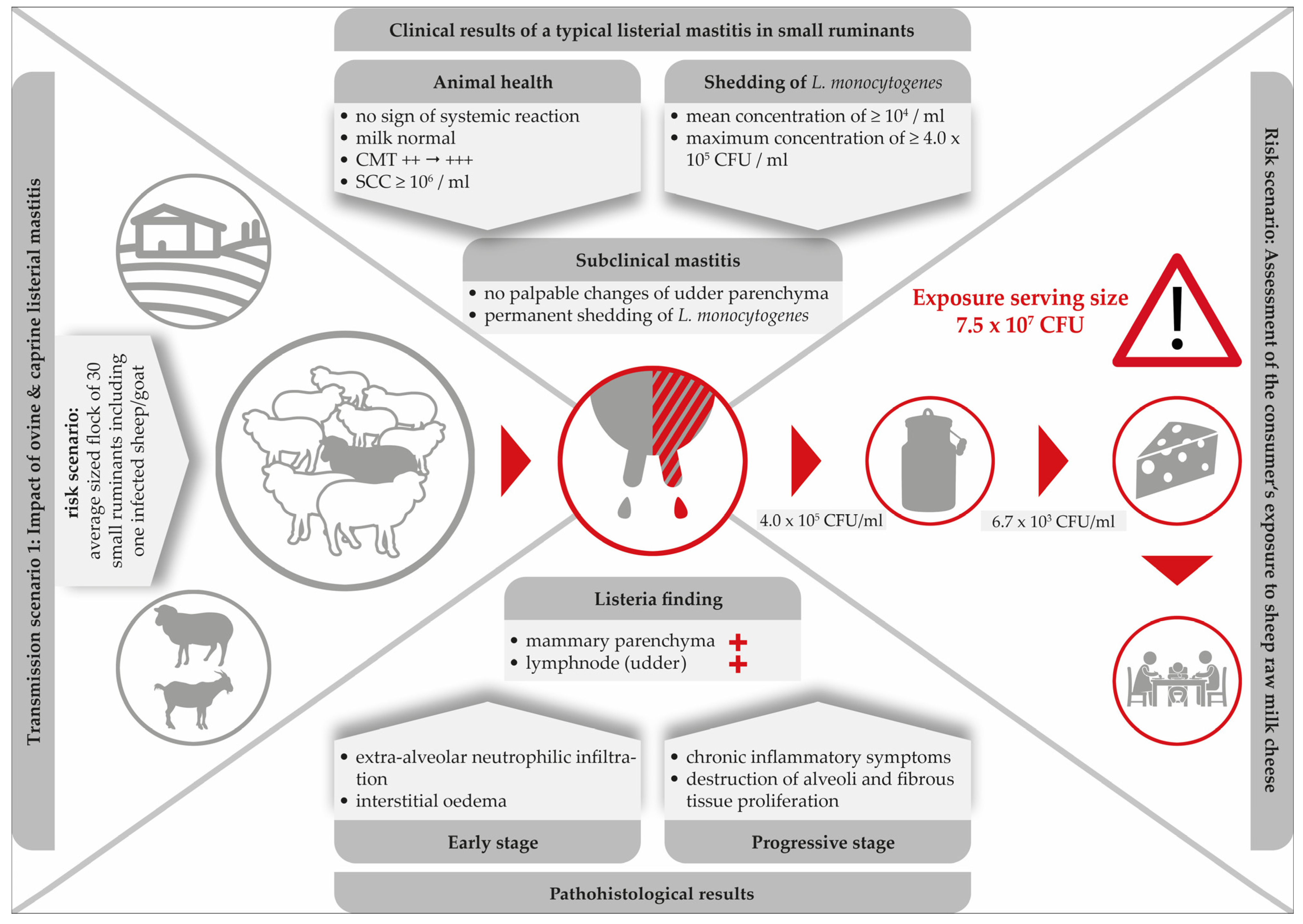
Figure 2. Clinical and histopathological findings in a typical listerial mastitis in small ruminants and consequences for the safety of cheese produced on-farm.
Clearly, mastitis attributed to L. monocytogenes is especially dangerous due to its subclinical nature. While milk from infected udders remains visually unchanged and the udders show no clinical signs, L. monocytogenes continues to be shed up to concentrations of 4.0 × 105 CFU/mL [13]. With respect to food safety, listerial mastitis has two main consequences: firstly, the direct contamination of bulk milk and raw milk cheese with high loads of the pathogen and, secondly, the increase of environmental colonization of the farm and the cheese processing environment. Furthermore, within the last decades, hypervirulent L. monocytogenes strains were found to be significantly associated with subclinical mastitis and were more commonly isolated from dairy products [58].
Remarkably, merely three single studies have been published demonstrating the consequences of ovine listerial mastitis on the further processing of milk to cheese [13][15][16]. Based on two cases of ovine mastitis, a risk scenario was designed in order to assess the consumer’s exposure to L. monocytogenes per serving size of sheep raw milk cheese [15]. Various cheese-making procedures were performed. The results were alarming: the final level of contamination was up to 7.5 × 107 CFU/serving size. Certainly, such an extremely high dose qualifies the cheese to be hazardous for consumers (Figure 2).
Clearly, there is an urgent need to screen small ruminant farms for the presence of cases of subclinical L. monocytogenes mastitis by implementing CMT at least once per week [13]. With regard to caprine mastitis, however, milk SCC is a less reliable indicator of inflammation than in other dairy animals [59]. Therefore, the routine control of subclinical mastitis cases by SCC monitoring, such as with the CMT, is less meaningful than in cows or ewes [17]. In conclusion, Addis et al. [17] emphasized that the milk of all goats of a dairy farm should be screened for the presence of L. monocytogenes on a regular basis.
2. Transmission Scenario 2: Impact of Clinical Listeriosis
L. monocytogenes is a globally distributed pathogen with the ability to cause disease in a wide range of animal species, though sheep are particularly susceptible to infection. In the northern hemisphere, infections are typically seasonal and most common sporadically in winter and early spring in association with silage feeding. Meanwhile, in the southern hemisphere, most listeriosis cases in ruminants occur during the warmest months of the year and the transition from rainy to dry season. It can be assumed, that not only silage, but also feedstuff and water generally play an additional role in the mode of infection [60].
Listeriosis of small ruminants is well documented and there is a numerous number of case studies, including two comprehensive review articles. However, case studies focusing on the impact on milk processing are scarce [28][47][48] (Figure 1). The disease is clearly and most commonly caused by oral infection, but other entrance sites, such as the conjunctiva, microlesions of the skin, buccal and genital mucosa, or the teat canal have also been described [61]. After oral infection, L. monocytogenes is able to colonize the gastrointestinal tract. Animals either become asymptomatic carriers or they develop mild symptoms of a self-limiting enteritis. In both cases, the bacterium is shed with the faeces and is able to heavily contaminate the farm and milk processing environment (Figure 3), [1]. The interplay of environmental reservoirs outside the farm and of vectors and the farm animals is shown in Figure 3. Notably, the excretion of L. monocytogenes by the farm animals is not only a food safety and herd health issue, but can also contribute to infection of wildlife.
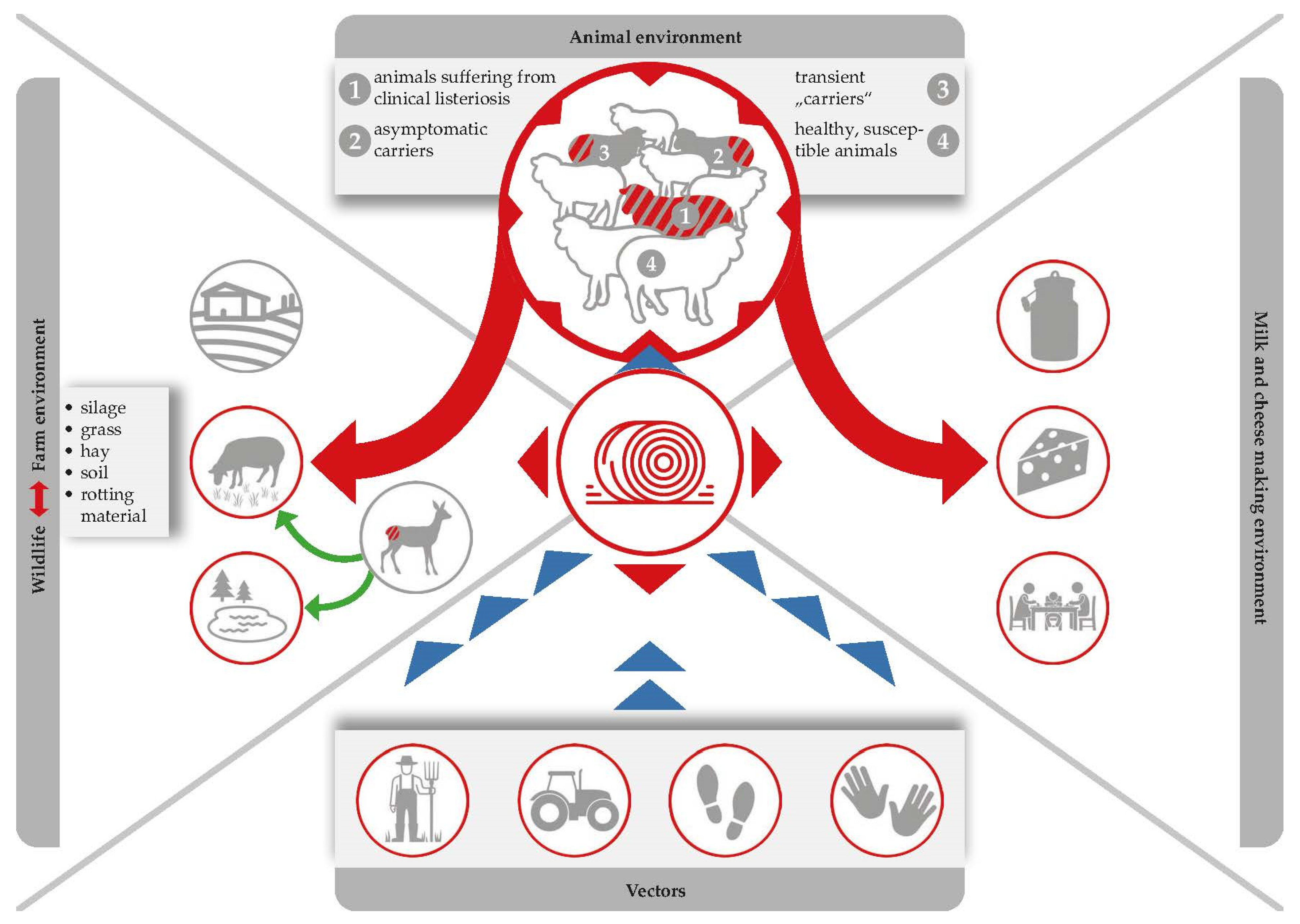
Figure 3. Silage serves as the most important Listeria reservoir. Ingestion of contaminated feed leads to the pathogen multiplying within animal hosts, and the bacteria are then excreted via faeces, which are in turn used as fertilizers, which forms a recurring cycle that favours the persistence of L. monocytogenes in both farm and natural environments.
Interestingly, there is a study describing the case of an orally infected sheep that carried L. monocytogenes in the spleen, liver and lymphoid organs without showing any clinical symptoms. The authors concluded that L. monocytogenes intestinal infection and translocation to visceral organs may occur asymptomatically [62]. Additionally, in the case of invasive listeriosis, the pathogen is able to cross the gastrointestinal barrier causing severe illnesses including abortion, septicaemia and rhombencephalitis—the so-called “circling disease”—which accounts for the vast majority of invasive clinical infections in small ruminants [60][61].
The incubation period in small ruminants varies according to pathogenesis. It can be as short as one to two days for septicaemia or gastrointestinal forms, two weeks for abortion, and between four and six weeks for the encephalitic form. The pathogen has a particular affinity for the central nervous system in sheep. The main clinical signs include apathy, fever, anorexia, head pressing or compulsive circling and unilateral or bilateral cranial nerve deficits. L. monocytogenes ascends the nervous system following peripheral traumatic lesions (e.g., ascending intra-axonal migration within the trigeminal nerve or other cranial nerves following small lesions of the buccal mucosa). Another route involves ascending infection via the sensory nerves of the skin [60].
Neurological symptoms leave little doubt as to their cause and affected animals can be removed from herds. The milk and meat of these affected animals is rather unlikely to enter the food chain [28][47][48]. However, especially during an outbreak event, massive contamination of the animal environment, both through contaminated feed and faecal shedding from exposed animals, may lead to cross-contamination of: (i) the milk processing and cheese-making environment; (ii) bulk tank milk; and (iii) subsequently, the cheese products themselves (Figure 3).
Following an outbreak of clinical listeriosis in sheep, Wagner et al. [48] sourced the infectious agent to grass silage feed, which was contaminated with 105 CFU/g L. monocytogenes. The investigation took place on a dairy farm producing raw milk cheese made of 50% ewe and 50% cow milk. Dairy cows were not affected by this outbreak, reflecting the high susceptibility of sheep to listerial infection. Interestingly, the clinical manifestation within the flock of 55 sheep was also quite variable. Although they were all fed from the same batch of silage, only one ewe was affected by central nervous symptoms caused by rhombencephalitis, four ewes suffered from septicaemia and a further nine animals delivered a combined total of 20 stillborn mature foetuses.
From the animal that had developed central nervous symptoms, L. monocytogenes could neither be recovered from the visceral organs nor in the faeces, but was found in a blood sample taken directly from the heart, brain and the nasal mucosa. The authors concluded that the infection had originated within the nasal mucosa and spread to the brain, but that the liver, spleen and other visceral organs remained clear. The route of infection in the other animals was most likely via feedborne transmission. Those with septicaemia suffered from accumulation of Listeria in the liver, spleen, heart and lung, with a median concentration of 5.9 × 105 to 6.4 × 106 CFU/g. L. monocytogenes could also be detected in the foetal liver, spleen, lung, heart and brain with values ranging from 3.1 × 103 to 5.6 × 105 CFU/g.
Samples from both the farm environment and the cheese production chain, which were randomly taken from ewes, cattle and all individuals who lived on the farm, were positive for L. monocytogenes, including 62% of faecal samples and the bulk tank of the cows. Interestingly, one farm worker tested positive for an isolate that was so similar to the outbreak clone that it could not be distinguished genetically, which clearly occurred through them consuming contaminated raw bovine milk. Due to intensive consultation and the fact that the most important countermeasures were immediately taken (silage had been discarded, affected animals had been separated and cleaning and disinfection of the cheese making facilities were implemented), L. monocytogenes was not detected in the cheese samples [48].
3. Molecular Epidemiological Aspects of Listeria monocytogenes
Bagatella et al. [60] provides a comprehensive overview of epidemiological and experimental studies, which highlight the genetic heterogeneity of L. monocytogenes in humans and in ruminants. A heterogeneity, which is likely linked to the variability observed in virulence and in clinical manifestations, as well as to the environmental distribution of listeriosis [63][64][65]. Research is currently ongoing in an attempt to identify the bacterial determinants driving variability and niche adaptation in L. monocytogenes, as well as the principally associated mechanisms [66]. Several bacterial subtypes have been characterized and efforts made to associate them with particular niches and relative virulence. Of the 13 serovars identified, types 1/2a, 1/2b, and 4b were those most frequently found in clinical isolates from both humans and animals. Meanwhile, in cases of ruminant neurolisteriosis and in major outbreaks of listeriosis, serotype 4b was the most dominant [30][37][67][68][69].
All 3 serotypes, apart from being implicated in disease, were additionally isolated from food, food processing and farm environments, and animal faeces [6][70][71][72]. Isolates can be linked to clinical outcomes, the environment and foods through molecular typing methods, including pulsed field gel electrophoresis, multilocus sequence typing (MLST) and whole genome sequencing (WGS). Using these techniques, four distinct lineages (I–IV) were identified and further subdivided into clonal complexes (CCs) and sequence types (STs), or sublineages (SLs) and core genome MLST types (CTs), respectively [73].
L. monocytogenes that can be frequently isolated from diverse sources are binned into two major lineages (I, II), with lineage I being overrepresented in human clinical isolates and ruminant neurolisteriosis cases as well as being the most genetically homogeneous, while L. monocytogenes that are sporadically isolated from animal infections are binned into two minor lineages (III, IV) [34][65][69][74][75][76].
Several CCs were found to be hypervirulent in experimental models, including CCs from lineage I belonging to serotype 4b (such as CC1, CC2, CC4, and CC6), these were also significantly linked to human clinical cases and well-adapted to host colonization compared to clones overrepresented in food and the environment (such as CC9 and CC121) [63][64][75].
Within clinical isolates and particularly neurolisteriosis isolates from ruminants, lineage I, specifically CC1 and CC4, were found to be significantly overrepresented compared with other clinical listeriosis syndromes in ruminants, such as abortion, mastitis or gastroenteritis. Additional isolates, from diseased animals and diseased animal environments, that are commonly found include isolates from both lineage I (CC2, CC217, CC6, CC191, CC59) and lineage II (CC7, CC11, CC14, CC37, CC204, CC412) [34][58][65][72].
Researchers can conclude that preventing disease in ruminants and its concomitant transmission to humans is a challenging task, requiring efficient surveillance and control measures. As ruminants, humans and the environment are indelibly connected, achieving a more comprehensive understanding of the pathogenesis of listeriosis and its molecular epidemiology within these domains is critical for developing methodologies to meet the challenge in congruence with the “Farm to Fork” strategy and One Health concepts.
4. Transmission Scenario 3: Impact of Farm Management and Feeding Practices
Transmission of foodborne pathogens frequently involves complex interactions among the pathogen, the environment and one or multiple host species [77]. L. monocytogenes is a ubiquitous pathogen that can be found in moist environments, soil, water and decaying vegetation [78]. However, does L. monocytogenes still have its origin and main habitat in the natural environment and wildlife? Or, can researchers assume that this major pathogen acts as a cultural successor, which has already successfully colonized the farm- and food-production environment, creating new reservoirs there? Interestingly, the prevalence of L. monocytogenes in the dairy cattle environment is well documented [79][80]). There are also numerous studies focusing on the occurrence of L. monocytogenes in small ruminant farms. However, the knowledge of Listeria transmission dynamics and ecology in on-farm dairies is limited (Figure 1).
L. monocytogenes prevalence is normally lower during the pasture season than it is during the indoor season [81][82]. Furthermore, the pathogen has been isolated from both clinically infected and clinically symptomless ruminants. In fact, L. monocytogenes can be shed by (i) healthy sheep and goats (so called transient “carriers” and asymptomatic carriers); and (ii) by ruminants suffering from a clinical listeriosis (Figure 3).
Faecal shedding of L. monocytogenes has several effects on food safety: (i) L. monocytogenes accumulation within the immediate environment of the barn increases the probability that more animals will become infected; (ii) contamination of feed and crops with L. monocytogenes can occur when the manure of infected animals is used as fertilizer in agriculture, whilst water sources can be contaminated by runoff from farms [83][84]; (iii) raw milk contamination may occur due to poor hygiene standards during the milking of animals in which infection has gone undetected (Figure 3).
Ingestion of contaminated feed, multiplication of the pathogen in animal hosts, and subsequent excretion of the bacterium via faeces, which are in turn used as fertilizers, form a recurring cycle which favours the persistence of L. monocytogenes (Figure 3), [79]. It cannot be denied that there is a high contamination pressure of L. monocytogenes on dairy farms and researchers have to admit that the problem is entirely self-generated. Alarmingly, L. monocytogenes may be present in 8% up to as much as 50% of faecal samples collected from dairy sheep and goats [21][24][28][49]. The shedding itself is associated with animal stress and is strongly connected to the contamination of silage [85]. While L. monocytogenes is rarely detected on growing grasses prior to processing, detection rates in clamp silages range from between 2.5% and 5.9% and reach up to 22.2% in large bales. This further increases to an alarming 44% in mouldy silage samples [86]. Alternatively, use of inadequately fermented silage (pH of 5.0 to 5.5) contaminated by soil and tainted crops can permit subsequent amplification of L. monocytogenes numbers to high levels. In this way, field studies consistently highlight silage feeding as the main factor associated with farm animal exposure. However, the pathogen could also be isolated from a number of other sources, including bedding material, feed bunks, and water troughs [87][88].
Once ingested via feed, L. monocytogenes transforms its metabolism and colonises the ruminant gastrointestinal tract intracellularly as a cytosol-adapted pathogen, thereby escaping immune defence. According to Zundel and Bernard [62], L. monocytogenes multiplied in the rumen of sheep who were asymptomatic carriers due to the favourable environment of the organ (pH 6.5–7.2 and body temperatures from 38.0 to 40.5 °C). Thus, the rumen content serves as an important reservoir for Listeria.
However, there is still the widespread opinion that grass and soil are initially contaminated by wildlife such as deer and birds, which means that dairy farm animals are mainly subsequently challenged with L. monocytogenes, either during grazing or after consumption of silage: Indeed, asymptomatic carriage of L. monocytogenes is thought to be prevalent in up to 36% of wild birds. This includes a variety of species, such as crows, gulls, pheasants, pigeons, rooks, and sparrows [1]. Interestingly, it was suggested that birds may be somewhat responsible for spreading strains of L. monocytogenes within the human food chain, as there were often similarities in the pulsotypes isolated from the birds with those found in the food chain [89]. However, the findings do not explain if the birds are infected when feeding on fertilized fields contaminated with Listeria, if birds contaminate the environment or if both situations apply. Additionally, a wide range of mammals, such as red fox (3.5%), wild boars (25%), and deer (42%), also harbour L. monocytogenes [1]. Silage winter feeding is a common practice for free-living [90][91]) as well as farmed [92] wild ruminants in alpine regions, and it remains to be explored to what extent this practice contributes to Listeria infections in wildlife. Again, there is considerable evidence that the high prevalence rate in wild animals is entirely self-generated.
Finally, faecal transmission of L. monocytogenes is not exclusively driven by animals, either wild or domestic, as it has been shown to occur regularly in humans also [1]. A number of studies have investigated such transmission within specific occupational groups. Laboratory technicians had a 77% high cumulative prevalence rate of faecal carriage. However, prevalence was also very high (62%) in office workers, who were not occupationally exposed to L. monocytogenes [93]. Furthermore, 16% of swab samples from the hands of farmers [94] and 5.7% of swab samples from hands and working clothes of abattoir workers [95] were positive for L. monocytogenes [1].
Faecal shedding of L. monocytogenes by asymptomatic farm animals increases its presence within the farm environment, which leads to an increased risk of feed and food contamination (Figure 3). Therefore, the ecology of L. monocytogenes within the agricultural environment should be thorough analysed and Listeria reservoirs should be identified and removed as part pathogen reduction programs [71].
Hence, in order to gain a more comprehensive understanding of the transmission dynamics and ecology of L. monocytogenes, a prevalence study was conducted in the dairy-intensive region of Austria, focusing on small ruminant on-farm dairies [24]. The study focused on dairy farms that manufactured cheese from raw caprine and ovine milk, and aimed to identify the routes of transmission of Listeria spp. and to investigate the link between L. monocytogenes mastitis and the contamination of raw milk. A total of 5799 samples were taken from 53 Austrian dairy farms, and the pathogen was found in 0.9% of them. However, none of the samples taken from the udders of the sheep or goats tested positive, meaning that raw milk contamination was not significantly impacted by listerial mastitis.
The prevalence levels from swab samples of working boots and faecal samples were 15.7% and 13.0%, respectively. The investigators concluded that silage feeding practices correlated significantly with the prevalence of L. monocytogenes in the farm and milk processing environments. Again, silage was a main culprit, such that L. monocytogenes was between three to seven times more likely to be present in farms that fed silage to animals year-round than in farms that did not use silage [24].
Appraisal of state-of-the-art studies now leads researchers to conclude that silage and the rumen itself serve as the most important Listeria reservoirs. While the pathogen persists in a cyclic infection (from faecal excretion to contamination of feed to multiplication in the gastrointestinal tract) [79], it can enter the food chain either by contaminating raw milk or by being excreted from the udder of an infected animal. In turn, this contamination can spread silently to the milk and cheese processing environment (Figure 3); once contaminated, milk and cheese processing devices and premises can act as a reservoir for Listeria and contaminate product batches that were originally free from the pathogen.
References
- Schoder, D.; Guldimann, C.; Märtlbauer, E. Asymptomatic Carriage of Listeria monocytogenes by Animals and Humans and Its Impact on the Food Chain. Foods 2022, 11, 3472.
- Gitter, M.; Bradley, R.; Blampied, P.H. Listeria monocytogenes in bovine mastitis. Vet. Rec. 1980, 107, 390–393.
- Sharp, M.W. Bovine mastitis and Listeria monocytogenes. Vet. Rec. 1989, 125, 512–513.
- Fedio, W.M.; Schoonderwoerd, M.; Shute, R.H.; Jackson, H. A case of bovine mastitis caused by Listeria monocytogenes. Can. Vet. J. 1990, 31, 773–775.
- Bourry, A.; Poutrel, B.; Rocourt, J. Bovine mastitis caused by Listeria monocytogenes: Characteristics of natural and experimental infections. J. Med. Microbiol. 1995, 43, 125–132.
- Jensen, N.E.; Aarestrup, F.M.; Jensen, J.; Wegener, H.C. Listeria monocytogenes in bovine mastitis. Possible implication for human health. Int. J. Food Microbiol. 1996, 32, 209–216.
- Wagner, M.; Podstatzky-Lichtenstein, L.; Lehner, A.; Asperger, H.; Baumgartner, W.; Brandl, E. Prolonged excretion of Listeria monocytogenes in a subclinical case of mastitis. Milchwissenschaft 2000, 55, 3–6.
- Rawool, D.B.; Malik, S.V.; Shakuntala, I.; Sahare, A.M.; Barbuddhe, S.B. Detection of multiple virulence-associated genes in Listeria monocytogenes isolated from bovine mastitis cases. Int. J. Food Microbiol. 2007, 113, 201–207.
- Hunt, K.; Drummond, N.; Murphy, M.; Butler, F.; Buckley, J.; Jordan, K. A case of bovine raw milk contamination with Listeria monocytogenes. Ir. Vet. J. 2012, 65, 13.
- Bourry, A.; Cochard, T.; Poutrel, B. Serological diagnosis of bovine, caprine, and ovine mastitis caused by Listeria monocytogenes by using an enzyme-linked immunosorbent assay. J. Clin. Microbiol. 1997, 35, 1606–1608.
- Fthenakis, G.C.; Saratsis, P.; Tzora, A.; Linde, K. Naturally occurring subclinical ovine mastitis associated with Listeria monocytogenes. Small Rumin. Res. 1998, 31, 23–27.
- Tzora, A.; Fthenakis, G.C.; Linde, K. The effects of inoculation of Listeria monocytogenes into the ovine mammary gland. Vet. Microbiol. 1998, 59, 193–202.
- Schoder, D.; Winter, P.; Kareem, A.; Baumgartner, W.; Wagner, M. A case of sporadic ovine mastitis caused by Listeria monocytogenes and its effect on contamination of raw milk and raw-milk cheeses produced in the on-farm dairy. J. Dairy Res. 2003, 70, 395–401.
- Winter, P.; Schilcher, F.; Bagò, Z.; Schoder, D.; Egerbacher, M.; Baumgartner, W.; Wagner, M. Clinical and histopathological aspects of naturally occurring mastitis caused by Listeria monocytogenes in cattle and ewes. J. Vet. Med. Ser. B Infect. Dis. Vet. Public Health 2004, 51, 176–179.
- Schoder, D.; Zangana, A.; Paulsen, P.; Winter, P.; Baumgartner, W.; Wagner, M. Ovine Listeria monocytogenes mastitis and human exposure via fresh cheese from raw milk—The impact of farm management, milking and cheese manufacturing practices. Milchwissenschaft 2008, 63, 258–262.
- Pintado, C.M.; Grant, K.A.; Halford-Maw, R.; Hampton, M.D.; Ferreira, M.A.; McLauchlin, J. Association between a case study of asymptomatic ovine listerial mastitis and the contamination of soft cheese and cheese processing environment with Listeria monocytogenes in Portugal. Foodborne Pathog. Dis. 2009, 6, 569–575.
- Addis, M.F.; Cubeddu, T.; Pilicchi, Y.; Rocca, S.; Piccinini, R. Chronic intramammary infection by Listeria monocytogenes in a clinically healthy goat—A case report. BMC Vet. Res. 2019, 15, 229.
- Kulesh, R.; Shinde, S.V.; Khan, W.A.; Chaudhari, S.P.; Patil, A.R.; Kurkure, N.V.; Paliwal, N.; Likhite, A.V.; Zade, N.N.; Barbuddhe, S.B. The occurrence of Listeria monocytogenes in goats, farm environment and invertebrates. Biol. Rhythm. Res. 2022, 53, 831–840.
- Zhao, Q.; Hu, P.; Li, Q.; Zhang, S.; Li, H.; Chang, J.; Jiang, Q.; Zheng, Y.; Li, Y.; Liu, Z.; et al. Prevalence and transmission characteristics of Listeria species from ruminants in farm and slaughtering environments in China. Emerg. Microbes Infect. 2021, 10, 356–364.
- Condoleo, R.; Giangolini, G.; Chiaverini, A.; Patriarca, D.; Scaramozzino, P.; Mezher, Z. Occurrence of Listeria monocytogenes and Escherichia coli in Raw Sheep’s Milk from Farm Bulk Tanks in Central Italy. J. Food Prot. 2020, 83, 1929–1933.
- Hurtado, A.; Ocejo, M.; Oporto, B. Salmonella spp. and Listeria monocytogenes shedding in domestic ruminants and characterization of potentially pathogenic strains. Vet. Microbiol. 2017, 210, 71–76.
- Jamali, H.; Radmehr, B.; Thong, K.L. Prevalence, characterisation, and antimicrobial resistance of Listeria species and Listeria monocytogenes isolates from raw milk in farm bulk tanks. Food Control 2013, 34, 121–125.
- Al-Mariri, A.; Younes, A.A.; Ramadan, L. Prevalence of Listeria spp. in Raw Milk in Syria. Bulg. J. Vet. Med. 2013, 16, 112–122.
- Schoder, D.; Melzner, D.; Schmalwieser, A.; Zangana, A.; Winter, P.; Wagner, M. Important vectors for Listeria monocytogenes transmission at farm dairies manufacturing fresh sheep and goat cheese from raw milk. J. Food Prot. 2011, 74, 919–924.
- Ho, A.J.; Lappi, V.R.; Wiedmann, M. Longitudinal monitoring of Listeria monocytogenes contamination patterns in a farmstead dairy processing facility. J. Dairy Sci. 2007, 90, 2517–2524.
- D’Amico, D.J.; Groves, E.; Donnelly, C.W. Low incidence of foodborne pathogens of concern in raw milk utilized for farmstead cheese production. J. Food Prot. 2008, 71, 1580–1589.
- D’Amico, D.J.; Donnelly, C.W. Microbiological quality of raw milk used for small-scale artisan cheese production in Vermont: Effect of farm characteristics and practices. J. Dairy Sci. 2010, 93, 134–147.
- Nightingale, K.K.; Schukken, Y.H.; Nightingale, C.R.; Fortes, E.D.; Ho, A.J.; Her, Z.; Grohn, Y.T.; McDonough, P.L.; Wiedmann, M. Ecology and transmission of Listeria monocytogenes infecting ruminants and in the farm environment. Appl. Environ. Microbiol. 2004, 70, 4458–4467.
- Samkange, A.; van der Westhuizen, J.; Voigts, A.S.; Chitate, F.; Kaatura, I.; Khaiseb, S.; Hikufe, E.H.; Kabajani, J.; Bishi, A.S.; Mbiri, P.; et al. Investigation of the outbreaks of abortions and orchitis in livestock in Namibia during 2016–2018. Trop. Anim. Health Prod. 2022, 54, 346.
- Kotzamanidis, C.; Papadopoulos, T.; Vafeas, G.; Tsakos, P.; Giantzi, V.; Zdragas, A. Characterization of Listeria monocytogenes from encephalitis cases of small ruminants from different geographical regions, in Greece. J. Appl. Microbiol. 2019, 126, 1373–1382.
- Rissi, D.R.; Kommers, G.D.; Marcolongo-Pereira, C.; Schild, A.L.; Barros, C.S.L. Meningoencephalitis in sheep caused by Listeria monocytogenes . Pesqui. Vet. Bras. 2010, 30, 51–56.
- Hamidi, A.; Bisha, B.; Goga, I.; Wang, B.; Robaj, A.; Sylejmani, D.S. A case report of sporadic ovine listerial meningoencephalitis in Kosovo. Vet. Ital. 2020, 56, 205–211.
- Papić, B.; Kušar, D.; Zdovc, I.; Golob, M.; Pate, M. Retrospective investigation of listeriosis outbreaks in small ruminants using different analytical approaches for whole genome sequencing-based typing of Listeria monocytogenes. Infect. Genet. Evol. 2020, 77, 104047.
- Dreyer, M.; Thomann, A.; Böttcher, S.; Frey, J.; Oevermann, A. Outbreak investigation identifies a single Listeria monocytogenes strain in sheep with different clinical manifestations, soil and water. Vet. Microbiol. 2015, 179, 69–75.
- Wesley, I.V.; Larson, D.J.; Harmon, K.M.; Luchansky, J.B.; Schwartz, A.R. A case report of sporadic ovine listerial menigoencephalitis in Iowa with an overview of livestock and human cases. J. Vet. Diagn. Investig. 2002, 14, 314–321.
- Al-Dughaym, A.M.; Elmula, A.F.; Mohamed, G.E.; Hegazy, A.A.; Radwan, Y.A.; Housawi, F.M.; Gameel, A.A. First report of an outbreak of ovine septicaemic listeriosis in Saudi Arabia. Rev. Sci. Tech. 2001, 20, 777–783.
- Wiedmann, M.; Czajka, J.; Bsat, N.; Bodis, M.; Smith, M.C.; Divers, T.J.; Batt, C.A. Diagnosis and epidemiological association of Listeria monocytogenes strains in two outbreaks of listerial encephalitis in small ruminants. J. Clin. Microbiol. 1994, 32, 991–996.
- Meredith, C.D.; Schneider, D.J. An outbreak of ovine listeriosis associated with poor flock management practices. J. S. Afr. Vet. Assoc. 1984, 55, 55–56.
- Wardrope, D.D.; MacLeod, N.S. Outbreak of listeria meningoencephalitis in young lambs. Vet. Rec. 1983, 113, 213–214.
- Vandegraaff, R.; Borland, N.A.; Browning, J.W. An outbreak of listerial meningo-encephalitis in sheep. Aust. Vet. J. 1981, 57, 94–96.
- Grønstøl, H. Listeriosis in sheep. Isolation of Listeria monocytogenes from grass silage. Acta Vet. Scand. 1979, 20, 492–497.
- Du Toit, I.F. An outbreak of caprine listeriosis in the Western Cape. J. S. Afr. Vet. Assoc. 1977, 48, 39–40.
- McDonald, J.W. An outbreak of abortion due to Listeria monocytogenes in an experimental flock of sheep. Aust. Vet. J. 1967, 43, 564–567.
- Gitter, M.; Terlecki, S.; Turnbull, P.A. An outbreak of visceral and cerebral listeriosis in a flock of sheep in South East England. Vet. Rec. 1965, 77, 11–15.
- Schlech, W.F., III. New perspectives on the gastrointestinal mode of transmission in invasive Listeria monocytogenes infection. Clin. Investig. Med. 1984, 7, 321–324.
- Grønstøl, H. Listeriosis in sheep. Listeria monocytogenes excretion and immunological state in healthy sheep. Acta Vet. Scand. 1979, 20, 168–179.
- Nightingale, K.K.; Fortes, E.D.; Ho, A.J.; Schukken, Y.H.; Grohn, Y.T.; Wiedmann, M. Evaluation of farm management practices as risk factors for clinical listeriosis and fecal shedding of Listeria monocytogenes in ruminants. J. Am. Vet. Med. Assoc. 2005, 227, 1808–1814.
- Wagner, M.; Melzner, D.; Bagò, Z.; Winter, P.; Egerbacher, M.; Schilcher, F.; Zangana, A.; Schoder, D. Outbreak of clinical listeriosis in sheep: Evaluation from possible contamination routes from feed to raw produce and humans. J. Vet. Med. Ser. B Infect. Dis. Vet. Public Health 2005, 52, 278–283.
- Palacios-Gorba, C.; Moura, A.; Gomis, J.; Leclercq, A.; Gomez-Martin, A.; Bracq-Dieye, H.; Moce, M.L.; Tessaud-Rita, N.; Jimenez-Trigos, E.; Vales, G.; et al. Ruminant-associated Listeria monocytogenes isolates belong preferentially to dairy-associated hypervirulent clones: A longitudinal study in 19 farms. Environ. Microbiol. 2021, 23, 7617–7631.
- Chitura, T.; Shai, K.; Ncube, I.; van Heerden, H. Contamination of the environment by pathogenic bacteria in a livestock farm in Limpopo Province, South Africa. Appl. Ecol. Environ. Res. 2019, 17, 2943–2963.
- Cavicchioli, V.Q.; Scatamburlo, T.M.; Yamazi, A.K.; Pieri, F.A.; Nero, L.A. Occurrence of Salmonella, Listeria monocytogenes, and enterotoxigenic Staphylococcus in goat milk from small and medium-sized farms located in Minas Gerais State, Brazil. J. Dairy Sci. 2015, 98, 8386–8390.
- Sarangi, L.N.; Panda, H.K. Isolation, characterization and antibiotic sensitivity test of pathogenic listeria species in livestock, poultry and farm environment of Odisha. Indian J. Anim. Res. 2012, 46, 242–247.
- Sharif, J.; Willayat, M.M.; Sheikh, G.N.; Roy, S.S.; Altaf, S. Prevalence of Listeria monocytogenes in Organised Sheep Farms of Kashmir. J. Pure Appl. Microbiol. 2010, 4, 871–873.
- Soncini, G.; Valnegri, L. Analysis of bulk goats’ milk and milk-filters from Valtellina and Valchiavenna (Lombardy Prealps) for the presence of Listeria species. Small Rumin. Res. 2005, 58, 143–147.
- Schoder, D.; Zangana, A.; Wagner, M. Sheep and goat raw milk consumption: A hygienic matter of concern? Arch. Lebensm. 2010, 61, 229–234.
- Almeida, G.; Magalhães, R.; Carneiro, L.; Santos, I.; Silva, J.; Ferreira, V.; Hogg, T.; Teixeira, P. Foci of contamination of Listeria monocytogenes in different cheese processing plants. Int. J. Food Microbiol. 2013, 167, 303–309.
- Miller, B.A.; Lu, C.D. Current status of global dairy goat production: An overview. Asian-Australas. J. Anim. Sci. 2019, 32, 1219–1232.
- Papić, B.; Golob, M.; Kušar, D.; Pate, M.; Zdovc, I. Source tracking on a dairy farm reveals a high occurrence of subclinical mastitis due to hypervirulent Listeria monocytogenes clonal complexes. J. Appl. Microbiol. 2019, 127, 1349–1361.
- Moroni, P.; Pisoni, G.; Ruffo, G.; Boettcher, P.J. Risk factors for intramammary infections and relationship with somatic-cell counts in Italian dairy goats. Prev. Vet. Med. 2005, 69, 163–173.
- Bagatella, S.; Tavares-Gomes, L.; Oevermann, A. Listeria monocytogenes at the interface between ruminants and humans: A comparative pathology and pathogenesis review. Vet. Pathol. 2022, 59, 186–210.
- Brugère-Picoux, J. Ovine listeriosis. Small Rumin. Res. 2008, 76, 12–20.
- Zundel, E.; Bernard, S. Listeria monocytogenes translocates throughout the digestive tract in asymptomatic sheep. J. Med. Microbiol. 2006, 55, 1717–1723.
- Maury, M.M.; Bracq-Dieye, H.; Huang, L.; Vales, G.; Lavina, M.; Thouvenot, P.; Disson, O.; Leclercq, A.; Brisse, S.; Lecuit, M. Hypervirulent Listeria monocytogenes clones’ adaption to mammalian gut accounts for their association with dairy products. Nat. Commun. 2019, 10, 2488.
- Moura, A.; Criscuolo, A.; Pouseele, H.; Maury, M.M.; Leclercq, A.; Tarr, C.; Björkman, J.T.; Dallman, T.; Reimer, A.; Enouf, V.; et al. Whole genome-based population biology and epidemiological surveillance of Listeria monocytogenes. Nat. Microbiol. 2016, 10, 16185.
- Papić, B.; Pate, M.; Félix, B.; Kušar, D. Genetic diversity of Listeria monocytogenes strains in ruminant abortion and rhombencephalitis cases in comparison with the natural environment. BMC Microbiol. 2019, 19, 299.
- Disson, O.; Moura, A.; Lecuit, M. Making Sense of the Biodiversity and Virulence of Listeria monocytogenes. Trends Microbiol. 2021, 29, 811–822.
- Swaminathan, B.; Gerner-Smidt, P. The epidemiology of human listeriosis. Microbes Infect. 2007, 9, 1236–1243.
- Johnson, G.C.; Maddox, C.W.; Fales, W.H.; Wolff, W.A.; Randle, R.F.; Ramos, J.A.; Schwartz, H.; Heise, K.M.; Baetz, A.L.; Wesley, I.V.; et al. Epidemiologic evaluation of encephalitic listeriosis in goats. J. Am. Vet. Med. Assoc. 1996, 208, 1695–1699.
- Rocha, P.R.; Lomonaco, S.; Bottero, M.T.; Dalmasso, A.; Dondo, A.; Grattarola, C.; Zuccon, F.; Iulini, B.; Knabel, S.J.; Capucchio, M.T.; et al. Ruminant rhombencephalitis-associated Listeria monocytogenes strains constitute a genetically homogeneous group related to human outbreak strains. Appl. Environ. Microbiol. 2013, 79, 3059–3066.
- Borucki, M.K.; Gay, C.C.; Reynolds, J.; McElwain, K.L.; Kim, S.H.; Call, D.R.; Knowles, D.P. Genetic diversity of Listeria monocytogenes strains from a high-prevalence dairy farm. Appl. Environ. Microbiol. 2005, 71, 5893–5899.
- Esteban, J.I.; Oporto, B.; Aduriz, G.; Juste, R.A.; Hurtado, A. Faecal shedding and strain diversity of Listeria monocytogenes in healthy ruminants and swine in Northern Spain. BMC Vet. Res. 2009, 5, 2.
- Steckler, A.J.; Cardenas-Alvarez, M.X.; Townsend Ramsett, M.K.; Dyer, N.; Bergholz, T.M. Genetic characterization of Listeria monocytogenes from ruminant listeriosis from different geographical regions in the U.S. Vet. Microbiol. 2018, 215, 93–97.
- Datta, A.R.; Burall, L.S. Serotype to genotype: The changing landscape of listeriosis outbreak investigations. Food Microbiol. 2018, 75, 18–27.
- Orsi, R.H.; den Bakker, H.C.; Wiedmann, M. Listeria monocytogenes lineages: Genomics, evolution, ecology, and phenotypic characteristics. Int. J. Med. Microbiol. 2011, 301, 79–96.
- Maury, M.M.; Tsai, Y.H.; Charlier, C.; Touchon, M.; Chenal-Francisque, V.; Leclercq, A.; Criscuolo, A.; Gaultier, C.; Roussel, S.; Brisabois, A.; et al. Uncovering Listeria monocytogenes hypervirulence by harnessing its biodiversity. Nat. Genet. 2016, 48, 308–313.
- Orsi, R.H.; Wiedmann, M. Characteristics and distribution of Listeria spp., including Listeria species newly described since 2009. Appl. Microbiol. Biotechnol. 2016, 100, 5273–5287.
- Sauders, B.D.; Fortes, E.D.; Morse, D.L.; Dumas, N.; Kiehlbauch, J.A.; Schukken, Y.; Hibbs, J.R.; Wiedmann, M. Molecular subtyping to detect human listeriosis clusters. Emerg. Infect. Dis. 2003, 9, 672–680.
- Haase, J.K.; Didelot, X.; Lecuit, M.; Korkeala, H.; Group, L.m.M.S.; Achtman, M. The ubiquitous nature of Listeria monocytogenes clones: A large-scale Multilocus Sequence Typing study. Environ. Microbiol. 2014, 16, 405–416.
- Oliver, S.P.; Jayarao, B.M.; Almeida, R.A. Food-borne pathogens in milk and the dairy farm environment: Food safety and public health implications. Foodborne Pathog. Dis. 2005, 2, 115–129.
- Waak, E.; Tham, E.; Danielsson-Tham, M.-L. Prevalence and Fingerprinting of Listeria monocytogenes Strains Isolated from Raw Whole Milk in Farm Bulk Tanks and in Dairy Plant Receiving Tanks. Appl. Environ. Microbiol. 2022, 68, 3366–3370.
- Husu, J.R. Epidemiological studies on the occurrence of Listeria monocytogenes in the feces of dairy cattle. Zent. Vet. Ser. B 1990, 37, 276–282.
- Husu, J.R.; Seppanen, J.T.; Sivela, S.K.; Rauramaa, A.L. Contamination of raw milk by Listeria monocytogenes on dairy farms. Zent. Vet. Ser. B 1990, 37, 268–275.
- Wilkes, G.; Edge, T.A.; Gannon, V.P.J.; Jokinen, C.; Lyautey, E.; Neumann, N.F.; Ruecker, N.; Scott, A.; Sunohara, M.; Topp, E.; et al. Associations among pathogenic bacteria, parasites, and environmental and land use factors in multiple mixed-use watersheds. Water Res. 2011, 45, 5807–5825.
- Lyautey, E.; Lapen, D.R.; Wilkes, G.; McCleary, K.; Pagotto, F.; Tyler, K.; Hartmann, A.; Piveteau, P.; Rieu, A.; Robertson, W.J.; et al. Distribution and characteristics of Listeria monocytogenes isolates from surface waters of the South Nation River watershed, Ontario, Canada. Appl. Environ. Microbiol. 2007, 73, 5401–5410.
- Ivaneka, R.; Grohn, Y.T.; Ho, A.J.J.; Wiedmann, M. Markov chain approach to analyze the dynamics of pathogen fecal shedding—Example of Listeria monocytogenes shedding in a herd of dairy cattle. J. Theor. Biol. 2007, 245, 44–58.
- Fenlon, D.R. Wild birds and silage as reservoirs of Listeria in the agricultural environment. J. Appl. Bacteriol. 1985, 59, 537–543.
- Mohammed, H.O.; Stipetic, K.; McDonough, P.L.; Gonzalez, R.N.; Nydam, D.V.; Atwill, E.R. Identification of potential on-farm sources of Listeria monocytogenes in herds of dairy cattle. Am. J. Vet. Res. 2009, 70, 383–388.
- Walland, J.; Lauper, J.; Frey, J.; Imhof, R.; Stephan, R.; Seuberlich, T.; Oevermann, A. Listeria monocytogenes infection in ruminants: Is there a link to the environment, food and human health? A review. Schweiz Arch. Tierheilkd. 2015, 157, 319–328.
- Hellstrom, S.; Laukkanen, R.; Siekkinen, K.M.; Ranta, J.; Maijala, R.; Korkeala, H. Listeria monocytogenes contamination in pork can originate from farms. J. Food Prot. 2010, 73, 641–648.
- Deutz, A.; Gasteiner, J.; Buchgraber, K. Fütterung von Reh- und Rotwild: Ein Praxisratgeber; Leopold Stocker Verlag: Graz, Austria, 2009.
- Resch, R.; Pötsch, E.; Klansek, E.; Gahr, F.; Leitner, A.; Rothmann, G.; Stein, M.; Buchgraber, K. Beste Heu- und Silagequalitäten für Reh- und Rotwild. Tirol. Jagdaufseher 2017, 33.
- Bundesverband Österreichischer Wildhalter. Fütterung. Available online: https://wildhaltung.at/haltung/fuetterung/ (accessed on 10 November 2022).
- Schoder, D.; Wagner, M. Growing awareness of asymptomatic carriage of Listeria monocytogenes. Wien Tierarztl. Monat. 2012, 99, 322–329.
- Tahoun, A.; Abou Elez, R.M.M.; Abdelfatah, E.N.; Elsohaby, I.; El-Gedawy, A.A.; Elmoslemany, A.M. Listeria monocytogenes in raw milk, milking equipment and dairy workers: Molecular characterization and antimicrobial resistance patterns. J. Glob. Antimicrob. Resist. 2017, 10, 264–270.
- Akkaya, L.; Alisarli, M.; Cetinkaya, Z.; Kara, R.; Telli, R. Occurrence of Escherichia coli O157: H7/O157, Listeria monocytogenes and Salmonella spp. in beef slaughterhouse environments, equipment and workers. J. Muscle Foods 2008, 19, 261–274.
More
