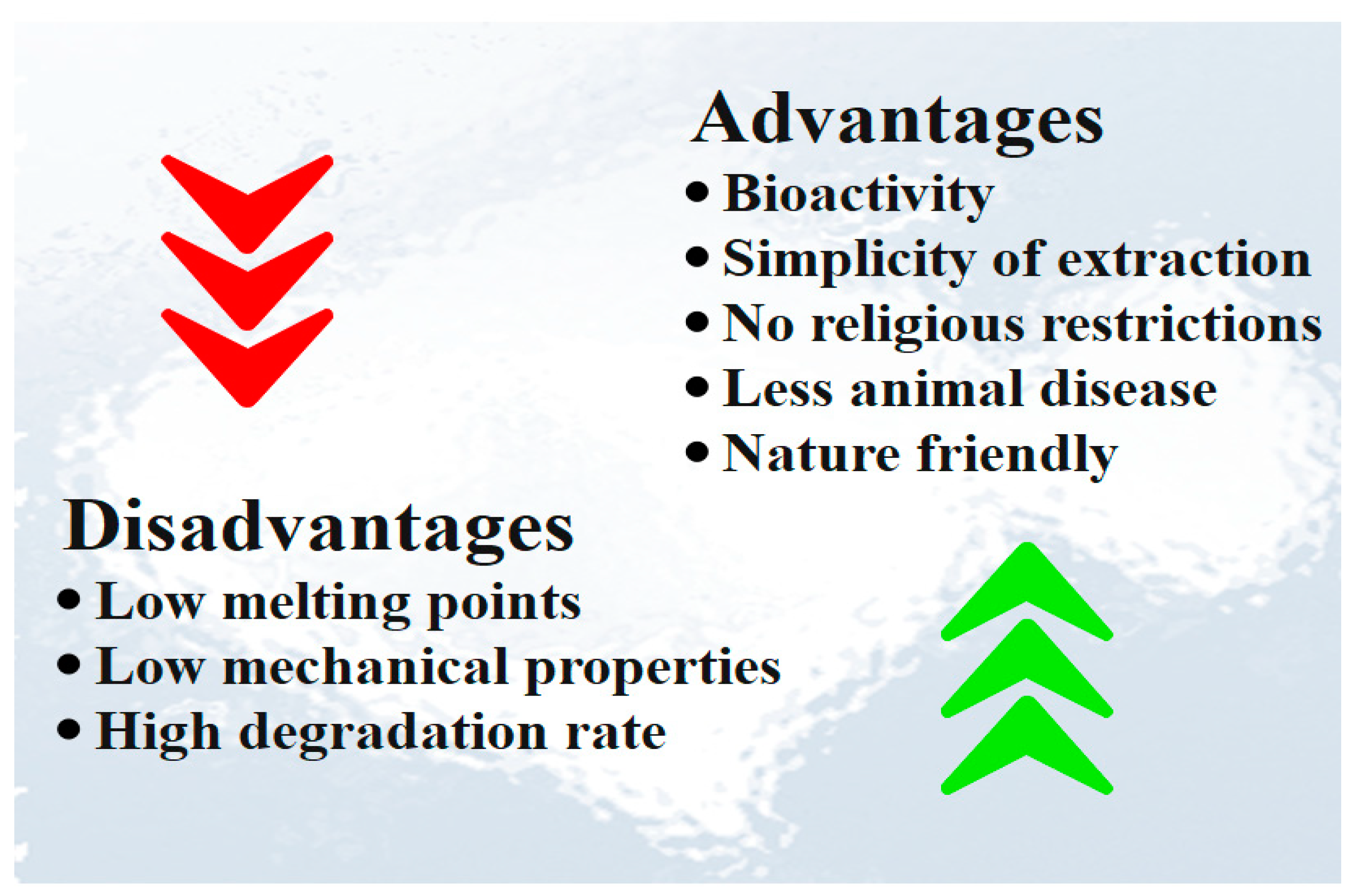
Fish collagen garnered significant academic and commercial featuring prospective applications in a variety of health-related industries, including food, medicine, pharmaceutics, and cosmetics. Due to its distinct advantages over mammalian-based collagen, including the reduced zoonosis transmission risk, the absence of cultural-religious limitations, the cost-effectiveness of manufacturing process, and its superior bioavailability, the use of collagen derived from fish wastes (i.e., skin, scales) quickly expanded. Moreover, by-products are low cost and the need to minimize fish industry waste’s environmental impact paved the way for the use of discards in the development of collagen-based products with remarkable added value.
- fish collagen
- fish industry waste
- collagen extraction
1. Introduction
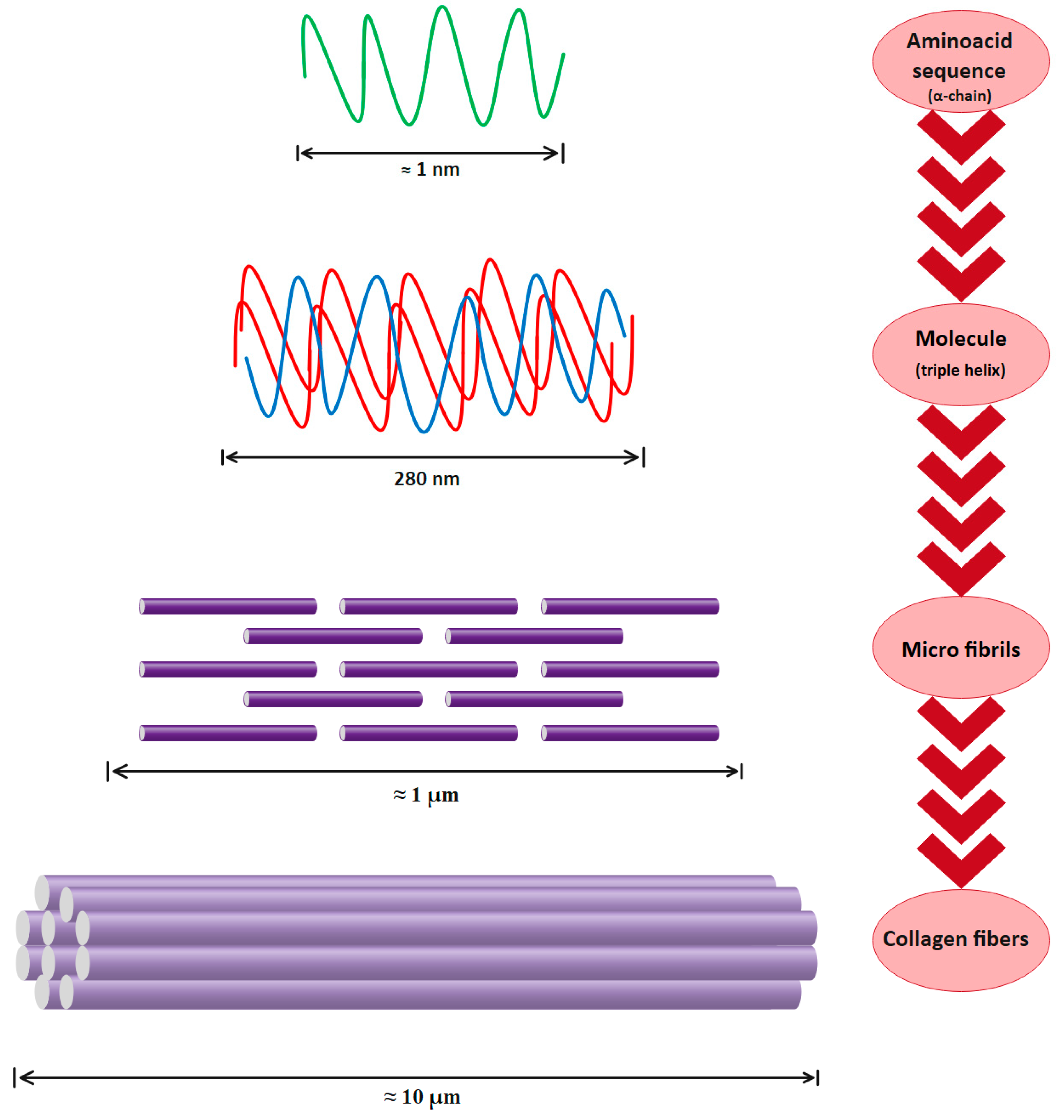
2. Collagen: Structure and Properties
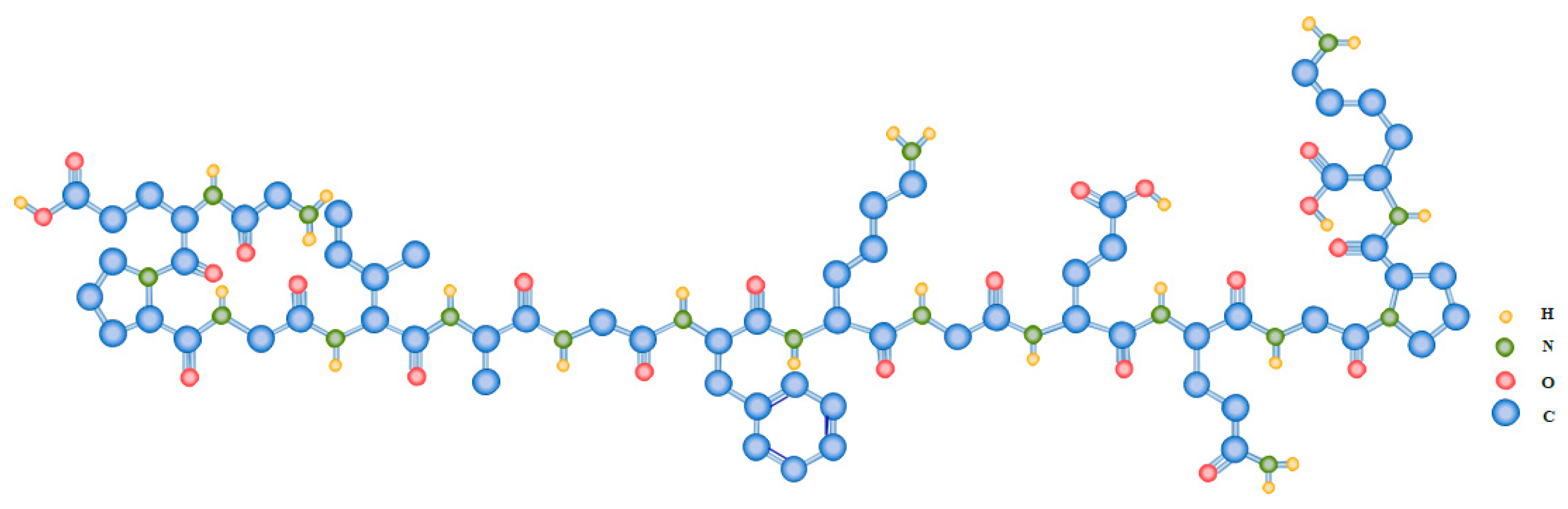
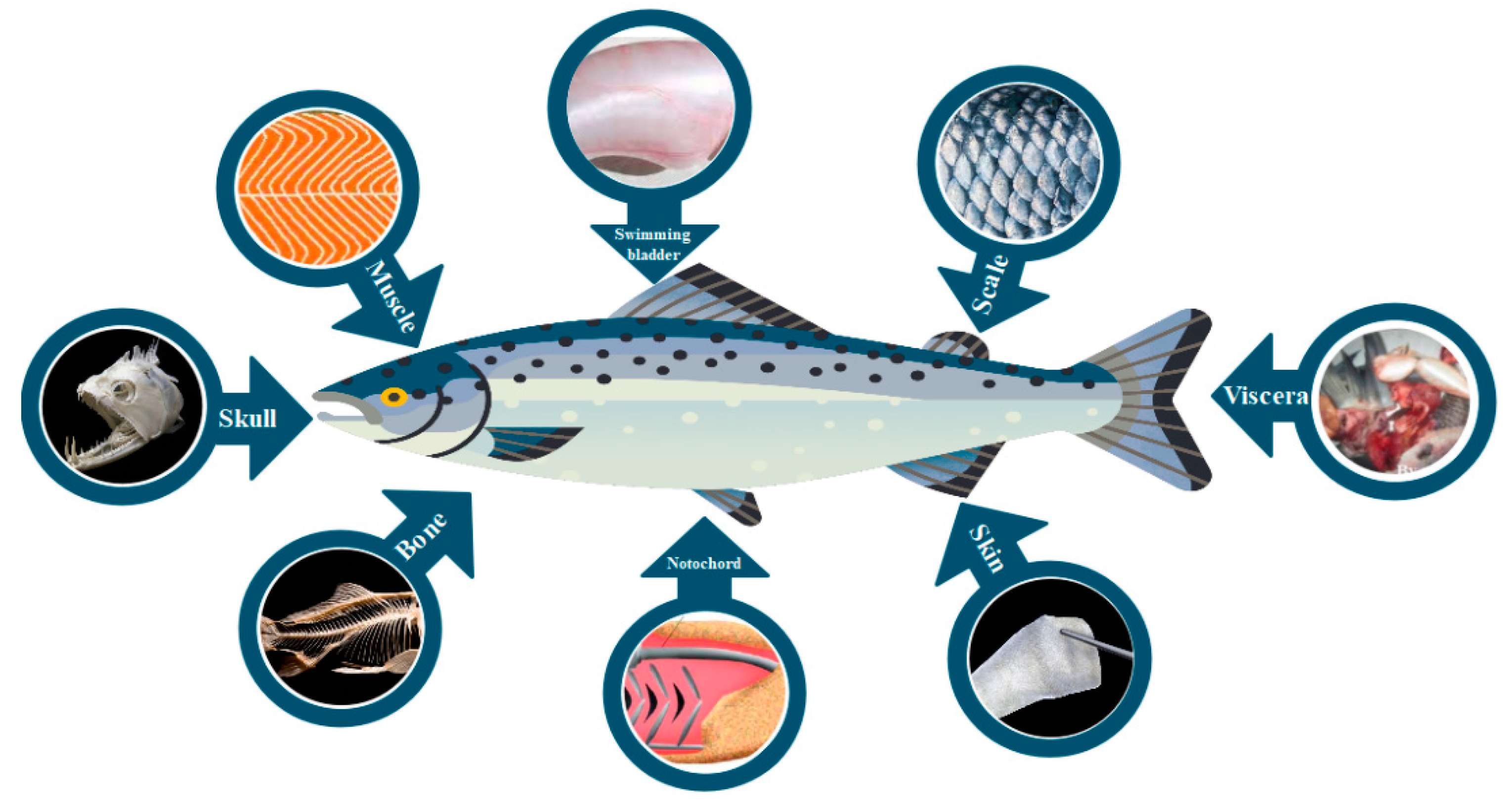
3. Fish Collagen
References
- Pal, G.K.; Nidheesh, T.; Suresh, P.V. Comparative study on characteristics and in vitro fibril formation ability of acid and pepsin soluble collagen from the skin of catla (Catla catla) and rohu (Labeo rohita). Food Res. Int. 2015, 76, 804–812.
- Jia, Y.; Wang, H.H.; Wang, H.H.; Li, Y.; Wang, M.; Zhou, J. Biochemical properties of skin collagens isolated from black carp (Mylopharyngodon piceus). Food Sci. Biotechnol. 2012, 21, 1585–1592.
- Wang, L.; An, X.; Yang, F.; Xin, Z.; Zhao, L.; Hu, Q. Isolation and characterisation of collagens from the skin, scale and bone of deep-sea redfish (Sebastes mentella). Food Chem. 2008, 108, 616–623.
- Wang, S.; Zhang, Y. Study of writing problem in college general english course—Reflection on the reform of college english course. J. Lang Teach. Res. 2017, 8, 176–183.
- Nagai, T.; Izumi, M.; Ishii, M. Fish scale collagen. Preparation and partial characterization. Int. J. Food Sci. Technol. 2004, 39, 239–244.
- Silvipriya, K.S.; Krishna Kumar, K.; Bhat, A.R.; Dinesh Kumar, B.; John, A.; Lakshmanan, P. Collagen: Animal Sources and Biomedical Application. J. Appl. Pharm. Sci. 2015, 5, 123–127.
- Shaw, C.; Knopf, K.; Kloas, W. Fish Feeds in Aquaponics and Beyond: A Novel Concept to Evaluate Protein Sources in Diets for Circular Multitrophic Food Production Systems. Sustainability 2022, 14, 4064.
- de Melo Oliveira, V.; Assis, C.R.D.; de Aquino Marques Costa, B.; de Araújo Neri, R.C.; Monte, F.T.D.; da Costa Vasconcelos Freitas, H.M.S.; Franca, R.C.P.; Santos, J.F.; de Souza Bezerra, R.; Figueiredo Porto, A.L. Physical, biochemical, densitometric and spectroscopic techniques for characterization collagen from alternative sources: A review based on the sustainable valorization of aquatic by-products. J. Mol. Struct. 2021, 1224, 129023.
- Sorushanova, A.; Delgado, L.M.; Wu, Z.; Shologu, N.; Kshirsagar, A.; Raghunath, R.; Mullen, A.M.; Bayon, Y.; Pandit, A.; Raghunath, M.; et al. The Collagen Suprafamily: From Biosynthesis to Advanced Biomaterial Development. Adv. Mater. 2019, 31, 1801651.
- Shavandi, A.; Hou, Y.; Carne, A.; McConnell, M.; Bekhit, A.E.-D.A. Marine Waste Utilization as a Source of Functional and Health Compounds. Adv. Food Nutr. Res. 2019, 87, 187–254.
- Furtado, M.; Chen, L.; Chen, Z.; Chen, A.; Cui, W. Development of fish collagen in tissue regeneration and drug delivery. Eng. Regen. 2022, 3, 217–231.
- Maschmeyer, T.; Luque, R.; Selva, M. Upgrading of marine (fish and crustaceans) biowaste for high added-value molecules and bio(nano)-materials. Chem. Soc. Rev. 2020, 49, 4527–4563.
- Hua, K.; Cobcroft, J.M.; Cole, A.; Condon, K.; Jerry, D.R.; Mangott, A.; Praeger, C.; Vucko, M.J.; Zeng, C.; Zenger, K.; et al. The Future of Aquatic Protein: Implications for Protein Sources in Aquaculture Diets. One Earth 2019, 1, 316–329.
- Mishra, P.K.; Gautam, R.K.; Kumar, V.; Kakatkar, A.S.; Chatterjee, S. Synthesis of Biodegradable Films Using Gamma Irradiation from Fish Waste. Waste Biomass Valorization 2021, 12, 2247–2257.
- Xu, C.; Nasrollahzadeh, M.; Selva, M.; Issaabadi, Z.; Luque, R. Waste-to-wealth: Biowaste valorization into valuable bio(nano)materials. Chem. Soc. Rev. 2019, 48, 4791–4822.
- Al Khawli, F.; Pateiro, M.; Domínguez, R.; Lorenzo, J.M.; Gullón, P.; Kousoulaki, K.; Ferrer, E.; Berrada, H.; Barba, F.J. Innovative Green Technologies of Intensification for Valorization of Seafood and Their By-Products. Mar. Drugs 2019, 17, 689.
- Caruso, G.; Floris, R.; Serangeli, C.; Di Paola, L. Fishery Wastes as a Yet Undiscovered Treasure from the Sea: Biomolecules Sources, Extraction Methods and Valorization. Mar. Drugs 2020, 18, 622.
- Nimni, M.E.; Harkness, R.D. Molecular Structure and Functions of Collagen. In Collagen; CRC Press: Boca Raton, FL, USA, 2018; Volume 1, pp. 1–78.
- Coppola, D.; Lauritano, C.; Esposito, F.P.; Riccio, G.; Rizzo, C.; De Pascale, D. Fish Waste: From Problem to Valuable Resource. Mar. Drugs 2021, 19, 116.
- Mahmood, A.; Patel, D.; Hickson, B.; DesRochers, J.; Hu, X. Recent progress in biopolymer-based hydrogel materials for biomedical applications. Int. J. Mol. Sci. 2022, 23, 1415.
- Castile, J.D.; Taylor, K.M.G.; Buckton, G. The influence of incubation temperature and surfactant concentration on the interaction between dimyristoylphosphatidylcholine liposomes and poloxamer surfactants. Int. J. Pharm. 2001, 221, 197–209.
- El Blidi, O.; Omari, N.E.l.; Balahbib, A.; Ghchime, R.; El Menyiy, N.; Ibrahimi, A.; Kaddour, K.B.; Bouyahya, A.B.; Chokairi, O.; Barkiyou, M. Extraction Methods, Characterization and Biomedical Applications of Collagen: A Review. Biointerface Res. Appl. Chem. 2021, 11, 13587–13613.
- Sbricoli, L.; Guazzo, R.; Annunziata, M.; Gobbato, L.; Bressan, E.; Nastri, L. Selection of collagen membranes for bone regeneration: A literature review. Materials 2020, 13, 786.
- Xu, Q.; Torres, J.E.; Hakim, M.; Babiak, P.M.; Pal, P.; Battistoni, C.M.; Nguyen, M.; Panitch, A.; Solorio, L.; Liu, J.C. Collagen- and hyaluronic acid-based hydrogels and their biomedical applications. Mater. Sci. Eng. R Rep. 2021, 146, 100641.
- Naomi, R.; Ridzuan, P.M.; Bahari, H.; Vallejo-Giraldo, C. Current Insights into Collagen Type I. Polymers 2021, 13, 2642.
- Coppola, D.; Oliviero, M.; Vitale, G.A.; Lauritano, C.; D’Ambra, I.; Iannace, S.; de Pascale, D. Marine Collagen from Alternative and Sustainable Sources: Extraction, Processing and Applications. Mar. Drugs 2020, 18, 214.
- Gallo, N.; Natali, M.L.; Sannino, A.; Salvatore, L. An Overview of the Use of Equine Collagen as Emerging Material for Biomedical Applications. J. Funct. Biomater. 2020, 11, 79.
- Jafari, H.; Lista, A.; Siekapen, M.M.; Ghaffari-Bohlouli, P.; Nie, L.; Alimoradi, H.; Shavandi, A. Fish Collagen: Extraction, Characterization, and Applications for Biomaterials Engineering. Polymers 2020, 12, 2230.
- Ikoma, T.; Kobayashi, H.; Tanaka, J.; Walsh, D.; Mann, S. Physical properties of type I collagen extracted from fish scales of Pagrus major and Oreochromis niloticas. Int. J. Biol. Macromol. 2003, 32, 199–204.
- Nomura, Y.; Sakai, H.; Ishii, Y.; Shirai, K. Preparation and some properties of type I collagen from fish scales. Biosci. Biotechnol. Biochem. 1996, 60, 20922094.
- Nagai, T.; Suzuki, N. Isolation of collagen from fish waste material—skin, bone and fins. Food Chem. 2000, 68, 277–281.
- Rauta, P.R.; Mohanta, Y.K.; Nayak, D. Nanotechnology in Biology and Medicine: Research Advancements & Future Perspectives; CRC Press: Boca Raton, FL, USA, 2019.
- Lo, S.; Fauzi, M.B. Current Update of Collagen Nanomaterials—Fabrication, Characterisation and Its Applications: A Review. Pharmaceutics 2021, 13, 316.
- Naskar, A.; Kim, K.S. Recent Advances in Nanomaterial-Based Wound-Healing Therapeutics. Pharmaceutics 2020, 12, 499.
- Ucar, B. Natural biomaterials in brain repair: A focus on collagen. Neurochem. Int. 2021, 146, 105033.
- Dong, C.; Lv, Y. Application of Collagen Scaffold in Tissue Engineering: Recent Advances and New Perspectives. Polymers 2016, 8, 42.
- Arun, A.; Malrautu, P.; Laha, A.; Luo, H.; Ramakrishna, S. Collagen Nanoparticles in Drug Delivery Systems and Tissue Engineering. Appl. Sci. 2021, 11, 11369.
- Neff, L.S.; Bradshaw, A.D. Cross your heart? Collagen cross-links in cardiac health and disease. Cell Signal. 2021, 79, 109889.
- Gelse, K.; Pöschl, E. Reviews TA-A drug delivery, 2003 U. Collagens—Structure, function, and biosynthesis. Adv. Drug Deliv. Rev. 2003, 55, 1531–1546.
- Schweitzer, M.H.; Suo, Z.; Avci, R.; Asara, J.M.; Allen, M.A.; Arce, F.T.; Horner, J.R. Analyses of Soft Tissue from Tyrannosaurus rex Suggest the Presence of Protein. Science 2007, 316, 277–280.
- Torres, J.M.; Borja, C.; Gibert, L.; Ribot, F.; Olivares, E.G. Twentieth-Century Paleoproteomics: Lessons from Venta Micena Fossils. Biology 2022, 11, 1184.
- Bächinger, H.P.; Mizuno, K.; Vranka, J.A.; Boudko, S.P. Collagen Formation and Structure. Compr. Nat. Prod II Chem. Biol. 2010, 5, 469–530.
- Ricard-Blum, S. The Collagen Family. Cold Spring Harb. Perspect. Biol. 2011, 3, a004978.
- Kadler, K.E.; Baldock, C.; Bella, J.; Boot-Handford, R.P. Collagens at a glance. J. Cell Sci. 2007, 120, 1955–1958.
- Inoue, Y.; Itoh, H.; Aoki, M.; Ogawa, S.; Yamane, T.; Baba, T.; Tachibana, N.; Kohno, M.; Oishi, Y.; Kobayashi-Hattori, K. Accelerating effect of soy peptides containing collagen peptides on type I and III collagen levels in rat skin. Biosci. Biotechnol. Biochem. 2012, 76, 1549–1551.
- Minor, A.J.; Coulombe, K.L.K. Engineering a collagen matrix for cell-instructive regenerative angiogenesis. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 2407–2416.
- Bornstein, P.; Sage, H. Structurally distinct collagen types. Annu. Rev. Biochem. 1980, 49, 957–1003.
- Salvatore, L.; Gallo, N.; Aiello, D.; Lunetti, P.; Barca, A.; Blasi, L.; Madaghiele, M.; Bettini, S.; Giancane, G.; Hasna, M.; et al. An insight on type I collagen from horse tendon for the manufacture of implantable devices. Int. J. Biol. Macromol/ 2020, 154, 291–306.
- Ignat’eva, N.Y.; Danilov, N.A.; Averkiev, S.V.; Obrezkova, M.V.; Lunin, V.V.; Sobol’, E.N. Determination of hydroxyproline in tissues and the evaluation of the collagen content of the tissues. J. Anal. Chem. 2007, 62, 51–57.
- Privalov, P.L.; Tiktopulo, E.I.; Tischenko, V.M. Stability and mobility of the collagen structure. J. Mol. Biol. 1979, 127, 203–216.
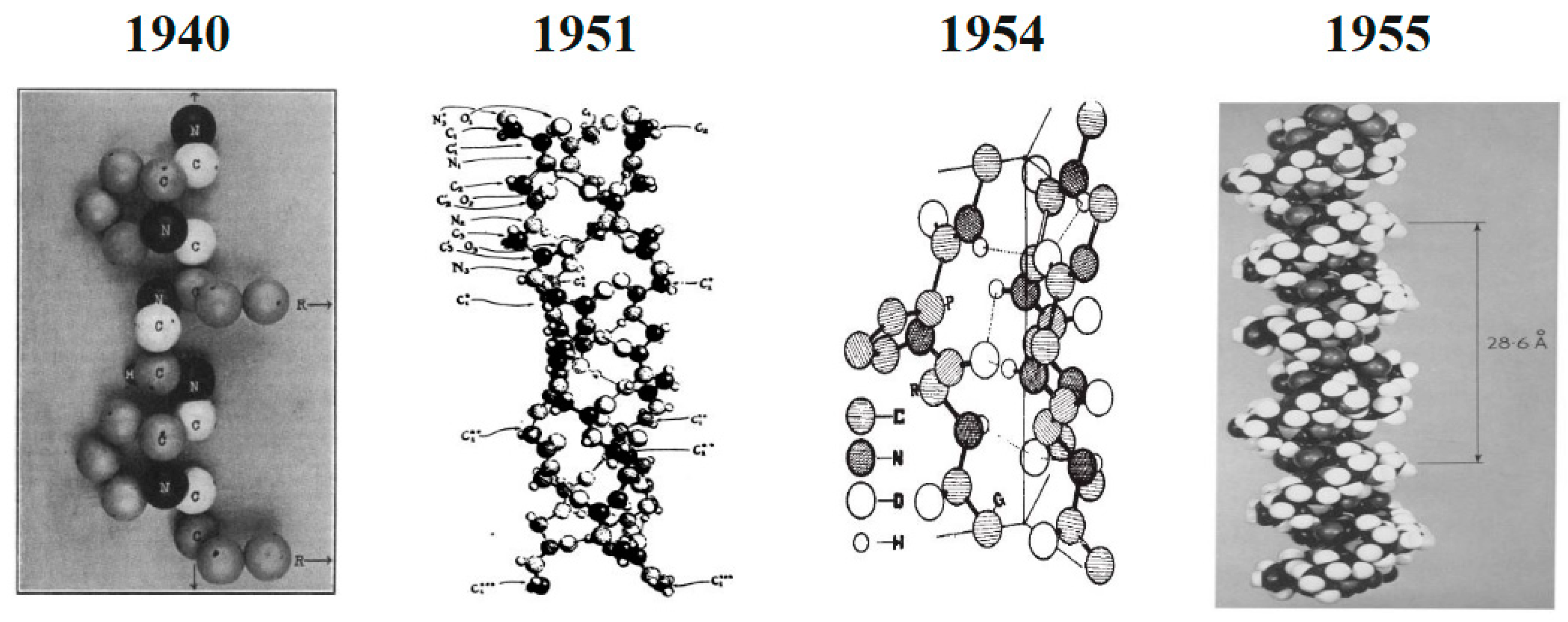
- Astbury, W.T.; Bell, F.O. Molecular Structure of the Collagen Fibres. Nature. 1940, 145, 421–422.
- Pauling, L.; Corey, R.B. The structure of fibrous proteins of the collagen-gelatin group. Proc. Natl. Acad. Sci. USA 1951, 37, 272–281.
- Ramachandran, G.N.; Kartha, G. Structure of Collagen. Nature 1954, 174, 269–270.
- Rich, A.; Crick, F.H.C. The molecular structure of collagen. J. Mol. Biol. 1961, 3, 483–506.
- Cowan, P.M.; Mcgavin, S.; North, A.C. The Polypeptide Chain Configuration of Collagen. Nature 1955, 176, 1062–1064.
- Bella, J. Collagen structure: New tricks from a very old dog. Biochem. J. 2016, 473, 1001–1025.
- Lim, Y.S.; Ok, Y.J.; Hwang, S.Y.; Kwak, J.Y.; Yoon, S. Marine collagen as a promising biomaterial for biomedical applications. Mar. Drugs 2019, 17, 467.
- Chen, L.; Cheng, G.; Meng, S.; Ding, Y. Collagen Membrane Derived from Fish Scales for Application in Bone Tissue Engineering. Polymers 2022, 14, 2532.
- León-López, A.; Morales-Peñaloza, A.; Manuel Martínez-Juárez, V.; Vargas-Torres, A.; Zeugolis, D.I.; Aguirre-Álvarez, G. Hydrolyzed Collagen—Sources and Applications. Molecules 2019, 24, 4031.
- Liu, X.; Gao, Y.; Long, X.; Hayashi, T.; Mizuno, K.; Hattori, S.; Fujisaki, H.; Ogura, T.; Wang, D.O.; Ikejima, T. Type i collagen promotes the migration and myogenic differentiation of C2C12 myoblasts: Via the release of interleukin-6 mediated by FAK/NF-κB p65 activation. Food Funct. 2020, 11, 328–338.
- Song, Z.; Liu, H.; Chen, L.L.; Chen, L.L.; Zhou, C.; Hong, P.; Deng, C. Characterization and comparison of collagen extracted from the skin of the Nile tilapia by fermentation and chemical pretreatment. Food Chem. 2021, 340, 128139.
- Peng, X.; Cui, Y.; Chen, J.; Gao, C.; Yang, Y.; Yu, W.; Rai, K.; Zhang, M.; Nian, R.; Bao, Z.; et al. High-Strength Collagen-Based Composite Films Regulated by Water-Soluble Recombinant Spider Silk Proteins and Water Annealing. ACS Biomater. Sci. Eng. 2022, 8, 3341–3353.
- Bao, Z.; Sun, Y.; Rai, K.; Peng, X.; Wang, S.; Nian, R.; Xian, M. The promising indicators of the thermal and mechanical properties of collagen from bass and tilapia: Synergistic effects of hydroxyproline and cysteine. Biomater. Sci 2018, 6, 3042–3052.
- Bao, Z.; Gao, M.; Fan, X.; Cui, Y.; Yang, J.; Peng, X.; Xian, M.; Sun, Y.; Nian, R. Development and characterization of a photo-cross-linked functionalized type-I collagen (Oreochromis niloticus) and polyethylene glycol diacrylate hydrogel. Int. J. Biol. Macromol. 2020, 155, 163–173.
- Shoulders, M.D.; Raines, R.T. Collagen Structure and Stability. Annu. Rev. Biochem. 2009, 78, 929–958.
- Buehler, M.J. Nature designs tough collagen: Explaining the nanostructure of collagen fibrils. Proc. Natl. Acad. Sci. USA 2006, 103, 12285–12290.
- Shen, Y.; Levin, A.; Kamada, A.; Toprakcioglu, Z.; Rodriguez-Garcia, M.; Xu, Y.; Knowles, T.P. From Protein Building Blocks to Functional Materials. ACS Nano 2021, 15, 5819–5837.
- Lin, J.; Shi, Y.; Men, Y.; Wang, X.; Ye, J.; Zhang, C. Mechanical roles in formation of oriented collagen fibers. Tissue Eng.-Part B Rev. 2020, 26, 116–128.
- Yang, L.; Van Der Werf, K.O.; Fitié, C.F.C.; Bennink, M.L.; Dijkstra, P.J.; Feijen, J. Mechanical properties of native and cross-linked type I collagen fibrils. Biophys. J. 2008, 94, 2204–2211.
- Tihăuan, B.-M.; Pircalabioru, G.G.; Axinie Bucos, M.; Marinaș, I.C.; Nicoară, A.-C.; Măruțescu, L.; Oprea, O.; Matei, E.; Maier, S.S. Crosslinked Collagenic Scaffold Behavior Evaluation by Physico-Chemical, Mechanical and Biological Assessments in an In Vitro Microenvironment. Polymers 2022, 14, 2430.
- Hossain, M.S.; Ebrahimi, H.; Ghosh, R. Fish scale inspired structures—A review of materials, manufacturing and models. Bioinspir. Biomim. 2022, 17, 061001.
- Sharma, S.; Dwivedi, S.; Chandra, S.; Srivastava, A.; Vijay, P. Collagen: A Brief Analysis. Oral. Maxillofac. Pathol. J. 2019, 10, 11–17.
- Salvatore, L.; Gallo, N.; Natali, M.L.; Campa, L.; Lunetti, P.; Madaghiele, M.; Blasi, F.S.; Corallo, A.; Capobianco, L.; Sannino, A. Marine collagen and its derivatives: Versatile and sustainable bio-resources for healthcare. Mater. Sci. Eng. C 2020, 113, 110963.
- Ferraro, V.; Gaillard-Martinie, B.; Sayd, T.; Chambon, C.; Anton, M.; Santé-Lhoutellier, V. Collagen type I from bovine bone. Effect of animal age, bone anatomy and drying methodology on extraction yield, self-assembly, thermal behaviour and. Int. J. Biol. Macromol. 2017, 97, 55–66.
- Davison-Kotler, E.; Marshall, W.S.; García-Gareta, E. Sources of Collagen for Biomaterials in Skin Wound Healing. Bioengineering 2019, 6, 56.
- Paschou, A.M.; Katsikini, M.; Christofilos, D.; Arvanitidis, J.; Ves, S.; Katsikini, C.M. High pressure Raman study of type-I collagen. Wiley Online Libr. 2018, 285, 2641–2653.
- Bhuimbar, M.V.; Bhagwat, P.K.; Dandge, P.B. Extraction and characterization of acid soluble collagen from fish waste: Development of collagen-chitosan blend as food packaging film. J. Environ. Chem. Eng. 2019, 7, 102983.
- Subhan, F.; Hussain, Z.; Tauseef, I.; Shehzad, A.; Wahid, F. A review on recent advances and applications of fish collagen. Crit. Rev. Food Sci. Nutr. 2021, 61, 1027–1037.
- Heidari, M.G.; Rezaei, M. Extracted pepsin of trout waste and ultrasound-promoted method for green recovery of fish collagen. Sustain. Chem. Pharm. 2022, 30, 100854.
- Morikawa, T.; Reátegui-Pinedo, N.; Salirrosas, D.; Sánchez-Tuesta, L.; Quiñones, C.; Jáuregui-Rosas, S.R.; Barraza, G.; Cabrera, A.; Ayala-Jara, C.; Miliani Martinez, R.; et al. Characterization of Collagen from Three Genetic Lines (Gray, Red and F1) of Oreochromis niloticus (Tilapia) Skin in Young and Old Adults. Molecules 2022, 27, 1123.
- Felician, F.; Xia, C.; Qi, W. Collagen from marine biological sources and medical applications. Wiley Online Libr. 2018, 15, e1700557.
- Nirmal, N.P.; Santivarangkna, C.; Rajput, M.S.; Benjakul, S.; Maqsood, S. Valorization of fish byproducts: Sources to end-product applications of bioactive protein hydrolysate. Compr. Rev. Food Sci. Food Saf. 2022, 21, 1803–1842.
- Fassini, D.; Wilkie, I.C.; Pozzolini, M.; Ferrario, C.; Sugni, M.; Rocha, M.S.; Giovine, M.; Bonasoro, F.; Silva, T.H.; Reis, R.L. Diverse and productive source of biopolymer inspiration: Marine collagens. Biomacromolecules 2021, 22, 1815–1834.
- Sanchez, A.; Blanco, M.; Correa, B.; Perez-Martin, R.I.; Sotelo, C.G. Effect of fish collagen hydrolysates on type I collagen mRNA levels of human dermal fibroblast culture. Mar. Drugs 2018, 16, 144.
- Lu, W.-C.; Chiu, C.-S.; Chan, Y.-J.; Guo, T.-P.; Lin, C.-C.; Wang, P.-C.; Po-Yu Lin, P.-Y.; Mulio, A.T.; Li, P.-H. An In Vivo Study to Evaluate the Efficacy of Blue Shark (Prionace glauca) Cartilage Collagen as a Cosmetic. Mar. Drugs 2022, 20, 633.
- Ahn, H.; Gong, D.J.; Lee, H.H.; Seo, J.Y.; Song, K.M.; Eom, S.J.; Yeo, S.Y. Mechanical Properties of Porcine and Fish Skin-Based Collagen and Conjugated Collagen Fibers. Polymers 2021, 13, 2151.
- Liu, S.; Lau, C.S.; Liang, K.; Wen, F.; Teoh, S.H. Marine collagen scaffolds in tissue engineering. Curr. Opin. Biotechnol. 2022, 74, 92–103.
