Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Camila Xu and Version 1 by António Carrapiço.
Metal nanoparticles (MNPs) are especially interesting due to properties such as optical polarizability, electrical conductivity, photocatalysis and antimicrobial activity, which makes them useful for many applications in areas from electronics to pharmaceutics.
- green synthesis
- NPs
- metallic nanoparticles
- metal-based nanoparticles
1. Introduction
It is known that it is possible to synthesize MNPs by adding metal salts to both plant extracts and cell-free supernatants of liquid microbial cultures. However, despite being similar, there are little variations in the biosynthesis of different MNPs. For instance, different precursors lead to MNPs with distinct characteristics [1], while variations in the concentration of elements, such as molecular oxygen (O2) or chloride (Cl−), may result in the formation of metal oxide nanoparticles (e.g., Ag2ONPs) or metal chloride nanoparticles (e.g., AgClNPs) instead of metal nanoparticles (e.g., AgNPs) [2]. Moreover, it has also been shown that several reaction conditions, such as temperature, oxygenation, pH, precursor (metal salt) concentration, microbial growth phase (upon supernatant collection), incubation time and irradiation, highly influence both the yield of the reaction and the properties of the MNPs [3][4][5]. However, the mechanism behind these phenomena is not yet fully understood. Notwithstanding, several studies using plant extracts and microorganisms’ cell-free supernatants have been performed in an attempt to identify the molecules responsible for the reduction and stabilization (capping agents) of these NPs [6][7][8][9][10][11][12][13][14][15][16][17][18][19][20][21][22][23][24][25]. In these studies, several techniques have been applied.
2. Techniques Employed in the Study of Metal Nanoparticles’ Biosynthesis Mechanisms
Aiming toward the identification of the functional groups that may be involved in the reactions, Fourier transform infrared spectroscopy (FTIR) analysis of the reaction media before and after synthesis has been the most used technique [6][7][8][9][10][11][12][14][20]. X-ray photoelectron spectroscopy (XPS) was also used for the same purpose [8]. One thermogravimetric study was also conducted [6]. Chromatographic techniques, such as gas chromatography (GC) [8], high-performance liquid chromatography (HPLC) [11], ultra-high-performance liquid chromatography (UPLC) [13], liquid chromatography (LC) [19] and gel permeation chromatography (GPC) [20], have been employed to separate and identify the several compounds present in the reaction solutions before and after synthesis. The latter are usually followed by identification techniques, such as mass spectrometry (MS) [8][19] and high-resolution mass spectrometry (HRMS) [13]. One study also used matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF-MS), aiming to identify the capping agents of MNPs [18]. Separation of proteins present in the media using sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) has also been performed [19] to try to prove their role in the reaction, as well as to determine their molecular weight. Quantifications of several compounds in both the media and nanoparticle suspensions were performed to understand their role in the reactions, as well as their relative contribution to the synthesis—e.g., DTNB, DNSA, DTNP and Folin-phenol assays [19][20]. Spectrophotometric analysis of the NPs plasmon peak associated with changes in the reaction media was generally performed [6][15][16][17][19][20][21] with the assumption that higher absorbance values are related to increases in concentration, and the shifts in the peak toward higher wavelengths (toward red) are due to the increase in the diameter of the nanoparticle [26]. Finally, cyclic voltammetry was used to try to prove the effective role of specific compounds (i.e., caffeine) in the reduction in metal ions [11]. The analysis of the data acquired from these techniques enabled researchers to propose mechanisms for nanoparticle synthesis using biological extracts (i.e., plant extracts and cell-free supernatant of microorganism cultures).
3. Biosynthesis Mechanisms—State of the Art
It has initially been hypothesized that MNPs synthesis using cell-free supernatants of microorganisms was achievable due to the secretion of enzymes responsible for the reduction in compounds bound to the metal—e.g., reduction in nitrate from silver nitrate using nitrate reductase [15][16][18]. However, this hypothesis has been rebutted by other studies that show the role of other molecules (e.g., reducing sugars [19], nitrogenous biomolecules (e.g., proteins) [14][17][19][20][21], GSH [19][21], NADH and NADPH [19][21], polysaccharides [20], glycoproteins [20] and proteoglycans [20]). One study, aiming to ascertain the role and relative contribution of enzymes to the synthesis of NPs, used the cell-free denatured protein fraction of microorganism culture supernatant to prove that, despite contributing to the synthesis of NPs, enzyme catalysis is not mainly responsible or even required [17]. Moreover, studies on plant extracts that also led to the synthesis of MNPs evidenced the role of several molecules in the reduction (reducing sugars [6], flavonoids [6][7][13], proteins [6], polysaccharides [6], aldehydes [7], phenolic compounds [8][13] and alkaloids [11]) and stabilization (capping) (reducing sugars [6], flavonoids [6][7][8], phenolic compounds [7][13], alcohols [8], amines [8], alkanes [8] and alkaloids [11]) of MNPs, some of which are also present in cell-free supernatants of microorganism culture media [27][28][29]. The synthesis of MNPs using isolated compounds, such as cysteine [22], flavonols (DMY) [23], caffeic acid [24] and alginate [25], also proved that enzymatic catalysis is not mandatory for metal nanoparticle synthesis.
4. Evidence-Based Proposed Biosynthesis Mechanisms
Several studies referenced above used plant extracts and cell-free supernatants of microorganism cultures to synthesize MNPs (plant extracts—Au, Ag, Cu, Fe and Zn; microorganisms—Ag and Au) aiming toward the study of the molecules involved and proposal of synthesis mechanisms.
From these studies, some general principles were evidenced regarding the possible mechanisms that lead to metal nanoparticle synthesis using the cell-free supernatant of microorganism cultures (Figure 1). Firstly, the molecules detected in the reaction media, as well as their relative abundances, seem to be highly influenced by both the microorganisms used and the composition of the growth media. Secondly, the role of secreted enzymes, despite being evidenced by several studies [15][16][18][21], is not overwhelming when compared with other molecules or mandatory for synthesis to occur. Notwithstanding, enzymatic catalysis seems to contribute to the increase in the reaction speed.
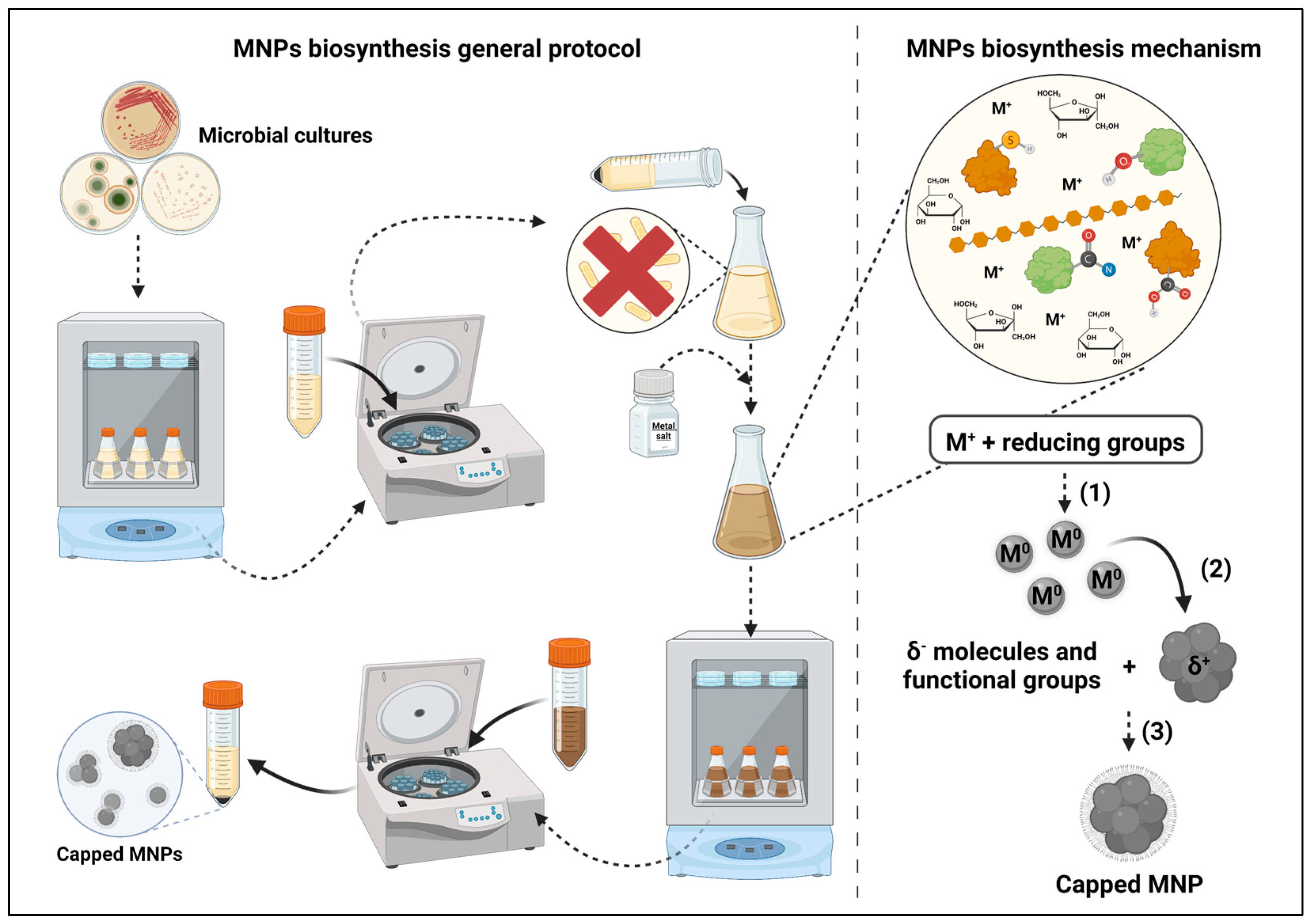
Figure 1. Metal nanoparticles biosynthesis’ mechanisms and general protocol. (1) Metal reduction by enzymes and other molecules from the supernatant; (2) reduced metal particle agglomeration; (3) MNPs capping by negatively charged molecules from the supernatant. Created with BioRender.com.
There seems to be strong evidence that the sole requirement for metal nanoparticle synthesis to occur is the presence of molecules with reducing groups, such as carboxyl, amide, thiol and hydroxyl. The latter seems to be of great importance given its presence in most molecules, which have been determined to play a role in metal reduction, and based on the evidence of the reduction in its signal after synthesis in FTIR analysis of the reaction media. Nevertheless, despite further research being required, the effect of the presence of reducing enzymes on the speed of reaction seems to be relevant. However, given that most of the studies do not report the yield of the reaction, it is impossible to accurately compare syntheses with different reaction conditions. Reporting that the synthesis occurred based solely on spectrophotometric analysis and microscopy (e.g., scanning electron microscopy (SEM) and transmission electron microscopy (TEM)) does not give enough information to hypothesize about the real importance of the different compounds present in the reaction media. On the other hand, the presence of negatively charged groups (e.g., carbonyl, amine) or atoms (e.g., nitrogen) seems to be indispensable for the adsorption of capping molecules to the MNPs, which are responsible for their stabilization and influence their antimicrobial activity [30]. However, analogously to the yield, not all studies determine the antimicrobial properties of the NPs, which does not enable the determination of the impact of the different capping molecules on these properties.
References
- Chang, H.; Kim, B.H.; Lim, S.G.; Baek, H.; Park, J.; Hyeon, T. Role of the Precursor Composition in the Synthesis of Metal Ferrite Nanoparticles. Inorg. Chem. 2021, 60, 4261–4268.
- Jeevanandam, J.; Chan, Y.S.; Danquah, M.K. Biosynthesis of Metal and Metal Oxide Nanoparticles. ChemBioEng Rev. 2016, 3, 55–67.
- Zikalala, N.; Matshetshe, K.; Parani, S.; Oluwafemi, O.S. Biosynthesis Protocols for Colloidal Metal Oxide Nanoparticles. Nano-Struct. Nano-Objects 2018, 16, 288–299.
- Salem, S.S.; Fouda, A. Green Synthesis of Metallic Nanoparticles and Their Prospective Biotechnological Applications: An Overview. Biol. Trace Elem. Res. 2021, 199, 344–370.
- Huq, M.A.; Ashrafudoulla, M.; Rahman, M.M.; Balusamy, S.R.; Akter, S. Green Synthesis and Potential Antibacterial Applications of Bioactive Silver Nanoparticles: A Review. Polymers 2022, 14, 742.
- Zhan, G.; Huang, J.; Lin, L.; Lin, W.; Emmanuel, K.; Li, Q. Synthesis of Gold Nanoparticles by Cacumen Platycladi Leaf Extract and Its Simulated Solution: Toward the Plant-Mediated Biosynthetic Mechanism. J. Nanoparticle Res. 2011, 13, 4957–4968.
- Mittal, A.K.; Bhaumik, J.; Kumar, S.; Banerjee, U.C. Biosynthesis of Silver Nanoparticles: Elucidation of Prospective Mechanism and Therapeutic Potential. J. Colloid Interface Sci. 2014, 415, 39–47.
- Liu, Y.; Jin, X.; Chen, Z. The Formation of Iron Nanoparticles by Eucalyptus Leaf Extract and Used to Remove Cr(VI). Sci. Total Environ. 2018, 627, 470–479.
- Yang, B.; Qi, F.; Tan, J.; Yu, T.; Qu, C. Study of Green Synthesis of Ultrasmall Gold Nanoparticles Using Citrus Sinensis Peel. Appl. Sci. Switz. 2019, 9, 2423.
- Buazar, F.; Sweidi, S.; Badri, M.; Kroushawi, F. Biofabrication of Highly Pure Copper Oxide Nanoparticles Using Wheat Seed Extract and Their Catalytic Activity: A Mechanistic Approach. Green Process. Synth. 2019, 8, 691–702.
- Bandeira, M.; Possan, A.L.; Pavin, S.S.; Raota, C.S.; Vebber, M.C.; Giovanela, M.; Roesch-Ely, M.; Devine, D.M.; Crespo, J.S. Mechanism of Formation, Characterization and Cytotoxicity of Green Synthesized Zinc Oxide Nanoparticles Obtained from Ilex Paraguariensis Leaves Extract. Nano-Struct. Nano-Objects 2020, 24, 100532.
- Biswal, A.K.; Misra, P.K. Biosynthesis and Characterization of Silver Nanoparticles for Prospective Application in Food Packaging and Biomedical Fields. Mater. Chem. Phys. 2020, 250, 123014.
- Pradeep, M.; Kruszka, D.; Kachlicki, P.; Mondal, D.; Franklin, G. Uncovering the Phytochemical Basis and the Mechanism of Plant Extract-Mediated Eco-Friendly Synthesis of Silver Nanoparticles Using Ultra-Performance Liquid Chromatography Coupled with a Photodiode Array and High-Resolution Mass Spectrometry. ACS Sustain. Chem. Eng. 2022, 10, 562–571.
- Mukherjee, P.; Roy, M.; Mandal, B.P.; Dey, G.K.; Mukherjee, P.K.; Ghatak, J.; Tyagi, A.K.; Kale, S.P. Green Synthesis of Highly Stabilized Nanocrystalline Silver Particles by a Non-Pathogenic and Agriculturally Important Fungus T. Asperellum. Nanotechnol. 2008, 19, 075103.
- Vaidyanathan, R.; Gopalram, S.; Kalishwaralal, K.; Deepak, V.; Pandian, S.R.K.; Gurunathan, S. Enhanced Silver Nanoparticle Synthesis by Optimization of Nitrate Reductase Activity. Colloids Surf. B Biointerfaces 2010, 75, 335–341.
- Moteshafi, H.; Mousavi, S.M.; Shojaosadati, S.A. The Possible Mechanisms Involved in Nanoparticles Biosynthesis. J. Ind. Eng. Chem. 2012, 18, 2046–2050.
- Maliszewska, I.; Juraszek, A.; Bielska, K. Green Synthesis and Characterization of Silver Nanoparticles Using Ascomycota Fungi Penicillium Nalgiovense AJ12. J. Clust. Sci. 2014, 25, 989–1004.
- Singh, D.K.; Kumar, J.; Sharma, V.K.; Verma, S.K.; Singh, A.; Kumari, P.; Kharwar, R.N. Mycosynthesis of Bactericidal Silver and Polymorphic Gold Nanoparticles: Physicochemical Variation Effects and Mechanism. Nanomedicine 2018, 13, 191–207.
- Ma, L.; Lv, S.; Tang, J.; Liu, J.; Li, W.; Deng, J.; Deng, Y.; Du, J.; Liu, X.; Zeng, X. Study on Bioactive Molecules Involved in Extracellular Biosynthesis of Silver Nanoparticles by Penicillium Aculeatum Su1. Mater. Express 2019, 9, 475–483.
- Wanarska, E.; Maliszewska, I. The Possible Mechanism of the Formation of Silver Nanoparticles by Penicillium Cyclopium. Bioorganic Chem. 2019, 93, 102803.
- Krishnan, S.; Jayakumar, D.; Madhyastha, H.; Chadha, A. The Complexity of Microbial Metal Nanoparticle Synthesis: A Study of Candida Parapsilosis ATCC 7330 Mediated Gold Nanoparticles Formation. BioNanoScience 2021, 11, 336–344.
- Roy, M.; Mukherjee, P.; Mandal, B.P.; Sharma, R.K.; Tyagi, A.K.; Kale, S.P. Biomimetic Synthesis of Nanocrystalline Silver Sol Using Cysteine: Stability Aspects and Antibacterial Activities. RSC Adv. 2012, 2, 6496–6503.
- Guo, Q.; Guo, Q.; Yuan, J.; Zeng, J. Biosynthesis of Gold Nanoparticles Using a Kind of Flavonol: Dihydromyricetin. Colloids Surf. Physicochem. Eng. Asp. 2014, 441, 127–132.
- Kim, H.-S.; Seo, Y.S.; Kim, K.; Han, J.W.; Park, Y.; Cho, S. Concentration Effect of Reducing Agents on Green Synthesis of Gold Nanoparticles: Size, Morphology, and Growth Mechanism. Nanoscale Res. Lett. 2016, 11, 1–9.
- Xiang, S.; Ma, X.; Shi, H.; Ma, T.; Tian, C.; Chen, Y.; Chen, H.; Chen, X.; Luo, K.; Cai, L.; et al. Green Synthesis of an Alginate-Coated Silver Nanoparticle Shows High Antifungal Activity by Enhancing Its Cell Membrane Penetrating Ability. ACS Appl. Bio Mater. 2019, 2, 4087–4096.
- Haiss, W.; Thanh, N.T.K.; Aveyard, J.; Fernig, D.G. Determination of Size and Concentration of Gold Nanoparticles from UV-Vis Spectra. Anal. Chem. 2007, 79, 4215–4221.
- Lim, H.S.; Yeu, J.E.; Hong, S.P.; Kang, M.S. Characterization of Antibacterial Cell-Free Supernatant from Oral Care Probiotic Weissella Cibaria, CMU. Molecules 2018, 23, 1984.
- Fuochi, V.; Coniglio, M.A.; Laghi, L.; Rescifina, A.; Caruso, M.; Stivala, A.; Furneri, P.M. Metabolic Characterization of Supernatants Produced by Lactobacillus spp. With in Vitro Anti-Legionella Activity. Front. Microbiol. 2019, 10, 1403.
- Assal, N.; Rennie, B.; Walrond, L.; Cyr, T.; Rohonczy, L.; Lin, M. Proteome Characterization of the Culture Supernatant of Mycobacterium Bovis in Different Growth Stages. Biochem. Biophys. Rep. 2021, 28, 101154.
- Wang, L.; Hu, C.; Shao, L. The Antimicrobial Activity of Nanoparticles: Present Situation and Prospects for the Future. Int. J. Nanomed. 2017, 12, 1227–1249.
More
