Metal ions can be involved in signaling processes within the cell in both physiological and pathological conditions. Magnesium and calcium are the most recognized signaling agents among metals. Zinc, copper, and iron can also play key roles in signaling pathways. There are many systems in which changes in intra- and extra-cellular zinc and copper concentrations have been linked to important downstream events, especially in nervous signal transduction. Iron signaling is mostly related with its homeostasis. However, it is also involved in a recently discovered type of programmed cell death, ferroptosis.
- cell signaling, metal homeostasis, ferroptosis
1. Introduction
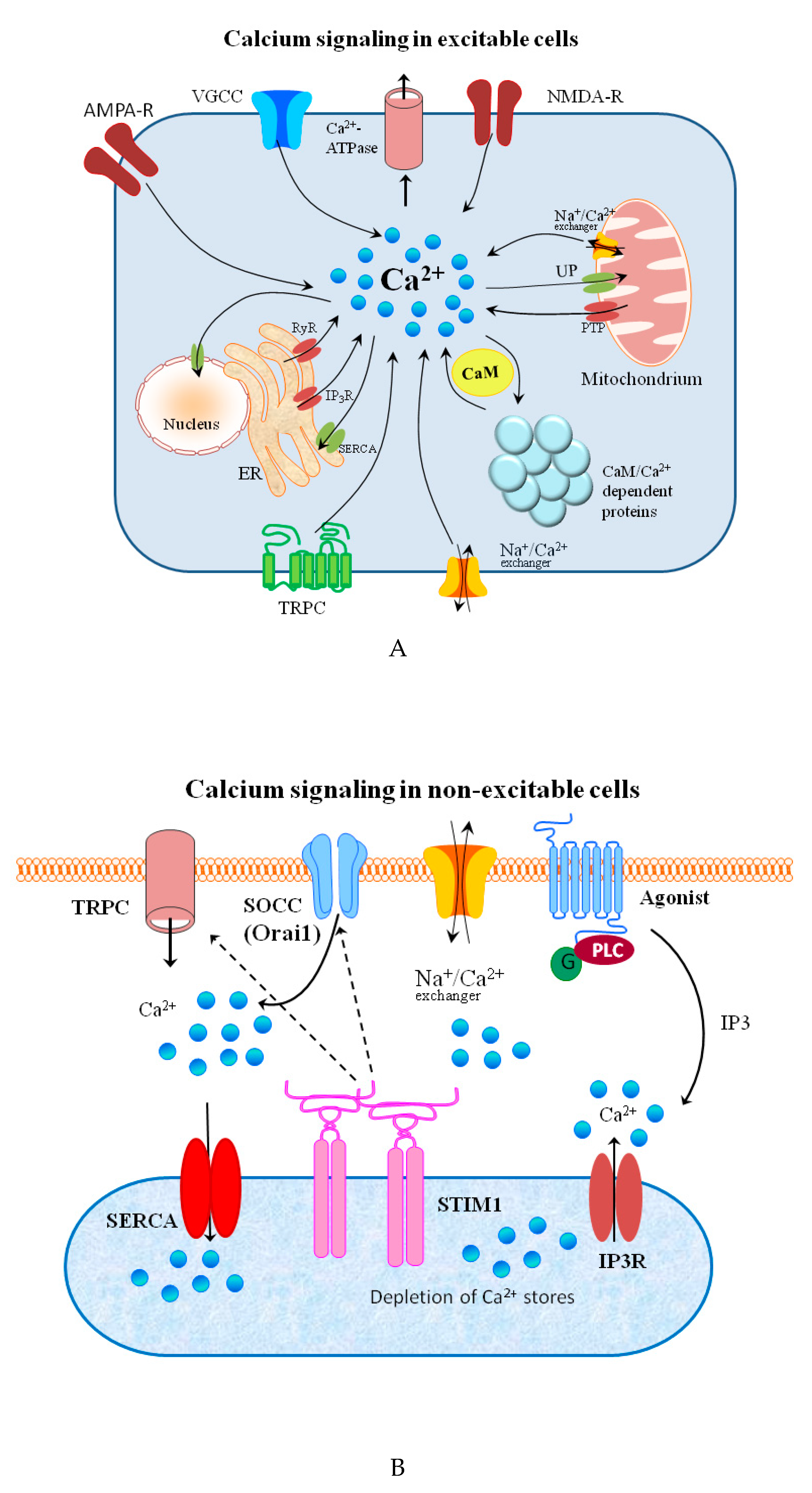
Signal transduction and spreading is a key cellular process in maintaining life and its development. Typical chemical signaling comprises the release of a transmitter from one cell and its interaction with selected detectors on the surface of another. If the transmitter is taken up into the cell, it may bind to receptors on the inner part of cell membrane and, thus, stimulate it to react in a required manner to this signal. The molecular mechanisms of reaction to the signal are regulated by a strict spatiotemporal dynamic [1]. The signal effectiveness and transduction differ on the extra and intracellular side of the cell. In general, extracellular signaling depends on exposure to a sufficient carrier concentration. Simultaneously, the changes in the timing and frequency of a messenger are crucial for the intracellular signals [2]. Changes in the concentration of metal ions affect the signaling processes in both excitable and non-excitable cells on both sides of the cellular membrane [3][4][5][6].
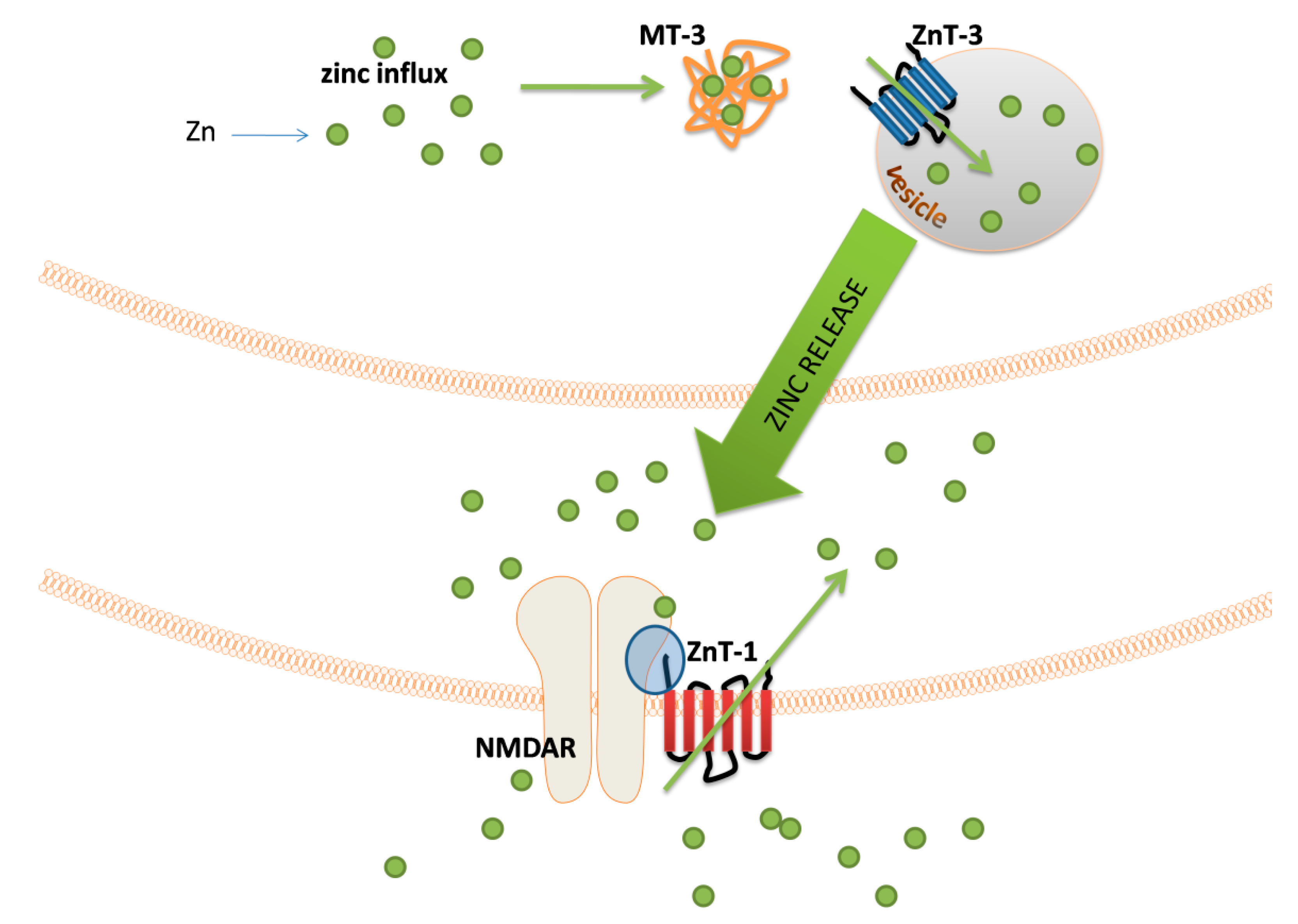
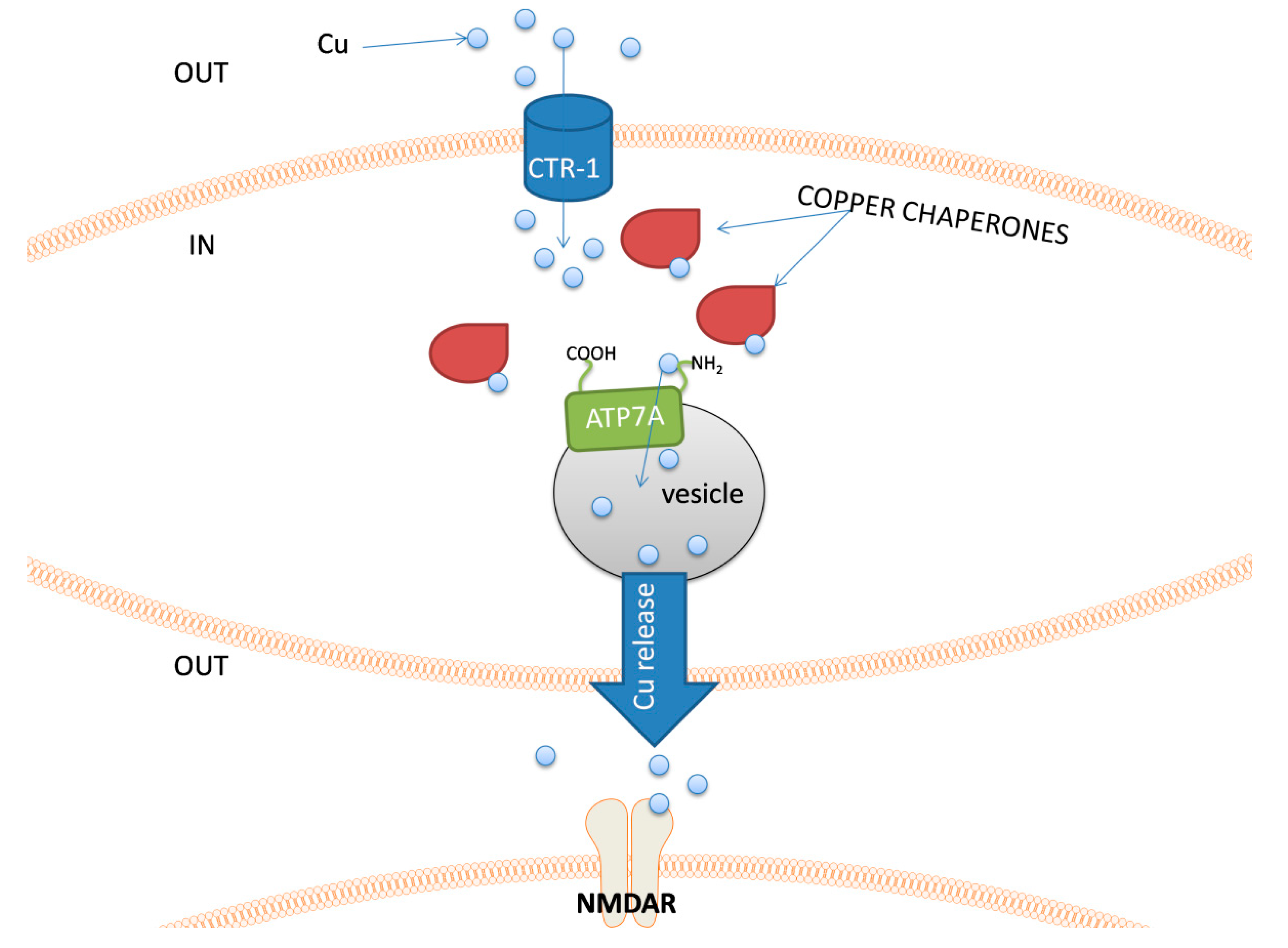
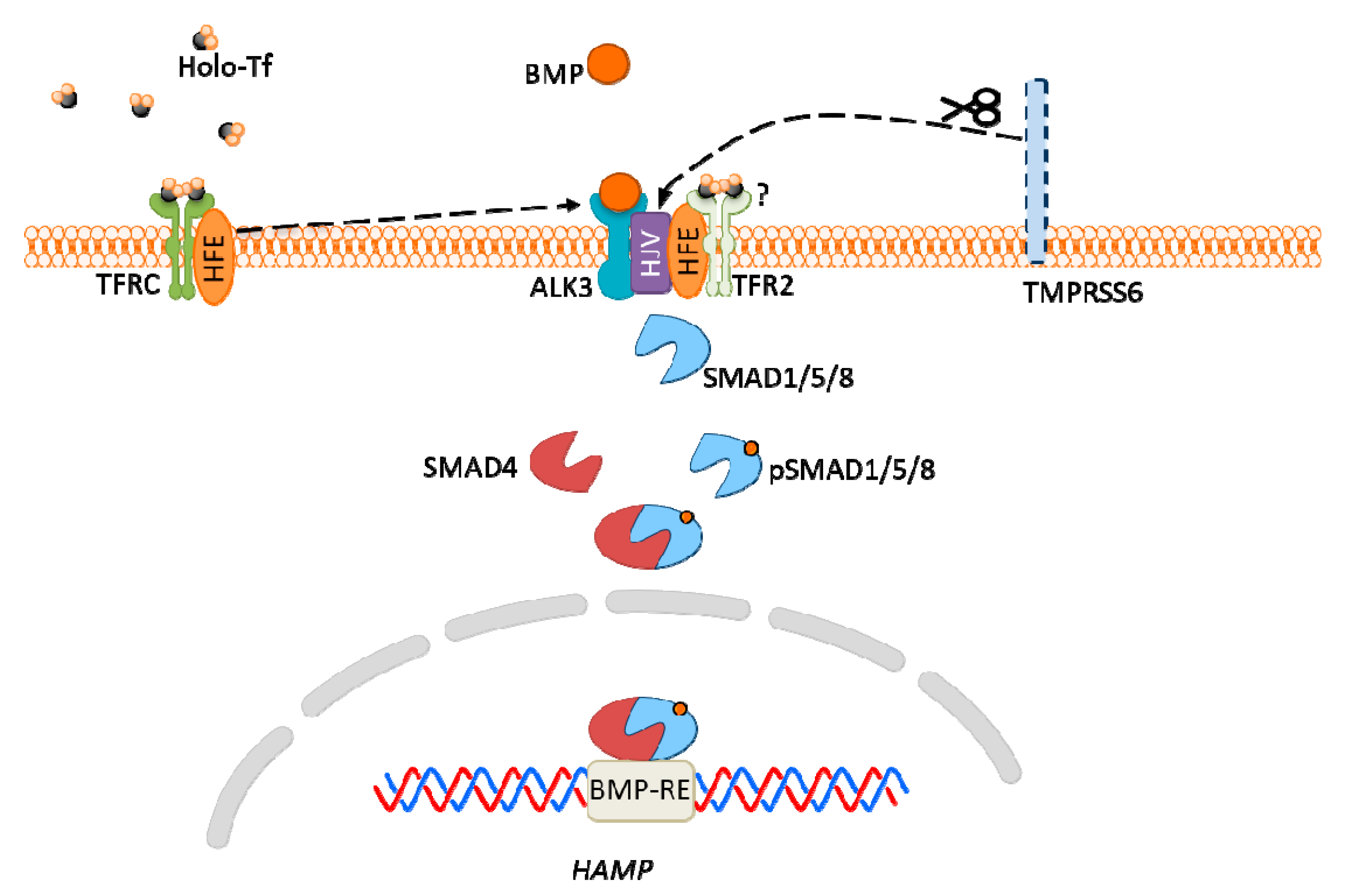
The major groups of chemical neurotransmitters in excitable cells are amino acids, amines, or neuropeptides. Recent studies also indicate that metal ions, such as zinc and copper, may be released to the synaptic cleft [7][8][9]. This phenomenon is still not fully understood; however, it is a very interesting example of signaling involving metal ions. Many new studies and conclusions have appeared in this field recently. Studies show that both the normal aging of the brain and the development of diseases, such as neurodegenerative and psychiatric disorders, are manifested by deregulation of the management of metal ions such as iron, zinc, and copper. That disproportion can have a direct impact on the neurotransmission coordinated by these ions and cellular processes necessary for proper functioning of nerve cells [10][11]. Furthermore, iron ions, along with reactive oxygen species (ROS), were connected with a recently discovered form of programmed cell death named ferroptosis [12]. While the pathological role of metal ions is still under investigation, it actually may arise from homeostasis disorders, which, in turn, may be related to disorders of metal ions sensing by different cells.
2. Metal Ion Signaling Examples
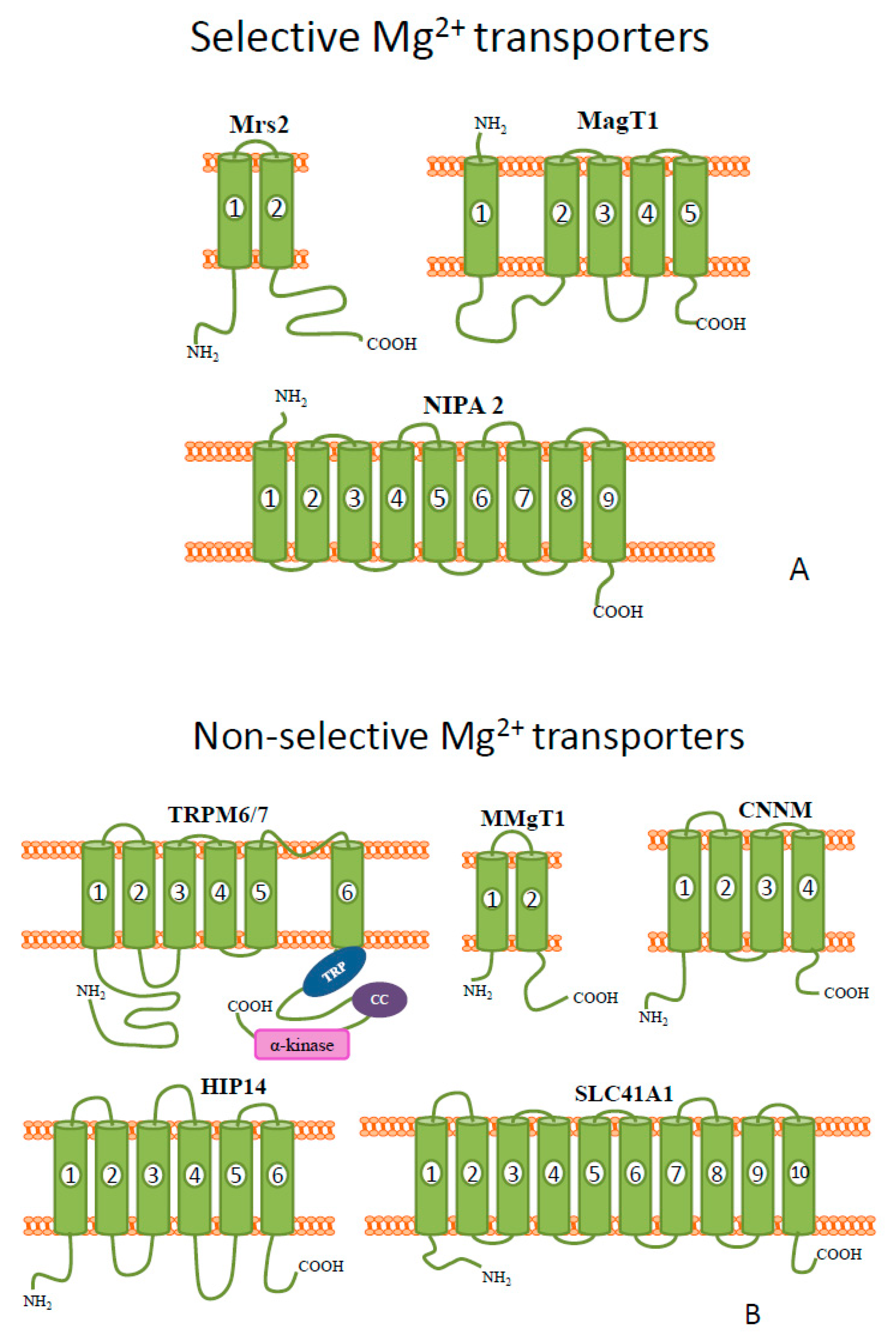
Figure 1. Schematic diagrams of the representative members of mammalian (A) selective and (B) non-selective Mg2+ transporters.
References
- Ross, B.; Mehtal, S.; Zhang, J. Molecular tools for acute spatiotemporal manipulation of signal transduction. Curr. Opin. Chem. Biol. 2016, 34, 135–142.
- Lqbal, J.; Zaidi, M.; Avadhani, N.G. Cell signaling. Mol. Integr. Physiol. Musculoskelet. Syst. 2010, 1211, 3–8.
- Penner, R.; Neher, E. The role of calcium in stimulus-secretion coupling in excitable and non-excitable cells. J. Exp. Biol. 1988, 139, 329–345.
- Hojyo, S.; Fukada, T. Roles of Zinc Signaling in the Immune System. J. Immunol. Res. 2016, 2016, 6762343.
- Tamano, H.; Koike, Y.; Nakada, H.; Shakushi, Y.; Takeda, A. Significance of synaptic Zn2+ signaling in zincergic and non-zincergic synapses in the hippocampus in cognition. J. Trace Elem. Med. Biol. 2016, 38, 93–98.
- Kardos, J.; Heja, L.; Simon, A.; Jablonkai, I.; Kovacs, R.; Jemnitz, K. Copper signalling: Causes and consequences. Cell Commun. Signal. 2018, 16, 71.
- Wolf, C.; Weth, A.; Walcher, S.; Lax, C.; Baumgartner, W. Modeling of Zinc Dynamics in the Synaptic Cleft: Implications for Cadherin Mediated Adhesion and Synaptic Plasticity. Front. Mol. Neurosci. 2018, 11.
- Tamano, H.; Morioka, H.; Nishio, R.; Takeuchi, A.; Takeda, A. Blockade of Rapid Influx of Extracellular Zn2+ into Nigral Dopaminergic Neurons Overcomes Paraquat-Induced Parkinson’s Disease in Rats. Mol. Neurobiol. 2019, 56, 4539–4548.
- D’Ambrosi, N.; Rossi, L. Copper at synapse: Release, binding and modulation of neurotransmission. Neurochem. Int. 2015, 90, 36–45.
- Ashraf, A.; Michaelides, C.; Walker, T.A.; Ekonomou, A.; Suessmilch, M.; Sriskanthanathan, A.; Abraha, S.; Parkes, A.; Parkes, H.G.; Geraki, K.; et al. Regional Distributions of Iron, Copper and Zinc and Their Relationships With Glia in a Normal Aging Mouse Model. Front. Aging Neurosci. 2019, 11.
- Pal, A.; Prasad, R. Regional Distribution of Copper, Zinc and Iron in Brain of Wistar Rat Model for Non-Wilsonian Brain Copper Toxicosis. Indian J. Clin. Biochem. 2016, 31, 93–98.
- Dixon, S.; Lemberg, K.; Lamprecht, M.; Skouta, R.; Zaitsev, E.; Gleason, C.; Patel, D.; Bauer, A.; Cantley, A.; Yang, W.; et al. Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death. Cell 2012, 149, 1060–1072.