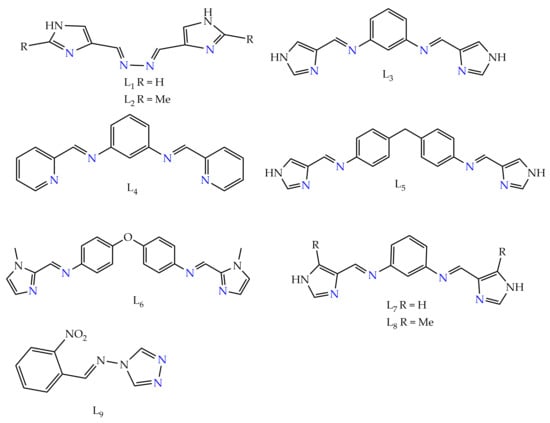
2. Schiff Base Ligand System in Supramolecular Architectures
Schiff bases, named after Hugo Schiff
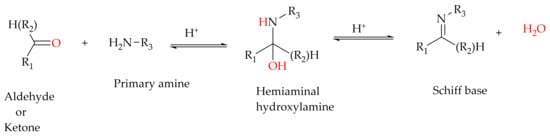
[25], are organic ligands that are synthesized from primary amines and carbonyl compounds (aldehydes/ketones) and known as imine or azomethine. Structurally, a Schiff base is an analogue of a ketone or aldehyde in which the carbonyl group (-C=O) is converted by a primary amine into an imine or azomethine (-HC=N-) functionality
[26] (
Scheme 1). Schiff bases are well-known ligands in constructing supramolecular architecture and other complexes through coordination bond, hydrogen bond, halogen bond or π-π interactions
[27]. Because of the simple reaction conditions to promote the synthesis of a wide variety of Schiff base ligands with different chelating abilities, flexibility or chirality, they are widely used to design and construct supramolecular architecture for specific purposes
[27]. Moreover, depending on the shape of the formyl and amine precursors and/or the metal-ion template used, the supramolecular architecture of the macrocyclic molecules of helicate/meso-helicate/spiral, grid/square cage and cube-containing imine groups can be formed through cyclization reactions as well. Schiff bases are classical organic ligands for the metal ions of p, d, and f blocks, which have significantly contributed to the development of coordination chemistry on both basic and application aspects
[28].
Scheme 1. General schematic synthesis of Schiff base ligands.
3. Magnetic Properties of Iron(II) Spin-Crossover Schiff Base Supramolecular Architectures
SCO supramolecular complexes are a fascinating class of materials revealing molecular bi-stability with their varying potential applications
[29]. The molecular bi-stability is due to the ability of electronic state switching between high spin (HS) and low spin (LS) when external stimuli such as change in temperature, pressure, light irradiation or guest presence/absence occurs
[30].
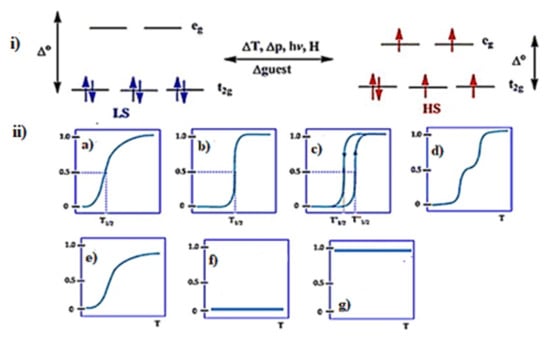
SCO can be observable for octahedral 3d transition metal (3d
4–3d
7) ions. There are two ways of electron distributions in the t
2g and e
g orbitals of octahedral iron(II) complexes. Depending on the nature of the ligand field splitting energy and pairing energy (P), electrons can be populated, as shown in (
Figure 2i). When a strong ligand field is applied, there will be large Δo, and electrons will remain in the lower energy state of the t
2g set and pair up, and it is a LS state (Δo > P), thus diamagnetic complexes can be observed. On the other hand, if weak field ligands are applied, Δo is small due to a very small energy difference between the t
2g and eg sets and the maximum number of unpaired electrons, i.e., the HS state occurs (Δo < P)
[31].
Figure 2. (
i) The d orbital splitting diagram for an octahedral iron(II) ion. (
ii) Various types of SCO behavior: (
a) gradual (
b) abrupt (
c) with hysteresis (
d) two-step transition (plateau) (
e) incomplete (
f) low spin and (
g) high spin. Reproduced with modification with permission from
[32]; Published by Royal Society of Chemistry, 2019.
When Δo is of a comparable magnitude to P, a possibility of inducing a switching between the HS and LS states arises (i.e., SCO) by perturbing the system through the application of external stimuli (
Figure 2i), by pressure, magnetic or electric field, light irradiation, and the presence/ absence of guest molecules. However, the most common perturbation is a change in temperature, due in large part to facile application and measurement
[33].
The SCO/spin switching/spin state in complexes are classified as gradual, abrupt, hysteretic, stepwise, incomplete, low-spin and high-spin, as shown in (
Figure 2ii). From spin switching in supramolecular complexes, hysteresis is the more focused one because of the cooperative first-order spin-state switching which is termed bi-stable. The supramolecular complexes in hysteresis spin switching can exist in two stable electronic states. The bi-stability of SCO supramolecular complexes, which shows a relatively large thermal hysteresis (ΔT ≥ 45 K) centered around room temperature (RT), is desired for various applications—especially for obtaining molecule-based switching and memory devices
[34][35][34,35].
SCOs at the metal centers of supramolecular architectures cause the bond between metal and ligand to contract or expand by 5–10% in length
[36]. This causes a change in the overall size and shape that leads to local distortions of the architecture. The distortions occurring in particular areas can trouble the lattice pressure at the nearest neighbor-switching sites that help transition propagating through the molecules. The efficiency of this propagation in the architecture determines the cooperativity of the metal centers in spin switching that exhibits thermal hysteresis
[36][37][38][36,37,38]. Hence, the designing of hysteretic SCO materials for specific application requires controlling of the interactions in metal ion switching sites in the architecture. Materials that exhibit large SCO hysteresis are mostly molecular complexes
[39][40][41][39,40,41], and many of the compounds are iron(II) complexes of N-donor ligands such as Schiff bases that are suited to cooperative spin transitions as they show large structural differences between HS and LS states
[42][43][44][42,43,44].
SCO systems, from a materials point of view, are verified as a potential molecular switch components in memory storage devices. Spin switching research is targeted towards the direction of molecular switch and memory applications that lead towards SCO materials that have complete, abrupt, hysteretic and switchable characters regarding RT. Above all, complete transitions are preferred as they are easier in the detection of distinct fully HS and LS spin-switch states
[45]. Abrupt transitions allow for a large output (color) for small inputs (small change in temperature). Hysteresis provides a thermal history of the complexes by the detection of temperature in the hysteresis loop; the system is bi-stable, and hence, there is a binary potential storage. Supramolecular array ligands that are designed for the purposes of giving exact Δo in the SCO, and also which facilitate cooperativity within or between the metal centers of complexes, are the main goal of many SCO chemists in getting abrupt and /or hysteretic spin transitions
[46].
Mononuclear iron(II) SCO complexes that are constructed by different kinds of coordination environments have been studied extensively, while di-nuclear to poly-nuclear structures are rare
[32][47][48][32,47,48].
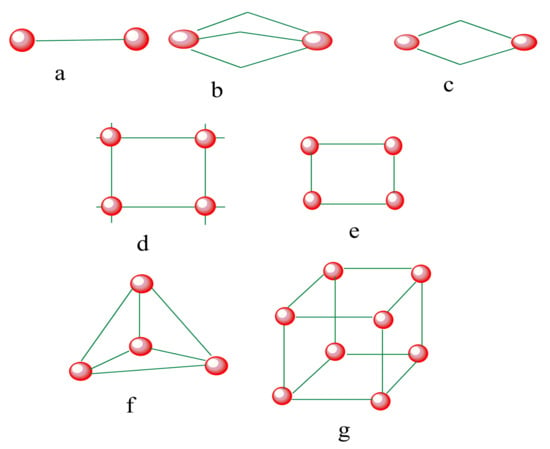
Figure 3 shows the different geometric possibilities of the SCO iron(II) supramolecular complexes of di-nuclear (spiral, helicate mesocate), tetra-nuclear (grid, square, tetrahedral) and octa-nuclear (cube). Di-nuclear and poly-nuclear have advantage over monomeric complexes because of multiple metal centers that can be covalently bridged in enhancing cooperativity in SCO systems
[49] with a designed and controlled synthetic protocol
[50][51][50,51].
Figure 3. SCO-active complexes of di-nuclear (a–c); grid (d), square (e), tetrahedral cage (f), octahedral cube (g). Green = di- to polydentate of N-donor and O-donor Schiff base ligands. Red = iron (II) metal centers.
Time-temperature integration on SCO compounds with thermal hysteresis has been described by Cavallini et al.
[52]. It is inferred from their review that in devices, the temperature band controllers (TBCs) are capable for monitoring the temperature changes depending on the spin states.
Also, in a recent review
[53] Cavallini et al., described the integration of SCO compounds as thin films on surfaces for technological application, proving the advancement made on SCO compounds in thin film growth and the self-assembly of the materials on surfaces.
Iron(II) SCO Compounds with Spiral Architectures
Spiral architectures of iron(II) are an interesting area to investigate due to the fascinating structure and cooperative SCO behavior, as metal centers in those will lead up to three different spin states LS-LS, LS-HS and HS-HS
[54][55][56][54,55,56]. These spin states can be related to cooperativity. Negative cooperativity can lead to a two-step spin transition, as the first SCO can stabilize the LS-HS mixed spin state in (
Figure 2d) and the second SCO takes place at different temperatures. Spiral iron(II) SCO architectures are interesting due to the fact that they have three-dimensional structures. As positive cooperativity occurs between metal centers, the SCO behaviors have been found in different forms compared as mononuclear analogues
[32][54][55][56][32,54,55,56]. Positive cooperativity can lead to one-step spin switching of both metal centers (i.e., [HS-HS] to [LS-LS] or vice versa) in which a sharp, abrupt and/or hysteretic loop SCO can be attained (
Figure 2b,c)
[57][58][59][60][57,58,59,60].
Recently, a helicate-like di-nuclear macrocyclic Schiff base iron(II) complex was reported by Zhang et al.
[61] showing an antiferromagnetic interaction between the metal centers. In the literature, there have been many iron(II) macrocyclic Schiff base complexes reported
[61][62][63][64][65][66][61,62,63,64,65,66] which were not formed by self-assembly and did not show any SCO properties.
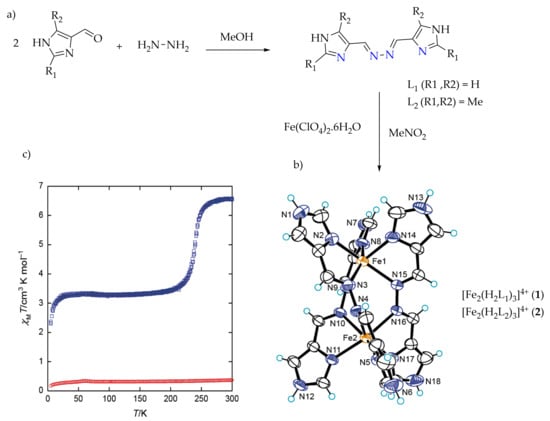
Kojima et al., reported on the SCO of spiral iron(II) complexes of L
1 and L
2 of (
Chart 1)
[57][59][57,59]. The di-nuclear spiral iron(II) complexes [Fe
2(H
2L
1)
3]
4+ (
1), [Fe
2(H
2L
2)
3]
4+ (
2) were synthesized by the reaction of respective ligands (
Figure 4a) and Fe(ClO
4)
2·6H
2O in nitromethane in a 3:2 molar ratio (
Figure 4b). The magnetic behaviors of complexes (
1) and (
2) are shown in (
Figure 4c). The complex from L
2 stayed in the LS state over the entire temperature range, and this result is in accordance with the strong ligand field strength of H
2L
2 caused by the presence of electron-donating methyl groups. The complex from L
1 exhibited an abrupt SCO behavior at about 240 K.
Chart 1. Ligands known to form SCO-active iron(II) dinuclear supramolecular architectures. Blue = binding pocket of the ligand to central metal.
Figure 4. (a) L1 and L2 used to synthesize spiral iron(II) complexes. (b) X-ray molecular structure of the cation of complexes (1) and (2). Color code: orange = Fe; blue = N; black = C; light blue = H. (c) Magnetic behaviors of complex (1), (blue) and (2) (red) in the form of a χMT vs. T plot.
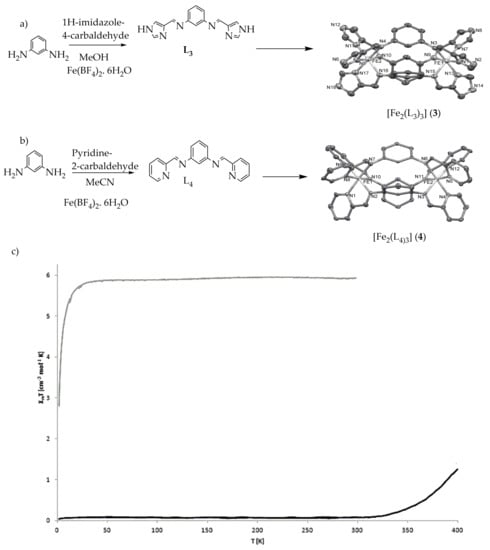
Lutzen et al., reported iron(II) spiral architectures of meso-helicate/mesocate rigid Schiff base ligands from 1
H-imidazole-4-carbaldehyde L
3 and Pyridine-2-carbaldehyde L
4 with 1,3-benzene diamine (
Figure 5a,b)
[67]. The complex designed as [Fe
2(L
3)
3] (
3) remained high-spin without undergoing SCO, while the complex [Fe
2(L
4)
3] (
4) remained low-spin up to around 350 K. However, upon heating to above 350 K, the magnetic moment began to increase. At 400 K (the limit of the magnetometer used), a magnetic moment of 1.26 cm
–3Kmol
–1 was reached, which indicates that roughly 20% of the metal centers had been switched to the high-spin state. The lack of SCO in complex (
3) was due to the reason that mechanical strain, imparted by strong CH-π interactions and ligand rigidity (which is locking the bond length of Fe-N) leads to a stabilizing of the high-spin state of the complex (
Figure 5c)
[68][69][68,69].
Figure 5. (a) [Fe2(L3)3] (3) [L3 = N,N′-bis(1H-imidazol-4-ylmethylene)benzene-1,3-diamine] (b) [Fe2(L4)3] (4) [L4 = N,N′-bis(pyridin-2-ylmethylene)benzene-1,3-diamine] (c) Magnetic behavior of complexes (3) blue line and (4) red line complexes as χMT vs. T plot.
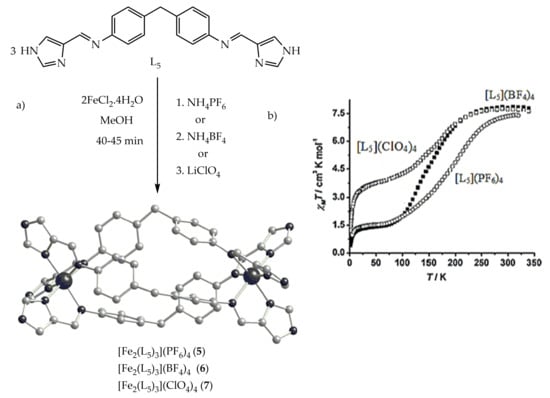
The intramolecular and intermolecular interactions such as hydrogen bonding and π-π interactions can affect the SCO which leads to difficulty in designing cooperative SCO systems. As a result, the same iron(II) spiral architecture can have a different SCO behavior depending on solvent or counter anions. Hannon et al.
[70] studied the effects of counter anions such as PF
6−, BF
4− and ClO
4− on SCO iron(II) spiral architecture with L
5 (
Chart 2), which has free imidazole N-H groups for hydrogen bonding to solvent and anions (
Figure 6a).
Figure 6. (a) The crystal structure of [Fe2(L5)3]X4 complexes (X= PF6 (5) or BF4 (6) and ClO4 (7) (b). Magnetic susceptibility χMT vs. T plot for spiral complexes of 5, 6 and 7 with different counter anions.
Chart 2.
The three bis-terdentate ligands known to form SCO-active Fe
4
L
4
grids. All ligands provide N
6
and N
4
O
2
donor sets to the octahedral iron(II) centers.
The PF
6− and BF
4−spiral complexes exhibited incomplete SCO at different T
1/2 values. The χ
MT value for [Fe
2(L
5)
3](PF
6)
4 (
5) at room temperature was 7.40 cm
3Kmol
−1, which corresponds to 2HS iron(II) centers. As the temperature is lowered down to 55 K, the χ
MT product decreases continuously (
Figure 6b), with a slight inflation at around 165 K. At this temperature, χ
MT is about 3.57 cm
3Kmol
−1, which corresponds to what is expected when 50% of the iron(II) ions undergo a thermally induced spin conversion from the HS to the LS. The spiral complex of [Fe
2(L
5)
3](BF
4)
4 (
6) behaves similar to complex (
5), but the SCO is more rapid. At room temperature, χ
MT is equal to 7.83 cm
3Kmol
−1, which is in the range of values expected for two non-interacting iron(II) centers. The χ
MT product remains almost constant when cooling to 250 K (
Figure 6b) and then decreases rapidly to reach the value of 1.90 cm
3Kmol
−1 at 100 K. The plateau observed in the temperature region 70–25 K corresponds to 1.40 cm
3Kmol
−1, suggesting an incomplete HS to LS transition with about 20% of the high-spin molar fraction trapped at low temperatures.
For [Fe
2(L
5)
3](ClO
4)
4 (
7), the χ
MT product is 7.70 cm
3Kmol
−1 in the temperature range 340–260 K, consistent with 100% of iron(II) centers in the HS state. As the temperature is lowered, χ
MT diminishes rapidly to reach a value of 3.81 cm
3Kmol
−1 at 60 K (
Figure 6). Between 60 and 40 K, the χ
MT product remains almost constant and finally drops rapidly down to 1.2 cm
3Kmol
−1 at 1.8 K. These features reveal an incomplete HS to LS spin conversion occurring in two steps. The first χ
MT drop is consistent with around 50% of iron(II) ions undergoing HS to LS spin conversion. The second χ
MT drop may be understood as arising from the combined effect of the electronic SCO and zero-field splitting of the remainder of the HS iron(II) ions.
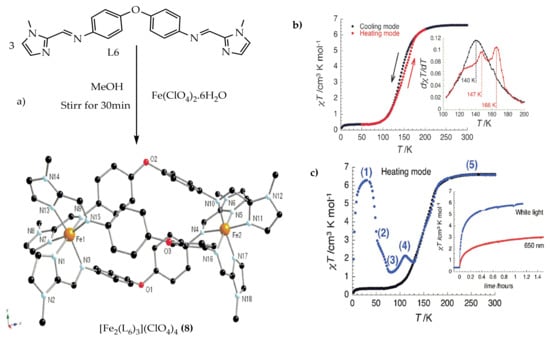
Kruger and co-workers
[71] reported the effect of solvents on the SCO behavior of an iron(II) spiral complex of L
6 (
Chart 2). The bis-bidentate L
6 was synthesized by the methanolic condensation of two equivalents of 1-methyl-2-imidazole carboxaldehyde and one equivalent of 4,4′-oxydianiline. Then, the corresponding iron(II) bi-nuclear triple helicate complex of [Fe
2(L
6)
3](ClO
4)
4 (
8) was obtained in good yield by stirring three equivalents of L
6 with two equivalents of Fe(ClO
4)
2.6H
2O in methanol for 30 min (
Figure 7a). Complex
8. 2MeCN showed complete SCO with hysteresis from HS-HS to LS-LS spin states at a low temperature of cooling mode at T
1/2 = 140 K. However, heating reproduces two-step transitions, which were observed between 147 and 166 K (
Figure 7b). Applying white light irradiation between 280 K–10 K, the spectral reflections at solid-state were collected. When irradiating with a white light or red light of 650 nm at 10 K, the HS-HS state could be accessed from the LS-LS spin state through LIESST shown in (
Figure 7c-inset). The spiral Iron(II) complex showed relaxation behavior in which two different relaxation mechanisms were proposed between points of peak 1 and valley 3. By way of heating, the first SCO was observed to have a metastable HS-HS spin state at point 4 and, upon heating, could lead to HS-HS at point 5.
Figure 7. (a) Molecular structure of the triple helicate of complex (8) (b) χMT vs. T plot for 8.2MeCN at 1000 Oe at a sweep rate of 0.6 K/min. Inset: dxT/dT vs. T plot (c) Temperature dependence of the χMT product after white light irradiation at 10 K for (8). Inset: χMT vs. T plot at 10 K under 650 nm and white light irradiations at 10 K (at T sweep rate of 0.6 K/min).
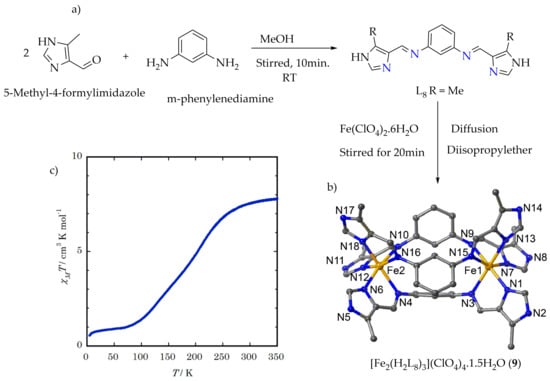
Sunatsuki et al.
[72] studied the effect of substitutions on the SCO behavior of a di-nuclear iron(II) mesocate complex reported by Lutzen et al.
[67] by changing the counter anion from BF
4– to ClO
4–. The bis-bidentate L
8 (
Figure 8a) was synthesized by the methanolic condensation of two equivalents of 5-methyl-4-formylimida-zole and one equivalent of m-phenylenediamine at room temperature. The corresponding triple mesocate complexes of [Fe
2(H
2L
8)
3](ClO
4)
4.1.5H
2O (
9) were obtained by stirring three equivalents of L
8 with two equivalents of Fe(ClO
4)
2.6H
2O (
Figure 8b).
Figure 8. (a) Synthesis root of ligand L8. (b) Crystal structure of the complex 9.1.5H2O. (c) The χMT vs. T plot of desolvated complex 9.
The χ
MT vs. T plot of desolvated complex
9 is shown in (
Figure 8c). The χ
MT value of complex
9 at 350 K is 7.8 cm
3 Kmol
–1 which clearly indicates that both iron(II) ions are in the HS states. The χ
MT values decreased gradually below 280 K and reached a plateau near 1.0 cm
3 K mol
–1 at 80 K, which is higher than that of the di-nuclear LS iron(II) complex. The χ
MT value at 80 K shows an incomplete (mixed) spin transition with 12.5% 2Fe (HS)-88.5% 2Fe (LS). The χ
MT measurement of this complex even decreased further below 20 K because of the remaining HS iron(II) ions’ orbital contributions. The SCO in the temperature region of 280 K–80 K shows a two-step spin state transition. The T
1/2 values for both two steps obtained by the first derivation of the χ
MT vs. T plot are 212 K and 134 K. There are two possible SCO processes for the two-step SCO of this di-nuclear mesocate iron(II) complex, i.e., [LS-LS] ↔ [LS-HS] ↔ [HS-HS] and the process such as [LS-LS] ↔ 50% [LS-LS] + 50% [HS-HS] ↔ 100% [HS-HS]. Generally, it is determined that crystalline complex
9 is in the HS state at 180 K. This fact indicates that the desolvation of crystalline enhances the essential SCO nature of complex
9 cations. The thermal hysteresis is not observed between cooling and heating scan modes of magnetic measurement but shows promising SCO behavior comparable with the report of Lutzen et al., (complex
8), where the similar di-nuclear mesocate shows no SCO behavior and remains HS state
[67]. This is due to the fact that the ligand H
2L
8 has a suitable ligand-field strength for the SCO of iron(II) due to the introduction of the electron-donating methyl groups on imidazole rings. Generally from the reports, it is clearly shown that by changing the substituent on the ligand, the iron(II) SCO nature can be enhanced specially to get the desired property for better applications.
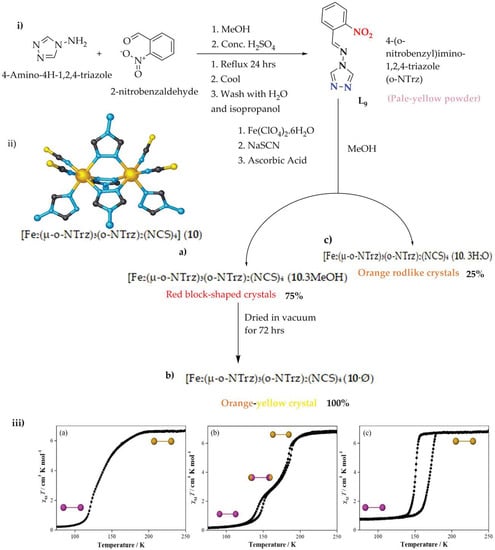
One of the great advantages of the di-nuclear iron(II) supramolecular complex is the tendency of adaptability with guests through binding pockets
[73][74][75][76][77][78][79][73,74,75,76,77,78,79] or the intra-spiral cavity
[80][81][80,81]. Neville et al., studied and reported on the guest adaptability of the SCO properties in iron(II) di-nuclear species underpinned by supramolecular interactions between the central metal cation and Schiff base ligand
[82]. The corresponding ligand L
9 (4-(
o-nitrobenzyl)imino-1,2,4-triazole (
o-NTrz)) was synthesized from 4-amino-4
H-1,24-triazole and 2-nitrobenzaldehyde by MeOH condensation (
Figure 9i) schematic procedures. Then, the di-nuclear iron(II) Schiff base complex of [Fe
2(μ-
o-NTrz)
3(
o-NTrz)
2(NCS)
4] (
10) (the solvated (
10 3MeOH), desolvate (10·Ø) and hydrated (
10. 3H
2O) were synthesized from L
9 Schiff base ligands as observed in
Figure 9ii(a–c).
Figure 9. (i) o-NTrz, 4-(onitrobenzyl)imino-1,2,4-triazole (blue: N-Fe binding groups, red: functionality). (ii) Di-nuclear [Fe2(μ-o-NTrz)3(o-NTrz)2(NCS)4] (10) core structure (a) 10·3MeOH, (b) 10·Ø, and (c) 10·3H2O (iii) χMT vs. T plot of the three complexes (a) 10·3MeOH, (b) 10·Ø, and (c) 10·3H2O (250−50−250 K; sweep mode; scan rate: 2 K min−1). Inset: Spin-state per di-nuclear unit at the indicated plateau region, as determined by structural analysis (Fe-Fe; HS: gold; LS: purple).
The χ
MT vs. T magnetic measurements were performed for the three di-nuclear complexes
10·3MeOH,
10·Ø and
10·3H
2O that show a broad diversity of SCO transition profile characteristics (
Figure 9iii(a–c)). The di-nuclear iron(II) Schiff base complex (
10·3MeOH) in
Figure 9 iii(a) shows the χ
MT value of 6.64 cm
−3Kmol
−1 which is consistent with two HS iron(II) ions at 250 K. This value remains constant up to 200 K, then shows a gradual decrease in χ
MT values to 150 K, followed by a more abrupt decrease to 115 K. The χ
MT values remain constant again at 0.25 cm
−3 K mol
−1, below 100 K, which is consistent with two LS iron(II) ions. There is no thermal hysteresis observed over the cooling and heating modes; rather, a gradual and complete one-step SCO transition for
10·3MeOH is observed at a transition temperature (T
1/2) of 135 K.
The χ
MT values of the di-nuclear complex (
10·Ø) (
Figure 9iii(a)) remain constant from 250 to 200 K at 6.80 cm
−3Kmol
−1, which is inconsistent with two HS iron(II) ions. From 200 to 175 K, the χ
MT values decrease quickly to 3.39 cm
−3Kmol
−1 and then again to 0.25 cm
−3Kmol
−1 over the range of 175−125 K, showing a two-step spin transition. The χ
MT values at an intermediate plateau at around 175 K show a 50%HS-50%LS for iron(II) centers. The χ
MT value 100 K remains constant at 0.25 cm
−3Kmol
−1, which indicates the conversion of fully HS to LS iron(II) ions that show complete and two-step SCO. Two narrow thermal hystereses is observed over the heating and cooling mode and the two iron(II) centers for the complex
10·Ø. One thermal hysteresis is observed at T
1/2↓↑ 181 and 185 K (ΔT = 4 K), and the second thermal hysteresis is at T
1/2↓↑ 141 and 148 K (ΔT = 7 K) during 2 Kmin
−1 scan rate.
The third di-nuclear iron(II) SCO complex
10·3H
2O (
Figure 9iii(c)) remains fully HS on the cooling mode from 250 K to 160 K as χ
MT values remain constant at 6.74 cm
−3Kmol
−1. From 160 K to 130 K, the χ
MT values show a sharp and abrupt decrease to 0.78 cm
−3Kmol
−1, which is responsible for a complete and one-step spin transition to fully LS iron(II) centers. A promising wide thermal hysteresis loop is observed over the cooling and heating mode. The complete and hysteretic one-step SCO transition of
10·3H
2O (2 Kmin
−1) is characterized by T
1/2↓↑:150, 172 K, ΔT= 22 K. This is the first evidence of the strong cooperativity of metal centers in the complex and one of the widest thermal hysteresis reported so far for a di-nuclear iron(II) SCO compound
[74][75][76][77][78][79][74,75,76,77,78,79]. Comparing the three complexes above, all of them show complete spin transition on both cooling and heating modes during the scan rate of 2 Kmin
−1, but complex
10·3H
2O shows a more promising SCO property. Generally, since it is promising research, further studies are recommended by changing external stimuli, guests such as counter anion, and/or substitutions to get a better thermal hysteresis loop as far as the application side is concerned.