Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Conner Chen and Version 1 by Ana Carolina Feltrin.
By their unique compositions and microstructures, the high-entropy materials (HEMs) exhibit outstanding properties and performance above the threshold of traditional materials. Wear- and erosion-resistant materials are of significant interest for different applications, such as industrial devices, aerospace materials, and military equipment, related to their capability to tolerate heavy loads during sliding, rolling, or impact events.
- high-entropy
- wear
- irradiation
1. Alloys
HEAs were introduced in 2004, culminating in an exponentially growing field of research. The goal of the high-entropy mixing system in alloys was generally directed to increase strength and ductility. The first developed composition is the CoCrFeMnNi alloy, termed Cantor alloy [2][1], formed in a face-centered cubic (fcc) system followed by other compositions with body-centered cubic (bcc) and hexagonal close-packed (hcp) structures, as exemplified in Figure 21a–c.
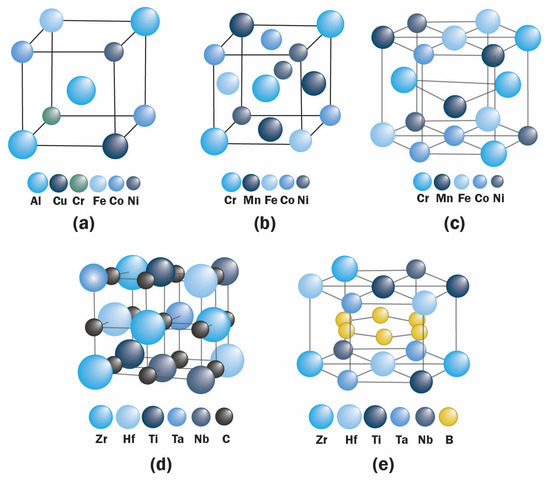
Figure 21. Proposed arrangement of atoms in the structures without considering lattice distortion of: (a) BCC CuCoNiCrAl2.8Fe [1][2], (b) FCC FeCrMnNiCo [2][1], (c) hcp CoCrFeMnNi [50][3], (d) rock-salt (Hf0.2Zr0.2Ti0.2Ta0.2Nb0.2)C [48][4], and (e) layered hexagonal (Hf0.2Zr0.2Ti0.2Ta0.2Nb0.2)B2 [27][5].
The primary production method of bulk HEAs is by powder metallurgy, using mechanical alloying followed by spark plasma sintering (SPS), hot pressing, or hot isostatic pressing (HIP) methods. More detailed information is presented in the review by Torralba et al. [29][6]. Additive manufacturing (AM) is also used for bulk HEAs production, using laser metal deposition (LMD), selective laser melting (SLM), and particular electron beam melting (SEBM). The main advantage of bulk HEAs produced by AM is the complex geometrical shapes and structure tailoring. A review of HEAs produced by AM is presented by Moghaddam et al. [30][7]. A way to take advantage of HEAs is by applying them as films and coatings for conventional materials. It is better economically, and a good combination of costs and properties can be achieved [31][8]. In the same way, HEAs coatings and films have great potential in solving the demand for high-performance surfaces applied in extreme conditions [32][9].
The deposition of HEA coatings can be carried out by different methods, such as the sputtering deposition technique [33[10][11],34], resistance seam welding [35][12], laser cladding [36][13], electrodeposition [37][14], etc. Due to the high heating temperature and fast cooling rate, the microstructure of deposited HEAs is homogeneous, and the formation of amorphous and nanocrystalline phases is observed [32][9].
2. Ceramics
HECs is a young research field, and its concept was created even before the definition of its name. After the description of HEAs in 2004, high-entropy nitride was synthesized in the same year [10][15], and in 2012 the term high-entropy alloy oxide appeared [38][16], describing the HECs family of materials. Nowadays, there are families of high-entropy nitrides [39][17], high-entropy carbides [40][18], high-entropy oxides [41][19], high-entropy borides [27[5][20],42], and more recently, high-entropy silicides [43][21], high-entropy borocarbides [11[22][23],44], and high-entropy oxyhalides [45][24]. Ceramic protective films and coatings are applied in industries as an alternative to bulk materials. The characteristics of high-entropy diborides, carbides, and nitrides as coatings and films have been reviewed and detailed elsewhere [42,46,47][20][25][26].
The crystal structure of HECs is characterized by the atomic disorder of metal elements occupying the cation position and non-metal elements occupying the anion position, resulting in compositional complexity and lattice distortion. The principal difference between HEAs and HECs is due to the lattice distortion that, in the first, occurs in the whole crystal structure, while in the latter, it occurs through the anion sublattice [48][4].
An Ideal HEC should have a lattice with long-range periodicity but compositional disorder. The reported formed structures of HECs are mostly single-phase rock-salt structure and layered hexagonal single-phase, as shown in Figure 2d,e. However, amorphous structure and dual-phase HECs have also been reported [49][27].
The SPS or other methods that apply pressure and temperature simultaneously are the primary preparation method for bulk high-entropy ceramics. On the other hand, the most adopted technique for coatings and films is the sputtering deposition technique.
Generally, synthesizing HECs aims to develop materials with improved electrical, thermoelectrical, and optical properties and as coatings on cutting tools and hard-facing materials. Among the different HECs, the (TaNbHfTiZr) carbide and nitride structures [48,51,52,53][4][28][29][30] have gained much attention as high-hardness coatings, high oxidation, and wear-resistant materials with good thermodynamic properties.
3. Other Types of HEMs
High-entropy composites: The addition of SiC as the second phase in HECs has been investigated. Wang et al. [54][31] prepared a high-entropy composite using (Hf0.2Ta0.2Zr0.2Ti0.2Nb0.2)C doped with SiC to improve oxidation resistance at high temperatures. A high-entropy B4(HfMo2TaTi)C with SiC composite was synthesized, and it was proved that the addition of the secondary phase (SiC) offered oxidation resistance at high temperatures [11][22]. The addition of 20% SiC improved the mechanical properties of the high-entropy (Ti0.2Zr0.2Hf0.2Nb0.2Ta0.2)C, and the primary toughening mechanism was considered to be the crack deflection by the SiC [55][32].
High-entropy polymers: An HEA-polymer composite was synthesized and presented high specific strength and excellent recoverability under compression [56][33], showing the potential field to induce future studies with this brand-new material. Huang et al. [57][34] also used the concept of high-entropy to prepare a polymer blend with five different polymers, which manifested excellent performance, suppressing phase separation in the material.
High-entropy metallic glasses: The first report on high-entropy metallic glass was made by Ma et al. in 2002 [58][35], and its general properties are discussed by Wang [59][36]. The high-entropy metallic glasses were processed to combine the advantages of HEAs and metallic glasses, as higher thermal stability and sluggish crystallization process [60][37], enabling the use as high-performance materials. For example, they can have a high application potential under extreme irradiation conditions [61][38].
High-entropy interlayers: Medium-entropy alloys can be added as an interlayer between Al and steel, forming a high-entropy interlayer during fusion welding by thermal diffusion of the metallic elements. This can help to avoid brittleness in the welded joint [62][39]. For example, Ding et al. [63][40] produced a CoCrFeMnNi high-entropy interlayer as a diffusion bonding of copper and titanium, and strong adhesion between the layers was achieved at temperatures from 800 °C to 900 °C.
High-entropy compositionally graded materials: Addictive manufacturing can be used for synthesizing compositionally graded materials by varying the composition of each layer of deposited material [64][41]. Gwalani et al. [65][42], for example, produced AlxCoCrFeNi high-entropy alloy, with x = 0.3–0.7 and obtained single phase FCC grains with elongated shapes (x = 0.3) and FCC with typical lamellar morphology of a eutectic (x = 0.7), in the same produced specimen.
References
- Cantor, B.; Chang, I.T.H.; Knight, P.; Vincent, A.J.B. Microstructural Development in Equiatomic Multicomponent Alloys. Mater. Sci. Eng. A 2004, 375–377, 213–218.
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured High-Entropy Alloys with Multiple Principal Elements: Novel Alloy Design Concepts and Outcomes. Adv. Eng. Mater. 2004, 6, 299–303.
- Zhang, F.; Wu, Y.; Lou, H.; Zeng, Z.; Prakapenka, V.B.; Greenberg, E.; Ren, Y.; Yan, J.; Okasinski, J.S.; Liu, X.; et al. Polymorphism in a High-Entropy Alloy. Nat. Commun. 2017, 8, 15687.
- Yan, X.; Constantin, L.; Lu, Y.; Silvain, J.F.; Nastasi, M.; Cui, B. (Hf0.2Zr0.2Ta0.2Nb0.2Ti0.2)C High-Entropy Ceramics with Low Thermal Conductivity. J. Am. Ceram. Soc. 2018, 101, 4486–4491.
- Gild, J.; Zhang, Y.; Harrington, T.; Jiang, S.; Hu, T.; Quinn, M.C.; Mellor, W.M.; Zhou, N.; Vecchio, K.; Luo, J. High-Entropy Metal Diborides: A New Class of High-Entropy Materials and a New Type of Ultrahigh Temperature Ceramics. Sci. Rep. 2016, 6, 37946.
- Torralba, J.M.; Alvaredo, P.; García-Junceda, A. High-Entropy Alloys Fabricated via Powder Metallurgy. A Critical Review. Powder Metall. 2019, 62, 84–114.
- Ostovari Moghaddam, A.; Shaburova, N.A.; Samodurova, M.N.; Abdollahzadeh, A.; Trofimov, E.A. Additive Manufacturing of High Entropy Alloys: A Practical Review. J. Mater. Sci. Technol. 2021, 77, 131–162.
- Shi, Y.; Yang, B.; Liaw, P.K. Corrosion-Resistant High-Entropy Alloys: A Review. Metals 2017, 7, 43.
- Li, J.; Huang, Y.; Meng, X.; Xie, Y. A Review on High Entropy Alloys Coatings: Fabrication Processes and Property Assessment. Adv. Eng. Mater. 2019, 21, 1900343.
- An, Z.; Jia, H.; Wu, Y.; Rack, P.D.; Patchen, A.D.; Liu, Y.; Ren, Y.; Li, N.; Liaw, P.K. Solid-Solution CrCoCuFeNi High-Entropy Alloy Thin Films Synthesized by Sputter Deposition. Mater. Res. Lett. 2015, 3, 203–209.
- Feng, X.; Tang, G.; Sun, M.; Ma, X.; Wang, L.; Yukimura, K. Structure and Properties of Multi-Targets Magnetron Sputtered ZrNbTaTiW Multi-Elements Alloy Thin Films. Surf. Coat. Technol. 2013, 228, S424–S427.
- Zhao, D.; Yamaguchi, T.; Wang, W. Fabrication and Wear Performance of Al0.8FeCrCoNi High Entropy Alloy Coating on Magnesium Alloy by Resistance Seam Welding. Mater. Lett. 2020, 265, 127250.
- Zhang, H.; Wu, W.; He, Y.; Li, M.; Guo, S. Formation of Core-Shell Structure in High Entropy Alloy Coating by Laser Cladding. Appl. Surf. Sci. 2016, 363, 543–547.
- Yao, C.Z.; Zhang, P.; Liu, M.; Li, G.R.; Ye, J.Q.; Liu, P.; Tong, Y.X. Electrochemical Preparation and Magnetic Study of Bi-Fe-Co-Ni-Mn High Entropy Alloy. Electrochim. Acta 2008, 53, 8359–8365.
- Chen, T.K.; Shun, T.T.; Yeh, J.W.; Wong, M.S. Nanostructured Nitride Films of Multi-Element High-Entropy Alloys by Reactive DC Sputtering. Surf. Coat. Technol. 2004, 188–189, 193–200.
- Tsau, C.H.; Yang, Y.C.; Lee, C.C.; Wu, L.Y.; Huang, H.J. The Low Electrical Resistivity of the High-Entropy Alloy Oxide Thin Films. Procedia Eng. 2012, 36, 246–252.
- Shen, W.J.; Tsai, M.H.; Tsai, K.Y.; Juan, C.C.; Tsai, C.W.; Yeh, J.W.; Chang, Y.S. Superior Oxidation Resistance of (Al 0.34 Cr 0.22 Nb 0.11 Si 0.11 Ti 0.22) 50 N 50 High-Entropy Nitride. J. Electrochem. Soc. 2013, 160, C531–C535.
- Dusza, J.; Švec, P.; Girman, V.; Sedlák, R.; Castle, E.G.; Csanádi, T.; Kovalčíková, A.; Reece, M.J. Microstructure of (Hf-Ta-Zr-Nb)C High-Entropy Carbide at Micro and Nano/Atomic Level. J. Eur. Ceram. Soc. 2018, 38, 4303–4307.
- Rost, C.M.; Sachet, E.; Borman, T.; Moballegh, A.; Dickey, E.C.; Hou, D.; Jones, J.L.; Curtarolo, S.; Maria, J.P. Entropy-Stabilized Oxides. Nat. Commun. 2015, 6, 8485.
- Mayrhofer, P.H.; Kirnbauer, A.; Ertelthaler, P.; Koller, C.M. High-Entropy Ceramic Thin Films; A Case Study on Transition Metal Diborides. Scr. Mater. 2018, 149, 93–97.
- Gild, J.; Braun, J.; Kaufmann, K.; Marin, E.; Harrington, T.; Hopkins, P.; Vecchio, K.; Luo, J. A High-Entropy Silicide: (Mo0.2Nb0.2Ta0.2Ti0.2W0.2)Si2. J. Mater. 2019, 5, 337–343.
- Zhang, H.; Hedman, D.; Feng, P.; Han, G.; Akhtar, F. A High-Entropy B4(HfMo2TaTi)C and SiC Ceramic Composite. Dalton Trans. 2019, 48, 5161–5167.
- Zhang, H.; Akhtar, F. Processing and Characterization of Refractory Quaternary and Quinary High-Entropy Carbide Composite. Entropy 2019, 21, 474.
- Wang, Q.; Sarkar, A.; Wang, D.; Velasco, L.; Azmi, R.; Bhattacharya, S.S.; Bergfeldt, T.; Düvel, A.; Heitjans, P.; Brezesinski, T.; et al. Multi-Anionic and -Cationic Compounds: New High Entropy Materials for Advanced Li-Ion Batteries. Energy Environ. Sci. 2019, 12, 2433–2442.
- Jansson, U.; Lewin, E. Carbon-Containing Multi-Component Thin Films. Thin Solid Films 2019, 688, 137411.
- Lewin, E. Multi-Component and High-Entropy Nitride Coatings—A Promising Field in Need of a Novel Approach. J. Appl. Phys. 2020, 127, 160901.
- Qin, M.; Gild, J.; Hu, C.; Wang, H.; Bin Hoque, M.S.; Braun, J.L.; Harrington, T.J.; Hopkins, P.E.; Vecchio, K.S.; Luo, J. Dual-Phase High-Entropy Ultra-High Temperature Ceramics. J. Eur. Ceram. Soc. 2020, 40, 5037–5050.
- Yang, L.; Ge, H.; Zhang, J.; Xiong, T.; Jin, Q.; Zhou, Y.; Shao, X.; Zhang, B.; Zhu, Z.; Zheng, S.; et al. High He-Ion Irradiation Resistance of CrMnFeCoNi High-Entropy Alloy Revealed by Comparison Study with Ni and 304SS. J. Mater. Sci. Technol. 2019, 35, 300–305.
- Braic, V.; Vladescu, A.; Balaceanu, M.; Luculescu, C.R.; Braic, M. Nanostructured Multi-Element (TiZrNbHfTa)N and (TiZrNbHfTa)C Hard Coatings. Surf. Coat. Technol. 2012, 211, 117–121.
- Feng, L.; Fahrenholtz, W.G.; Hilmas, G.E. Low-Temperature Sintering of Single-Phase, High-Entropy Carbide Ceramics. J. Am. Ceram. Soc. 2019, 102, 7217–7224.
- Wang, H.; Cao, Y.; Liu, W.; Wang, Y. Oxidation Behavior of (Hf0.2Ta0.2Zr0.2Ti0.2Nb0.2)C-XSiC Ceramics at High Temperature. Ceram. Int. 2020, 46, 11160–11168.
- Lu, K.; Liu, J.X.; Wei, X.F.; Bao, W.; Wu, Y.; Li, F.; Xu, F.; Zhang, G.J. Microstructures and Mechanical Properties of High-Entropy (Ti0.2Zr0.2Hf0.2Nb0.2Ta0.2)C Ceramics with the Addition of SiC Secondary Phase. J. Eur. Ceram. Soc. 2020, 40, 1839–1847.
- Zhang, X.; Yao, J.; Liu, B.; Yan, J.; Lu, L.; Li, Y.; Gao, H.; Li, X. Three-Dimensional High-Entropy Alloy-Polymer Composite Nanolattices That Overcome the Strength-Recoverability Trade-Off. Nano Lett. 2018, 18, 4247–4256.
- Huang, Y.J.; Yeh, J.W.; Chang-Mou Yang, A. “High-Entropy Polymers”: A New Route of Polymer Mixing with Suppressed Phase Separation. Materialia 2021, 15, 100978.
- Ma, L.; Wang, L.; Zhang, T.; Inoue, A. Bulk Glass Formation of Ti-Zr-Hf-Cu-M (M = Fe, Co, Ni) Alloys. Mater. Trans. 2002, 43, 277–280.
- Wang, W.H. High-Entropy Metallic Glasses. JOM 2014, 66, 2067–2077.
- Yang, M.; Liu, X.J.; Ruan, H.H.; Wu, Y.; Wang, H.; Lu, Z.P. High Thermal Stability and Sluggish Crystallization Kinetics of High-Entropy Bulk Metallic Glasses. J. Appl. Phys. 2016, 119, 245112.
- Wang, Y.; Zhang, K.; Feng, Y.; Li, Y.; Tang, W.; Zhang, Y.; Wei, B.; Hu, Z. Excellent Irradiation Tolerance and Mechanical Behaviors in High-Entropy Metallic Glasses. J. Nucl. Mater. 2019, 527, 151785.
- Waseem, O.A. Can High-Entropy Interlayers Develop Intermetallic-Free Welded Joints of Dissimilar Metals? Eng 2020, 1, 183–187.
- Ding, W.; Liu, N.; Fan, J.; Cao, J.; Wang, X. Diffusion Bonding of Copper to Titanium Using CoCrFeMnNi High-Entropy Alloy Interlayer. Intermetallics 2021, 129, 107027.
- Reichardt, A.; Shapiro, A.A.; Otis, R.; Dillon, R.P.; Borgonia, J.P.; McEnerney, B.W.; Hosemann, P.; Beese, A.M. Advances in Additive Manufacturing of Metal-Based Functionally Graded Materials. Int. Mater. Rev. 2021, 66, 1–29.
- Gwalani, B.; Gangireddy, S.; Shukla, S.; Yannetta, C.J.; Valentin, S.G.; Mishra, R.S.; Banerjee, R. Compositionally Graded High Entropy Alloy with a Strong Front and Ductile Back. Mater. Today Commun. 2019, 20, 100602.
More
