Methylmercury (MeHg) is the most important and the most abundant organic Hg pollutant in the aquatic ecosystem that can affect human health through biomagnification. It is the most toxic organic Hg form, which occurs naturally and by human-induced contamination in water and is further biomagnified in the aquatic food web. MeHg is the only Hg form that accumulates in living organisms and is able to cross the blood–brain barrier, presenting an enormous health risk. Anthropogenic activity increases eutrophication of coastal waters worldwide, which promotes algae blooms. Microalgae, as primary producers, are especially sensitive to MeHg exposure in water and are an important entrance point for MeHg into the aquatic food web. MeHg assimilated by microalgae is further transferred to fish, wildlife and, eventually, humans as final consumers. MeHg biomagnifies and bioaccumulates in living organisms and has serious negative health effects on humans, especially newborns and children.
- methylmercury
- mercury cycling
- microalgae
- biomagnification
1. Introduction
2. MeHg Interaction with Organic Matter (OM), S and Se Organic Compounds
A key aspect in MeHg bioaccumulation and toxicity is the degree to which MeHg interacts with complexing agents in solution (OH−, Cl−, organic sulfur compounds—especially thiols (ligands containing sulfhydryl group R–SH), OM, artificial chelating agents such as EDTA salts) [4][7][4,7]. OM that is naturally present in aqueous systems promotes Hg (II) methylation mainly because it contains metabolic substrate molecules essential for heterotrophic microbes and strong ligands that bind Hg(II) [8][11]. In the case of highly enclosed terrestrial water basins, such as the Baltic Sea, hypoxic and anoxic zones are caused by an increased biological oxygen demand (BOD) due to the excess of nutrients and OM runoffs where Hg binds to allochthonous (terrestrially discharged) OM in the surface layers of the water column and sinks to deeper waters, where it can be released in the microbial remineralization process of OM [9][10][11][12,13,14]. In natural waters where OM and reduced S species are absent, MeHg forms inorganic complexes, MeHgCl and MeHgOH, with chloride and hydroxide ions [12][15]. The chemical speciation of MeHg prior to cell exposure controls its bioavailability and is a key factor determining the uptake rate and accumulation in microalgae [13][14][15][10,16,17]. MeHg forms stronger complexes with organic thiols, which also have higher stability constants than complexes with OH− and Cl− groups [16][18]. The bioconcentration step of MeHg from water to the base of the food web is crucial for MeHg concentrations found in aquatic organisms higher up in the food web [5][12][17][5,15,19]. Uptake by microalgae is the first and largest step of MeHg bioaccumulation in aquatic food webs, which is influenced by many factors that drive seasonal changes in water MeHg concentrations [8][11]. OM is an important parameter of MeHg production and uptake by phytoplankton. Measurements from the Bothnian Sea in the Northern Baltic, taken as one of the few studied models of biogeochemical cycling of MeHg in natural aquatic environments, showed that MeHg concentrations can vary by up to an order of magnitude between years (from 103 ± 12 fM in September 2014 to 18 ± 9 fM in August 2016), which underlines the interannual variability in water column MeHg concentrations [8][11]. Both Hg(II) and MeHg preferentially bind to DOM over inorganic particles, which affects Hg bioavailability [8][11]. DOM can also decrease light attenuation in water, which decreases MeHg photodegradation. The association between MeHg and dissolved organic carbon (DOC) suggests that labile DOC is the important factor for the remineralization rate and Hg(II) methylation potential [8][11]. DOM promotes methylation of Hg(II) by (i) stimulating microbial activity and methylation processes, (ii) providing methyl groups for methylation, and (iii) enhancing the solubility of HgS(s) in mineral form [18][20]. Increased DOM inputs from terrestrial runoffs may lead to higher inputs of Hg and MeHg and increase MeHg formation in the aquatic ecosystem [18][20]. Hg methylation mainly takes place within 24 h after entering aquatic ecosystems, and to a greater extent under nonequilibrium conditions, before inorganic Hg becomes complexed with substances present in DOM [18][20]. It is often difficult to characterize DOM, and because ~50% of DOM is organic carbon, it is usually measured and expressed as DOC [14][16]. Natural levels of DOC vary in different aquatic environments: 4.0 ± 0.02 mg/L in the Baltic Sea; 3.78 ± 1.42 mg/L in Lake Titicaca (Bolivia), with peaks of up to 8 mg/L in some regions; 1.57–17.6 mg/L in the Sacramento River Delta (Sacramento, CA, USA) [14][19][20][21][16,21,22,23]. Humic matter forms part of allochthonous DOM in seawater [8][11]. In the case of the Northern Baltic, humic matter concentration decreases from the Bothnian Bay to the Bothnian Sea and further remains constant around 10 µg/L [8][11]. Humic content reduces microbial MeHg degradation by decreasing its bioavailability and decreases photodegradation of MeHg by increasing light attenuation [8][11]. Humic substances can also bind Hg (II) and correlate more strongly than DOC with Hg(II) concentration in water streams. Binding to humic substances makes Hg(II) less available to microbial reduction and methylation [22][24]. Humic substances are not easily remineralized; thus, an increased proportion of humic matter can decrease the activity of microorganisms that act by chemical reduction and methylation of Hg (II), which further decreases the rates of Hg transformation reactions [8][11]. In the Northern Baltic Sea, most Hg(II) was available for methylation by forming stable complexes with humic substances or by converting Hg(II) to elemental Hg [8][11]. The stability constants for Hg–ligand complexes drive metal internalization, thus determining rates of further chemical conversion of Hg species inside the cell. In this sense, it was demonstrated that a Hg–ligand (Hg–L) complex in the culture medium reacts with a biotic ligand (R) at the microalgae cell surface, forming a new complex (Hg–R) prior to metal internalization, and the rate for the formation of complexes at the cell surface is determined by the relative thermodynamic stability constants for Hg–L and Hg–R [13][10]. Similar to thiols (R–SH), organic selenium also has an affinity towards MeHg and can modify its toxicity by complexation [2]. Selenium belongs to the same group in the periodic table as S and exerts similar chemical affinities, so it can act as a S analogue in amino acids (selenomethionine—SeMet and selenocysteine—SeCys) as well as other organic compounds [23][25]. Some microalgae strains can take up Se(VI) salts from the medium, biotransform it to organic Se compounds, such as SeMet, which is less toxic to the cell than Se(VI), and accumulate it in the biomass [24][25][26,27]. A biologically important Se amino acid—SeMet [23][25]—significantly inhibits the uptake of MeHg by diatoms and mussels [26][28]. Detoxification of MeHg in seabirds and marine mammals involves its demethylation by reactive oxygen species (ROS) and the subsequent formation of high-molecular-weight Hg–Se–protein compounds, which are then degraded in lysosomes, forming insoluble Hg–Se compounds [27][29]. It was demonstrated that fish and wildlife living in environments with elevated Se levels exhibit lower MeHg accumulation [27][29]. There are a few proposed mechanisms for the protective role of Se against Hg toxicity, such as competition for binding sites or the formation of Hg–Se complexes. It was suggested that the increased Hg(II) uptake in the presence of SeMet is due to the formation of Hg–Se complexes that can be transported across the membrane at a faster rate [26][28].3. Biogeochemical Cycling of MeHg and Its Presence in Global Aquatic Ecosystem
Human exposure to MeHg comes predominately from diets containing Hg-contaminated fish and seafood [28][29][30,31]. Understanding the cycling of Hg in aquatic systems is essential to assess the environmental risks to human health. Hg cycling differs in freshwater and ocean ecosystems. Hg can be released into the atmosphere both by natural (e.g., volcano eruptions) and anthropogenic sources: mining and burning of fossil fuels [30][31][32,33]. However, the more toxic organic form, MeHg, is the dominant species found in rice paddies and fish [32][33][34][34,35,36]. Hg speciation in nature has three different forms: elemental Hg(0), Hg(II) bound to particulate OM and cationic inorganic Hg(II), mainly as HgCl2. Elemental Hg(0) travels around the globe, and cationic Hg is transported at intermediate distances, while particulate is not transported very far [35][37]. MeHg contamination is also associated with long-term emissions from fossil fuel combustion throughout the industrialized world [36][38]. The burning of fossil fuels, coal, the extraction of gold, smelting and chemical production increase atmospheric Hg input in terrestrial and aquatic ecosystems [37][39]. The burning of coal is the most abundant anthropogenic source of Hg emissions in the USA [36][38]. Hg leaching from fossil fuel combustion can contribute to a significant increase in Hg concentration in rivers that enter the sea [28][30]. Subsequently, Hg(II) reduces to elemental Hg(0), which is usually volatilized to the atmosphere, while a small portion of Hg(II) is converted to MeHg, which is the most toxic form of Hg and acts primarily as a neurotoxin in humans and wildlife [29][31]. It is known that Hg emissions are ubiquitous, and MeHg as a contaminant bioaccumulates to a great extent in the aquatic food web [35][37]. It was estimated that bioconcentration factors of MeHg are 106–108 fold from initial water MeHg concentrations, resulting in MeHg levels that are of global toxicological concern [30][35][32,37]. Biogeochemical cycling of Hg includes emissions of anthropogenic Hg, transport through the atmosphere, deposition into ocean, subsequent transformation into MeHg [38][40], and its incorporation into aquatic food webs, which depends on its absorption or modification by microorganisms (Figure 1a). Microorganisms perform four types of Hg transformation: reduction of Hg(II) to Hg(0), degradation of organic Hg compounds, methylation of Hg(II) and the oxidation of Hg(0) to Hg(II) [29][31]. Methylation of Hg occurs in wetlands and lakes as a biological process performed by a range of known heterotrophic microbes (iron- and sulfur-reducing bacteria, methanogens and nitrite oxidizers) [8][11]. The bulk of MeHg in natural ecosystems originates from methylation of atmospherically deposited Hg by sulfur-reducing bacteria within aquatic sediments [30][36][32,38]. Reducing anaerobic conditions within aquatic sediments that support the growth and Hg methylation activity of sulfur-reducing bacteria are in redox potential (Eh) and pH ranges of 0.4 ≤ Eh ≤ 0.0 V and 5 ≤ pH ≤ 10 [36][38]. MeHg is predominately formed in hypoxic and anoxic environments from its inorganic form, Hg(II), via biological reactions, and the methylation process has been mostly linked to the presence of sulfate- and iron-reducing bacteria [39][41]. However, more recently, methanogens and other microbes have been found to play important roles in the MeHg formation process [40][41][42][43][44][42,43,44,45,46].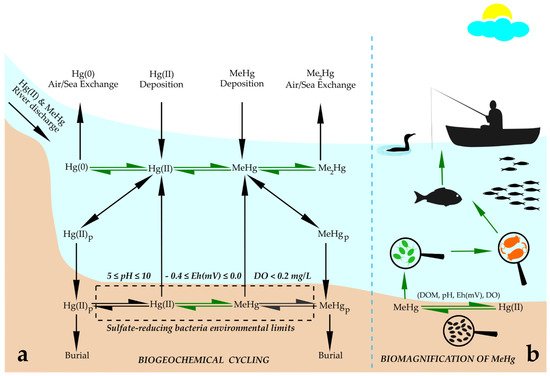
| Location | Total Hg | 1 | MeHg | References | ||
|---|---|---|---|---|---|---|
| - | pM or ng/L | pM, fM or ng/L | - | |||
| Southern Baltic Sea | 1.5 ± 0.7 pM | 23 ± 13 fM | [9] | [12] | ||
| Northern Baltic Sea | 1.0 ± 0.3 pM | 37 ± 15 fM; 21 ± 9 fM | [6][9] | [6,12] | ||
| Bothnian Bay (Baltic) | 1.24 ± 0.3 pM | 80 ± 25 fM | [8] | [11] | ||
| Bothnian Sea (Baltic) | 0.84 ± 0.24 pM | 21 ± 9 fM | [8] | [11] | ||
| Bothnian Bay (Baltic) | 11.5 ± 1.66 pM | 116–236 fM | [18] | [20] | ||
| Atlantic Ocean (Southern Polar Front) |
0.93 ± 0.69 ng/L | 0.26 ± 0.12 ng/L | [51] | [52] | ||
| Jiaozhou Bay (Yellow Sea) | 8.46–27.3 ng/L | 0.08–0.83 ng/L | [52] | [53] | ||
| Yellow Sea | 6.7–27.5 pM | – | 2 | [53] | [54] | |
| South China Sea | 0.8–2.3 ng/L | 0.05–0.22 ng/L | [54] | [55] | ||
| North Atlantic Ocean | 2.4 pM | – | [55] | [56] | ||
| Pacific Ocean | 1.2 pM | – | [56] | [57] | ||
| Mediterranean Sea | 1.0 pM; 2.5 pM | – | [38]] | [40 | [57 | ,58] |
| Average in oceans | 1.5 pM | – | [58] | [59] | ||
| Lake Titicaca (Bolivia) | – | 0.01–0.18 ng/L | [21] | [23] | ||
| Olt River (Romania) | 8–88 ng/L | 0.7 ng/L | [47] | [48] | ||
| Råne River estuary (Baltic) | 2.0–5.95 pM | 306 fM | [8] | [11] | ||
| Wetlands in Rouge Park, Canada |
1.45 ± 0.91 ng/L | 0.59 ± 0.45 ng/L | [48] | [49] | ||
| Lakes in Oil Sands Region, Canada |
0.4–5.3 ng/L | 0.01–0.34 ng/L | [37] | [39] | ||
| Lake Victoria, Africa | 3–15 ng/L | – | [59] | [60] | ||
| Average in surface water of lakes and rivers | – | 0.003–1.03 ng/L | [20] | [22] |
