Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Carmen Rodríguez García and Version 3 by Lindsay Dong.
Colorectal cancer (CRC) is one of the most common cancers worldwide. Its main modifiable risk factors are diet, alcohol consumption, and smoking. Thus, the right approach through lifestyle changes may lead to its prevention. Although cancer is a multi-factorial process, the study of post-translational modifications (PTMs) of proteins associated with CRC has recently gained interest, as inappropriate modification is closely related to the activation of cell signalling pathways involved in carcinogenesis.
- colorectal cancer
- (CRC)
- post-translational modifications
- (PTMs)
1. Introduction
Colorectal cancer (CRC) is currently the second type of cancer with the highest mortality rate in the population according to Global Cancer Statistics 2020 [1]. Metastatic CRC has a poor prognosis, with less than a 15% of five-year survival rate [2]. Its carcinogenesis is a process of many years of development and some early life risk factors are important contributors [3]. Among them, cigarette smoking, obesity, and a sedentary lifestyle are closely related to CRC incidence [4][5][4,5]. However, its quickly increasing incidence is mainly due to lifestyle westernization associated with changes in dietary behaviour such as heavy alcohol consumption and diets rich in sugars, saturated fats, and red and processed meat [6]. Thus, some protective lifestyle factors against CRC include a diet rich in minerals and vitamins, dairy, dietary fibre, fish, vegetables, and fruits. An alternative strategy for CRC prevention is the use of a chemopreventive supplement providing greater individual exposure to some nutrients than can be obtained from the diet (such as phytochemicals) [7].
The pathogenesis of CRC is a complex multi-stage process which includes gut microbiota imbalances, cell DNA disruption, and carcinogenic signalling pathways activation [8]. The aetiology underlying the mechanism of action of specific nutrients in CRC has been mainly attributed to their anti-inflammatory and antioxidant properties, and their modulation of gut microbiota populations, maintaining gut homeostasis and regulating the host immune response [9][10][9,10]. However, their effects on epigenetic modulation associated with CRC pathogenesis remains unknown. There is increasing evidence that the disruption of epigenetic control over gene expression has an important role in carcinogenesis [11][12][13][14][11,12,13,14]. Together with non-coding RNAs and DNA methylation, histone and protein post-translational modifications (PTMs) have an important role in carcinogenesis and gene regulation [15][16][15,16]. PTMs occur once the mRNA has been translated into the protein sequence in the ribosomes and produce marginal chemical modifications to lipoproteins and native proteins. Among these modifications, PTMs may mark proteins for degradation, inhibit or promote interactions with other proteins, redirect cellular protein localization, and modify enzyme activity [17][18][17,18]. Most PTMs are reversible, so normal cells use them as a switch to control proliferating or quiescent cells [19]. The role of PTMs in the onset and progression of diseases such as cancer has been investigated. Their involvement in the process of carcinogenesis could be due to their function in processes such as the cell cycle, cell survival, and cell proliferation [20]. Therefore, PTM-focused analysis of enzyme phosphorylation and the involvement of protein kinases in cancer formation and progression have led to the use of PTM-based therapeutic approaches (i.e., tyrosine kinase inhibitors) [21][22][21,22]. Furthermore, in the case of CRC, PTMs develop key role-playing as a tight junction protein and regulate the epithelial barrier function [23][24][23,24]. Thus, PTMs may be essential to work with the external impact and could provide an excellent opportunity for intervention through feeding and promoting clinical strategies for CRC patients regarding predictive, preventive, and personalized medicine.
2. Post-Translational Modifications in Colorectal Cancer
PTMs are protein-specific modifications that control many physiological processes to ensure the dynamic and quick response of cells to intracellular and extracellular stimuli [25][26]. Any proteome protein may be modified post-translationally or during translation. These reversible modifications may alter not only the protein’s stability, conformation, and charge state, but also its function modulating its intracellular conformation, its interactions, and the life span of the target protein [26][27]. In some cases, PTMs are inadequate and modulate positively some signal transduction pathways that are involved in tumourigenesis regulation and cancer development [27][28]. To date, more than 450 unique protein modifications have been described, including ubiquitination, acylation, SUMOylation, methylation, and phosphorylation [28][29]. In the case of CRC, the most important modifications involved have been summarized below (Figure 1).
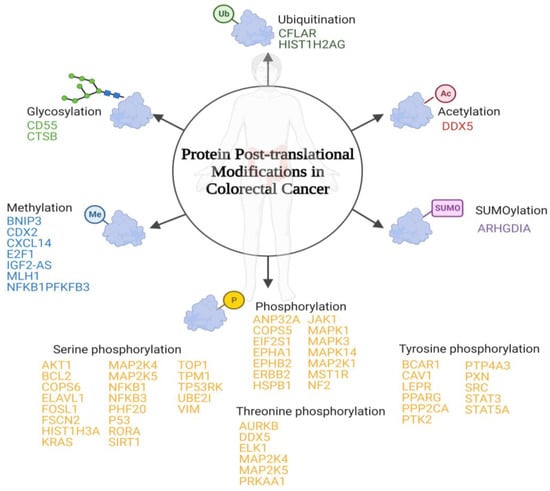
Figure 1. Schematic representation of the main post-translational modifications in colorectal cancer. Below each post-translational modification is a list of the identified proteins that suffer inappropriate post-translational modifications associated with colorectal cancer.
2.1. SUMOylation
Small ubiquitin-like modifiers (SUMO) are covalently attached to lysine residues [29][30]. The downregulated SUMOylation in lysine 138 of Rho GDP-dissociation inhibitor 1 has been observed in CRC cell lines. This protein is involved in Rho GTPases signalling regulation [30][31].
2.2. Glycosylation
A carbohydrate is attached to specific proteins. In mammals, there are two types: (1) O-glycosylation, where glycosyl groups are connected to tyrosine, hydroxylysine, serine, or threonine side chains with glycosidic linkages by glycosyltransferases, and (2) N-glycosylation, where glycosyl groups are connected to Asn side chains with amide linkages by oligosaccharyltransferase [31][32][32,33]. The upregulation of this PTM in complement decay-accelerating factor and cathepsin B has been identified in tumour tissue samples of CRC patients [33][34][34,35].
2.3. O-GlcNAcylation
There is a covalent attachment of N-acetylglucosamine residue O-linked to the hydroxyl group of threonine and serine residues of multiple cytosolic and nuclear proteins [35][36][36,37]. The upregulation of O-GlcNAcylation in ATP-dependent RNA helicase DDX5 has been associated with CRC in cell lines and murine models [37][38].
2.4. Ubiquitination
There is an attachment of ubiquitin molecules to the lysine residue of the substrate proteins. This process is based on an enzymatic cascade of ubiquitin-activating, ubiquitin-conjugating, and ubiquitin-ligase enzymes [38][39][39,40]. There have been two ubiquitination-susceptible proteins identified that are related to CRC: (1) caspase homolog that is an apoptosis regulator [40][41] and (2) histone H2A type 1 that is involved in chromosomal stability, DNA replication, and DNA repair [41][42].
2.5. Methylation
Methylation occurs mainly in arginine or lysine residues. One of the most biologically important roles of methylation is in histone modification [42][43]. Among the different proteins that suffer dysregulated post-translational methylation associated with CRC, the one that is involved in cell growth suppression has downregulated methylation (putative insulin-like growth factor 2 antisense gene protein) [43][44][44,45]. The other proteins identified have an upregulated methylation, among them are (1) BCL2/adenovirus E1B 19 kDa protein-interacting protein 3 that is involved in apoptosis [45][46]; (2) homeobox protein CDX-2 that is involved in the transcriptional regulation of different genes expressed in the intestine [46][47]; (3) C-X-C motif chemokine 14 that is involved in immunoregulatory and inflammatory processes [47][48]; (4) transcription factor E2F1 that participates in the cell cycle [48][49]; (5) DNA mismatch repair protein Mlh1 that participates in DNA repair [49][50]; (6) nuclear factor NF-kappa-B p105 subunit that is a pleiotropic transcription factor involved in several signal transduction events which are initiated by stimuli such as oxidative stress or inflammation [50][51]; and (7) 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 3 that is an essential protein for cell cycle progression and apoptosis prevention [51][52].
2.6. Phosphorylation
Phosphorylation is the most prevalent and widely studied type of PTM. It is inversely regulated by phosphatases and protein kinases in the amino acids’ hydroxyl tyrosine, threonine, or serine [31][52][32,60]. In the case of CRC, inadequate PTMs have been identified in the following proteins: (1) acidic leucine-rich nuclear phosphoprotein 32 family member A that is involved in cell growth [53][61]; (2) COP9 signalosome complex subunit 5 that develops an important role in the degradation of cyclin-dependent kinase inhibitor [54][62]; (3) eukaryotic translation initiation factor 2 subunit 1 that is a translation initiation factor [55][63]; (4) ephrin type-A receptor 1 and ephrin type-B receptor 2 that are members of the ephrin receptor subfamily of the protein tyrosine kinase family [56][64]; (5) receptor tyrosine-protein kinase erbB-2 that is a member of the epidermal growth factor receptor family [57][65]; (6) heat shock protein beta-1 which plays an important role in cancer cells proliferation [58][66]; (7) tyrosine-protein kinase JAK1 that is a tyrosine kinase of the non-receptor type [59][67]; (8) mitogen-activated protein kinase 1, 3, and 14 that are serine/threonine kinases that are essential components of the MAP kinase signal transduction pathway [60][61][62][63][68,69,70,71]; (9) dual specificity mitogen-activated protein kinase kinase 1 which acts as an essential component of the MAP kinase signal transduction pathway [64][72]; (10) macrophage-stimulating protein receptor that is a tyrosine kinase receptor [65][73]; and (11) merlin that plays a pivotal role in tumour suppression through apoptosis promotion [66][74].2.7. Serine Phosphorylation
Serine phosphorylation includes proto-oncogene c-Ak and Fos-related antigen 1 that regulates many processes including proliferation cell survival, growth, and angiogenesis [67][68][75,76]; apoptosis regulator Bcl-2 that is a regulator of apoptosis [69][77]; COP9 signalosome complex subunit 6 which is a component of the COP9 signalosome complex [70][78]; ELAV-like protein 1 that stabilizes mRNAs and regulates gene expression [71][79]; fascin-2 that acts as an actin bundling protein [72][80]; histone H3.1 which plays a central role in transcription regulation and DNA repair [73][81]; Kirsten rat sarcoma virus which is involved in the propagation of growth factors [74][82]; MAP kinase kinase 4 and 5 that are dual specificity protein kinase which act as an essential component of the MAP kinase signal transduction pathway [75][76][83,84]; NFKB1 and NFKB3 which are pleiotropic transcription factors involved in several signal transduction [77][78][85,86]; PHD finger protein 20 that contributes to p53 stabilization after DNA damage [79][87]; cellular tumour antigen p53 that acts as a tumour suppressor [80][88]; nuclear receptor ROR-alpha which is a key regulator of glucose metabolism [81][89]; sirtuin 1 that is an intracellular regulatory protein [82][90]; DNA topoisomerase 1 that releases the supercoiling tension of DNA introduced during the DNA replication [83][91]; tropomyosin-1 which is a member of the tropomyosin family of highly conserved proteins [84][92]; TP53-regulating kinase which is a protein kinase that phosphorylates ‘Ser-15’ of p53/TP53 protein [85][93]; SUMO-protein ligase that is essential for nuclear architecture and chromosome segregation [86][94]; and vimentin which is responsible for maintaining cell shape and stabilizing cytoskeletal interactions [87][95].2.8. Threonine Phosphorylation
Threonine phosphorylation includes Aurora kinase B which is a serine/threonine-protein kinase component of the chromosomal passenger complex [88][96]; probable ATP-dependent RNA helicase DDX5 which is involved in the alternative regulation of pre-mRNA splicing [89][97]; ETS domain-containing protein Elk-1 which is a transcription factor that binds to purine-rich DNA sequences [90][98]; dual specificity mitogen-activated protein kinase kinase 4 which is an essential component of the MAP kinase signal transduction pathway [75][83]; MAP kinase kinase 5 that acts as a scaffold for the formation of a ternary MAP3K2/MAP3K3-MAP3K5-MAPK7 signalling complex [76][84]; and 5′-AMP-activated protein kinase catalytic subunit alpha-1 which is the catalytic subunit of AMP-activated protein kinase that plays a key role in regulating cellular energy metabolism [91][99].2.9. Tyrosine Phosphorylation
Tyrosine phosphorylation includes breast cancer anti-estrogen resistance protein 1 which plays a central role in cell adhesion [92][93][100,101]; caveolin-1 that act as a scaffolding protein within caveolar membranes [94][102]; leptin receptor that mediates leptin central and peripheral effects [95][103]; peroxisome proliferator-activated receptor gamma that is a nuclear receptor [96][104]; serine/threonine-protein phosphatase 2A catalytic subunit alpha isoform which is the major phosphatase for microtubule-associated proteins [97][105]; focal adhesion kinase 1 which is a non-receptor protein-tyrosine kinase that plays an essential role in regulating cell migration and apoptosis [98][99][106,107]; protein tyrosine phosphatase type IVA 3 that stimulates progression from G1 into S phase during mitosis [100][108]; paxillin which is a cytoskeletal protein involved in actin-membrane attachment at sites of cell adhesion to the extracellular matrix [101][109]; proto-oncogene tyrosine-protein kinase Src that is a non-receptor protein tyrosine kinase [102][110]; signal transducer and activator of transcription 3 which mediates cellular responses to interleukins and other growth factors [103][104][111,112]; and signal transducer and activator of transcription 5A that is involved in signal transduction and activation of transcription [105][113].3. Relationship between Post-Translational Modifications Associated with Colorectal Cancer
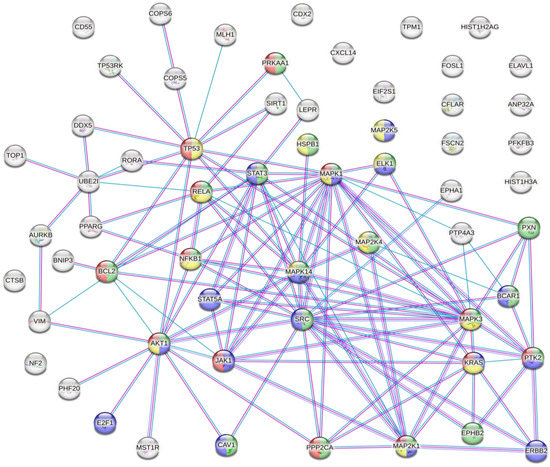
The results of the analysis showed that there were several interactions between some of the proteins susceptible to inappropriate PTMs associated with CRC (Figure 2).
Figure 2. Protein–protein interaction network. Coloured nodes in green: proteins involved in the VEGFA-VEGFR2 signalling pathway. Coloured nodes in blue: proteins involved in the EGF-EFGR signalling pathway. Coloured nodes in red: proteins involved in the MAPK signalling pathway. Coloured nodes in yellow: proteins involved in the PI3K-Akt signalling pathway. Coloured nodes in grey: proteins that are not involved in any of the signalling pathways mentioned above. Edges represent protein–protein associations. Pink line: association experimentally determined. Blue line: association determined from curated databases. Purple line: protein homology.
This analysis showed that there were strong interactions between TP53, AKT1, STAT3, STAT5A, JAK1, MAPK1, MAPK14, MAP2K1, and SRC. In fact, this network had significantly more interactions than expected, which means that proteins have more interactions among themselves than what would be expected from a random set of proteins, demonstrating that the proteins may be partially biologically connected as a group. This group of proteins is mainly involved in the PI3K-Akt, EGF-EFGR, MAPK, and VEGFA-VEGFR2 signalling pathways. On the one hand, PI3K-Akt is the classical signalling pathway involved in glucose metabolism that promotes cancer metabolic reprogramming by elevation of aerobic glycolysis (known as the “Warburg effect”) [106][107][114,115]. Both EGF-EGFR and MAPK signalling pathways are involved in proliferation, differentiation, and apoptosis. Its regulation in cancer cells allows the maintenance of proliferative signalling, promoting cancer cell survival [52][108][60,116]. On the other hand, VEGF and its receptors (such as VEGFR2) develop an important role in tumour-associated angiogenesis. This process is essential for tumour progression because it favours oxygen and nutrient uptake by cancer cells [109][110][117,118]. Therefore, the main PTMs identified in CRC are involved in cancer progression and cancer cell survival.
