Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Rita Xu and Version 1 by Yanmin Ju.
The misuse and mismanagement of antibiotics have made the treatment of bacterial infections a challenge. This challenge is magnified when bacteria form biofilms, which can increase bacterial resistance up to 1000 times. It is desirable to develop anti-infective materials with antibacterial activity and no resistance to drugs. With the rapid development of nanotechnology, anti-infective strategies based on metal and metal oxide nanomaterials have been widely used in antibacterial and antibiofilm treatments.
- bacterial infection
- metal and metal oxide nanomaterials
- antibacterial
1. Introduction
Bacterial infection with high morbidity and mortality rate have posed an increasingly serious threat to human health. The development of antibiotics has successfully resisted bacterial invasion. However, microorganisms have acquired drug resistance through genetic mutation or by receiving exogenous mutant genes because of the abuse of antibiotics [1,2][1][2]. They resist antibiotics by blocking drug–cell contact, rendering the drug inactive, altering their metabolic pathways, inhibiting the entry of drugs into the bacteria, or activating the efflux of drugs [2]. The era of antibiotics is progressively coming to an end due to the emergency of multidrug-resistant bacteria. It has been reported that multidrug-resistant bacteria cause at least 700,000 deaths worldwide each year, including 23,000 deaths in the United States and 25,000 deaths in the European Union [3]. The World Health Organization predicts that 10 million people worldwide will die from bacterial infections by 2050 if no efforts are made to reduce bacterial resistance or develop new antibiotics [4].
Infectious diseases caused by planktonic bacteria are common, such as sepsis and keratitis. When bacteria acquire drug resistance, removing the bacteria from the site of infection becomes a challenge. This challenge is amplified when bacteria form biofilm, which are one of the reasons bacteria develop drug resistance [5]. Bacterial biofilms are three-dimensional structure groups formed by bacteria embedded in their own secreted extracellular polymeric substance (EPS) [6,7,8][6][7][8]. Depending on the presence or absence of substrate during biofilm formation, they are classified into classical attached biofilms and type II nonattached biofilms. Biofilm formation is a cascading process, which can be divided into adhesion, reproduction, maturation, and shedding [9,10,11][9][10][11]. First, planktonic bacteria attach to the surface of the vector through outer membrane proteins and flagella, etc. Next, adhesion is enhanced by EPS secreted by the bacteria and which begins to develop into an irreversible adherent state by differentiation. They then develop into a mature three-dimensional structure through the aggregation of colonies. Finally, biofilms are hydrolyzed to release planktonic bacteria, which lead to the development of new infections. The formation of biofilm is beneficial to the survival of bacteria. The resistance capacity of biofilm reaches 1000 times that of planktonic bacteria. On the one hand, bacteria will undergo immune escape due to the protective effect of biofilm. On the other hand, the relevant components of the biofilm will interact with antibiotics to reduce their effective concentration. Furthermore, bacteria in biofilm can also resist the action of drugs through quorum sensing (QS) systems and dormant states [12,13,14][12][13][14]. Biofilms are commonly present on the surface of chronic wounds or implants, which can lead to delayed wound healing or implant removal [15]. The slow wound healing is attributed to decreased immune function, reduced cell proliferation, or reduced cellular reconstitution [16,17][16][17]. additionally, there are limited treatments available for implant infections, such as systemic antibiotic therapy or surgical treatment [12]. These will seriously affect the quality of life of the patient. Therefore, effective antibacterial and antibiofilm methods with no resistance are in urgent demand.
With the rapid development of nanotechnology, metal and metal oxide nanomaterials have emerged as promising therapeutics in anti-infective fields due to their excellent characteristics, including low cost and strong heat resistance. Importantly, they do not cause the development of drug resistance [18]. The anti-infective mechanisms of metal and metal oxide nanomaterials are mainly divided into three types, including the destruction of bacterial cytoskeleton, the generation of metal ions, and reactive oxygen species (ROS). They can not only kill planktonic bacteria, but also inhibit the formation of biofilm or destroy the formed biofilm by destroying EPS, inhibiting QS, and killing bacteria inside the biofilm. For example, He et al. constructed Au-Ag nanoshells to kill multidrug-resistant bacteria by high heat and silver ions (Ag+) [19]. Ali et al. pointed out that Au nanoparticles (NPs) can be used as nanoantibiotics to eliminate the biofilms of Pseudomonas aeruginosa [20]. From the aforementioned points, and taken together, metal and metal oxide nanomaterials provide a new weapon for the treatment of infective diseases.
It is essential to have a clear understanding of the antibacterial mechanism for developing new antibacterial materials. Compared to previous reports, this progress report concentrates on metal and metal oxide nanomaterials to summarize their anti-infective mechanism based on the components of bacteria and biofilms (Figure 1) [21,22,23,24][21][22][23][24].
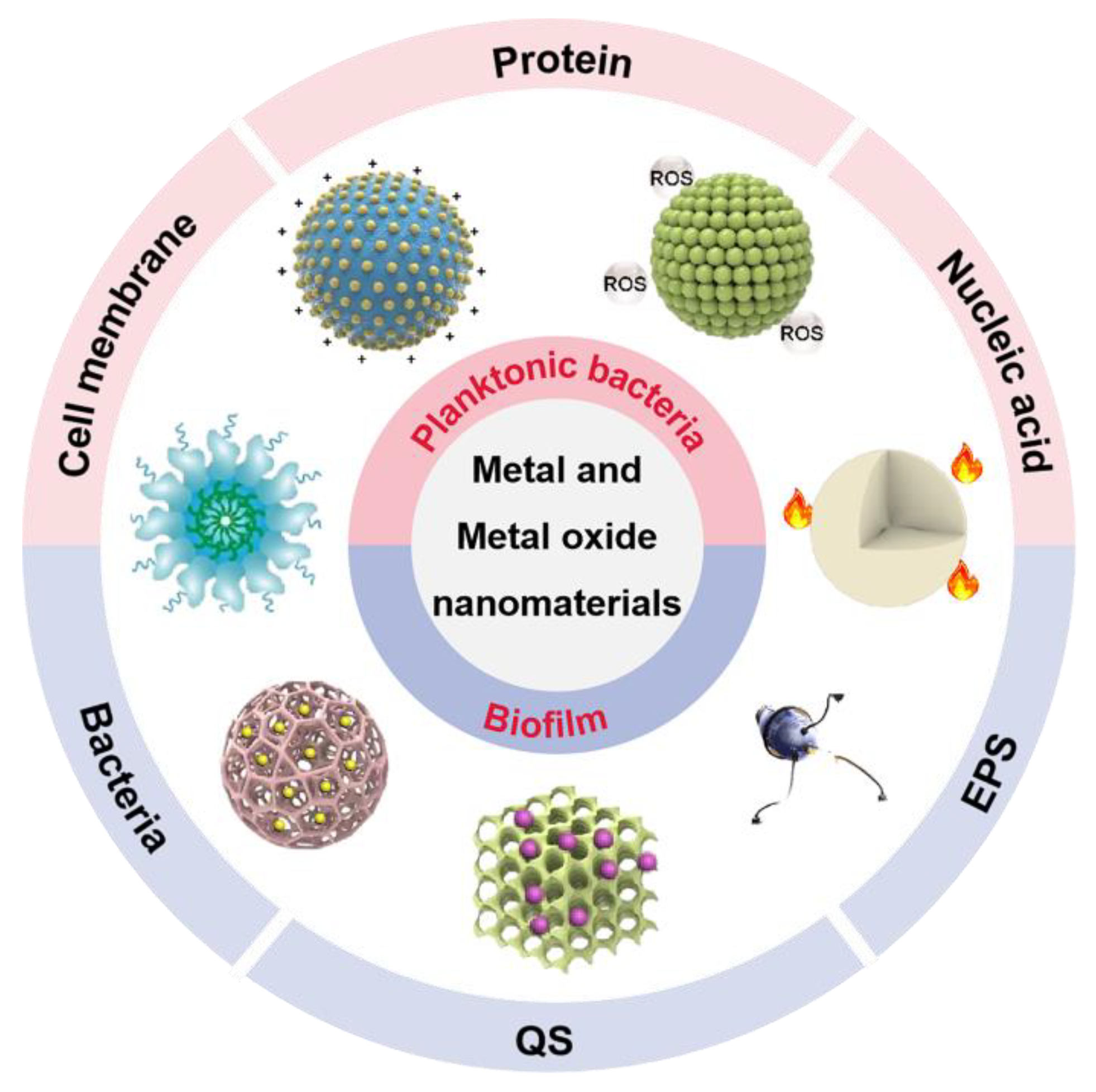
Figure 1. The antibacterial and antibiofilm application of metal and metal oxide nanomaterials. EPS: extracellular polymeric substance; QS: quorum sensing; ROS: reactive oxygen species.
2. Metal and Metal Oxide Nanomaterials
Metal and metal oxide nanomaterials refer to metals and alloys that form nanograins with small size effect, quantum effect, surface effect, and interface effect. They have unique physical and chemical properties compared to traditional metal and metal oxide materials, which have been widely investigated in recent years in different fields. Notably, they have been widely used in the anti-infective field due to their excellent anti-infective properties.
2.1. Metal Nanomaterials
Metal nanomaterials have been widely used in the field of anti-infection due to their excellent anti-infective efficacy. Among them, Ag, Au, and Cu are the most common anti-infective nanomaterial (Table 1). For thousands of years, the antibacterial properties of Ag have been discovered and used in everyday life, such as the use of silverware. The antibacterial properties of Ag nanomaterials mainly derive from Ag+ and depend largely on their size and shape. For example, Yang et al. used the displacement reaction between Zn and Ag+ to introduce Ag nanomaterials into the metal–organic framework. The antibacterial mechanism showed that massive release of Ag+ destroyed the bacterial contents and enhanced the effectiveness of the nanocomposite against S. aureus and E. coli [25]. Different from Ag nanomaterials, the antibacterial properties of Au nanomaterials are largely affected by their morphology and the antibacterial mechanisms are diverse. For example, Au NPs lack antibacterial activity on their own, but they showed favorable antibacterial activity by surface modification [26,27][26][27]. Based on this, Li et al. used 4,6-diamino-2-pyrimidinethiol modified Au NPs for the treatment of infections caused by E. coli [28]. In contrast, Au nanoclusters (NCs) with same surface ligand exhibit broad-spectrum antibacterial property by inducing the overaccumulation of ROS, not only against Gram-negative and Gram-positive bacteria, but also against their multidrug-resistant bacteria [29]. As another common anti-infective nanomaterial, Cu nanomaterials perform antibacterial properties mainly through generating ROS. On the one hand, Cu destroy the bacterial antioxidant system by causing the inactivation of glutathione reductase (GR), resulting in a surge of ROS [30]. On the other hand, cuprous ions originate from copper-based nanomaterials generated ROS due to Fenton-like activity. For example, Lin et al. constructed copper ion-loaded melanin and copper ion-loaded polydopamine to treat infective diseases induced by S. aureus and E. coli with the help of copper ion release and copper ion-induced ROS production [31]. Additionally, Pd and Pt nanomaterials can also act as nanozymes to produce ROS, resulting in broad-spectrum antibacterial activity against both Gram-negative and Gram-positive bacteria [32].
Table 1. The antibacterial mechanism and synthetic method of metal nanomaterials.
| Nanomaterials | Synthetic Method | Mode of Action | Bacterial Species | Ref. | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ref. | |||||||||||||||
| Ag NPs | Chemical reduction | Ag | + | E. coli | and | S. aureus | [33] | ||||||||
| ZnO NPs | Atmosphere arc discharge method | ROS | S. sanguinis | , | P. gingivalis | , and | S. mutans | [40] | |||||||
| Nano-Ag | Photoreduction | ||||||||||||||
| ZnO NPs | Ag | + | and ROS | E. coli | Biosynthetic method | ROS and Zn | + | E. coli | and | S. aureus | [34] | ||||
| P. aeruginosa | and | C. albicans | [47] | Ag NPs | Chemical reduction | ROS | Carbapenem-resistant | ||||||||
| Flower-shaped ZnO | K. pneumoniae | Wet chemical method | [ | 35] | |||||||||||
| ROS | E. coli | [ | 48 | ] | Ag NPs | Hydrothermal reaction | Ag | + | E. coli | , | S. aureus | , and | C. albicans | [36] | |
| [ | 37 | ||||||||||||||
| Nano-TiO | 2 | No information | ROS | E. coli | and | A. hydrophila | [49] | Au NPs | Seed-mediated growth | ROS | B. subtilis | and | E. coli | ] | |
| TiO | 2 | nanowire | Hydrothermal reaction | ROS | Gram-positive bacteria and Gram-negative bacteria | [50] | Au NPs | Chemical reduction | No information | E. coli | |||||
| TiO | 2 | NPs | Purchased from Nanostructured and Amorphous Materials Inc. and MK | [28] | |||||||||||
| Impex Corp., Division MK Nano | ROS | E. coli | [ | 51] | Au NCs | Chemical reduction | ROS | E. coli | , | S. aureus | , MDR | E. coli | , and MDR | S. aureus | [29] |
| Au NRs | Chemical reduction | ROS | E. coli | and | S. aureus | [38] | |||||||||
| Cu NPs | Solution casting method | Free radicals | E. coli | and | S. aureus | [39] | |||||||||
| Cu NPs | Atmosphere arc discharge method | ROS | S. sanguinis | , | P. gingivalis | , and | S. mutans | [40] | |||||||
| Cu NPs | Biosynthetic method | ROS | E. coli | and | S. aureus | [41] |
2.2. Metal Oxide Nanomaterials
Compared to metal nanomaterials, metal oxide nanomaterials have attracted the highest interest in the anti-infective community due to the better biological properties, such as TiO2 and ZnO (Table 2) [42]. As semiconductor nanomaterials, TiO2 and ZnO can generate highly toxic ROS by photocatalytic property, which are viewed as the promising tool for anti-infection therapy. However, their photocatalytic-based antibacterial properties were limited to the narrow response range of visible light and the easy recombination properties of photoinduced electron–hole pairs [43]. In order to improve their photocatalytic performance, combining with a semiconductor featuring narrow band gap has been reported. For example, Khan et al. reduced the band gap using Ag2S and decreased the rate of recombination for photoinduced charge carriers using graphene oxide (GO) [44]. The obtained Ag2S-ZnO/GO nanocomposite showed an outstanding photocatalytic property and remarkable antibacterial activity compared to pure ZnO nanomaterials. In addition, ZnO nanomaterials can also exert antibacterial properties through contact adsorption mechanism and metal ion dissolution mechanism. They all act by zinc ions derived from the degraded of ZnO nanomaterials in an acidic environment [45]. Zinc ions cause membrane potential disruption by adhering to the cell membrane. Moreover, they also act on the thiol group of the bacterial respiratory enzymes to increase the production of ROS, ultimately leading to bacterial death [46].
Table 2. The antibacterial mechanism and synthetic method of metal oxide nanomaterials.
| Nanomaterials | Synthetic Method | Mode of Action | Bacterial Species |
|---|
2.3. Synthetic Method
Different nanomaterials exhibit different degrees of anti-infective effect, which directly depend on their composition, morphology, and size. These characteristics are closely related to the synthetic method. There are various methods for the synthesis of metal and metal oxide nanomaterials, including chemical reduction, chemical precipitation, the Brust–Schiffrin method, seed-mediated growth, the hydrothermal reaction, and biosynthetic methods [52,53][52][53].
Chemical and biosynthetic methods have been reported for the preparation of metal-based nanomaterials. Among them, chemical reduction is the most common chemical method. However, the use of reducing agents raises the toxicity and cost of the method, as well as introduces impurities. As a simple and fast method, the chemical precipitation method has attracted a lot of attention, which allows for controlling the size and shape of the nanomaterials. The reaction rate and nucleation process are largely affected by reaction parameters such as pH, temperature, and reactant concentration. In addition, the size, shape, and properties of the nanomaterials will depend on the crystallization process. Based on this, Sondi et al. prepared well-dispersed Ag NPs with the size of 12.3 nm, using the reduction of AgNO3 by ascorbic acid [54]. Wang et al. synthesized morphologically controllable ZnO NPs nanoparticles with a particle size of about 20 nm using ZnCl2 as precursor and ammonium carbamate as precipitating agent [55]. As a nontoxic and environmentally friendly method, the biosynthetic method can be used to synthesize various nanomaterials with a wide range of size, physicochemical properties, shapes, and compositions, which has been widely reported. The sources of nanomaterials include plants, bacteria, and algae. For example, Mori et al. reported a simple and environmentally friendly route for the biosynthesis of Ag NPs [56]. The size of the Ag NPs was controlled by varying the glucose concentration. The final Ag NPs were produced with a controlled particle size range of 3.48 ± 1.83~20.0 ± 2.76 nm. Nasrollahzadeh et al. synthesized Cu NPs using the Euphorbia grandis leaf extract as a reducing and stabilizing agent without surfactants [52].
The Brust–Schiffrin method and the seed-mediated growth synthesis method can be employed to produce Au nanomaterials [57]. Brust–Schiffrin is the first special method that can generate thiolate-stabilized Au NPs. The Au NPs synthesized by this method have the following advantages: (1) high thermal and air stability, (2) no aggregation or decomposition occurring during repeated separation and redecomposition, (3) easy adjustment of the particle size of the synthesized gold nanoparticles with narrow dispersion range, (4) and relatively easy functionalization and ligand substitution modifications. For example, Selina Beatrice et al. prepared molecular tweezer-functionalized ultrafine Au NPs that selectively adsorb to lysine and arginine residues on protein surfaces using the Brust–Schiffrin method [58]. The process of the seed-mediated growth synthesis method of Au nanomaterials can be divided into two stages. First, small-sized Au NPs are synthesized as seeds. Second, the seeds are added to a “growth” solution consisting of HAuCl4, stabilizer, and a reducing agent. Then, Au0 produced by the reduction usually appears on the seed surface, and eventually a large amount of Au NPs is formed. Based on this method, MSc et al. obtained gold nanorods, gold nanostars, and gold nanospheres with a small size and a good dispersion [59].
The hydrothermal reaction is a method of synthesis using chemical reactions of substances in aqueous solutions at temperatures from 100 to 1000 °C and pressures from 1 MPa to 1 GPa. It can create new nanocompounds and nanomaterials that cannot be prepared by other methods because the homogeneous nucleation and nonhomogeneous nucleation mechanisms of the hydrothermal reaction are different from the diffusion mechanisms of the solid-phase reactions. Importantly, the products obtained by hydrothermal reaction have a high purity, good dispersion, and easy particle size control. For example, Wang et al. obtained well-dispersed copper nanowires using CuCl2·2H2O as the copper source and polyvinyl pyrrolidone as the dispersant. Ozga et al. used a modified hydrothermal reaction to obtain copper oxide films at less than 101 °C. Li et al. made ZnO nanoflowers by adjusting the ratio of zinc nitrate hexahydrate and cyclic hexamethylenetetramine [60]. Huang et al. prepared TiO2 nanotubes with high catalytic efficiency by the hydrothermal reaction.
To sum up, nanomaterials of the same component prepared by different methods may have different properties. It is important to choose an appropriate method by combining various factors.
References
- Christaki, E.; Marcou, M.; Tofarides, A. Antimicrobial resistance in bacteria: Mechanisms, evolution, and persistence. J. Mol. Evol. 2020, 88, 26–40.
- Munita, J.M.; Arias, C.A. Mechanisms of antibiotic resistance. Microbiol. Spectr. 2016, 4, 464–472.
- Yin, M.; Yang, M.; Yan, D.; Yang, L.; Wan, X.; Xiao, J.; Yao, Y.; Luo, J. Surface-charge-switchable and size-transformable thermosensitive nanocomposites for chemo-photothermal eradication of bacterial biofilms in vitro and in vivo. ACS Appl. Mater. Interfaces 2022, 14, 8847–8864.
- Lu, B.; Hu, E.; Xie, R.; Yu, K.; Lu, F.; Bao, R.; Wang, C.; Lan, G.; Dai, F. Magnetically guided nanoworms for precise delivery to enhance in situ production of nitric oxide to combat focal bacterial infection in vivo. ACS Appl. Mater. Interfaces 2021, 13, 22225–22239.
- Mahamuni-Badiger, P.P.; Patil, P.M.; Badiger, M.V.; Patel, P.R.; Thorat-Gadgil, B.S.; Pandit, A.; Bohara, R.A. Biofilm formation to inhibition: Role of zinc oxide-based nanoparticles. Mater. Sci. Eng. C Mater. 2020, 108, 110319.
- Ruhal, R.; Kataria, R. Biofilm patterns in gram-positive and gram-negative bacteria. Microbiol. Res. 2021, 251, 126829.
- Alves, P.J.; Barreto, R.T.; Barrois, B.M.; Gryson, L.G.; Meaume, S.; Monstrey, S.J. Update on the role of antiseptics in the management of chronic wounds with critical colonisation and/or biofilm. Int. Wound J. 2021, 18, 342–358.
- Li, X.; Chen, D.; Xie, S. Current progress and prospects of organic nanoparticles against bacterial biofilm. Adv. Colloid Interface Sci. 2021, 294, 102475.
- Rabin, N.; Zheng, Y.; Opoku-Temeng, C.; Du, Y.; Bonsu, E.; Sintim, H.O. Biofilm formation mechanisms and targets for developing antibiofilm agents. Future Med. Chem. 2015, 7, 493–512.
- Silva, N.B.S.; Marques, L.A.; Röder, D.D.B. Diagnosis of biofilm infections: Current methods used, challenges and perspectives for the future. J. Appl. Microbiol. 2021, 131, 2148–2160.
- Rodríguez-Merchán, E.C.; Davidson, D.J.; Liddle, A.D. Recent strategies to combat infections from biofilm-forming bacteria on orthopaedic implants. Int. J. Mol. Sci. 2021, 22, 10243.
- Johnson, C.T.; Wroe, J.A.; Agarwal, R.; Martin, K.E.; Guldberg, R.E.; Donlan, R.M.; Westblade, L.F.; Garcia, A.J. Hydrogel delivery of lysostaphin eliminates orthopedic implant infection by Staphylococcus aureus and supports fracture healing. Proc. Natl. Acad. Sci. USA 2018, 115, E4960–E4969.
- Flemming, H.C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575.
- Preda, V.G.; Sandulescu, O. Communication is the key: Biofilms, quorum sensing, formation and prevention. Discoveries 2019, 7, e100.
- Wang, C.; Wang, M.; Xu, T.; Zhang, X.; Lin, C.; Gao, W.; Xu, H.; Lei, B.; Mao, C. Engineering bioactive self-healing antibacterial exosomes hydrogel for promoting chronic diabetic wound healing and complete skin regeneration. Theranostics 2019, 9, 65–76.
- Bessa, L.J.; Fazii, P.; Di Giulio, M.; Cellini, L. Bacterial isolates from infected wounds and their antibiotic susceptibility pattern: Some remarks about wound infection. Int. Wound J. 2015, 12, 47–52.
- Mihai, M.M.; Holban, A.M.; Giurcaneanu, C.; Popa, L.G.; Buzea, M.; Filipov, M.; Lazar, V.; Chifiriuc, M.C.; Popa, M.I. Identification and phenotypic characterization of the most frequent bacterial etiologies in chronic skin ulcers. Rom. J. Morphol. Embryol. 2014, 55, 1401–1408.
- Alizadeh, N.; Salimi, A. Multienzymes activity of metals and metal oxide nanomaterials: Applications from biotechnology to medicine and environmental engineering. J. Nanobiotechnol. 2021, 19, 26.
- He, J.; Qiao, Y.; Zhang, H.; Zhao, J.; Li, W.; Xie, T.; Zhong, D.; Wei, Q.; Hua, S.; Yu, Y.; et al. Gold-silver nanoshells promote wound healing from drug-resistant bacteria infection and enable monitoring via surface-enhanced Raman scattering imaging. Biomaterials 2020, 234, 119763.
- Ali, S.G.; Ansari, M.A.; Alzohairy, M.A.; Alomary, M.N.; AlYahya, S.; Jalal, M.; Khan, H.M.; Asiri, S.M.M.; Ahmad, W.; Mahdi, A.A.; et al. Biogenic gold nanoparticles as potent antibacterial and antibiofilm nano-antibiotics against pseudomonas aeruginosa. Antibiotics 2020, 9, 100.
- Zhao, Y.; Chen, L.; Wang, Y.A.; Song, X.Y.; Li, K.Y.; Yan, X.F.; Yu, L.M.; He, Z.Y. Nanomaterial-based strategies in antimicrobial applications: Progress and perspectives. Nano Res. 2021, 14, 4417–4441.
- Weldick, P.J.; Wang, A.H.; Halbus, A.F.; Paunov, V.N. Emerging nanotechnologies for targeting antimicrobial resistance. Nanoscale 2022, 14, 4018–4041.
- Makabenta, J.M.V.; Nabawy, A.; Li, C.H.; Schmidt-Malan, S.; Patel, R.; Rotello, V.M. Nanomaterial-based therapeutics for antibiotic-resistant bacterial infections. Nat. Rev. Microbiol. 2021, 19, 23–36.
- Wang, Y.; Yang, Y.N.; Shi, Y.R.; Song, H.; Yu, C.Z. Antibiotic-free antibacterial strategies enabled by nanomaterials: Progress and perspectives. Adv. Mater. 2020, 32, 1904106.
- Yang, Y.; Wu, X.Z.; He, C.; Huang, J.B.; Yin, S.Q.; Zhou, M.; Ma, L.; Zhao, W.F.; Qiu, L.; Cheng, C.; et al. Metal-organic framework/Ag-based hybrid nanoagents for rapid and synergistic bacterial eradication. ACS Appl. Mater. Interfaces 2020, 12, 13698–13708.
- Ielo, I.; Rando, G.; Giacobello, F.; Sfameni, S.; Castellano, A.; Galletta, M.; Drommi, D.; Rosace, G.; Plutino, M.R. Synthesis, chemical-physical characterization, and biomedical applications of functional gold nanoparticles: A review. Molecules 2021, 26, 5823.
- Czechowska, J.; Cichoń, E.; Belcarz, A.; Ślósarczyk, A.; Zima, A. Effect of gold nanoparticles and silicon on the bioactivity and antibacterial properties of hydroxyapatite/chitosan/tricalcium phosphate-based biomicroconcretes. Materials 2021, 14, 3854.
- Li, J.J.; Cha, R.T.; Zhao, X.H.; Guo, H.B.; Luo, H.Z.; Wang, M.Z.; Zhou, F.S.; Jiang, X.Y. Gold Nanoparticles cure bacterial infection with benefit to intestinal microflora. ACS Nano 2019, 13, 5002–5014.
- Xie, Y.Z.Y.; Zhang, Q.; Zheng, W.F.; Jiang, X.Y. Small molecule-capped gold nanoclusters for curing skin infections. ACS Appl. Mater. Interfaces 2021, 13, 35306–35314.
- Zhang, X.C.; Zhang, Z.C.; Shu, Q.M.; Xu, C.; Zheng, Q.Q.; Guo, Z.; Wang, C.; Hao, Z.X.; Liu, X.; Wang, G.Q.; et al. Copper clusters: An effective antibacterial for eradicating multidrug-resistant bacterial infection in vitro and in vivo. Adv. Funct. Mater. 2021, 31, 2008720.
- Nie, X.L.; Wu, S.L.; Liao, S.Q.; Chen, J.F.; Huang, F.L.; Li, W.; Wang, Q.Q.; Wei, Q.F. Light-driven self-disinfecting textiles functionalized by PCN-224 and Ag nanoparticles. J. Hazard. Mater. 2021, 416, 125786.
- Wilke, C.M.; Wunderlich, B.; Gaillard, J.F.; Gray, K.A. Synergistic bacterial stress results from exposure to nano-Ag and nano-TiO2 mixtures under light in environmental media. Environ. Sci. Technol. 2018, 52, 3185–3194.
- Yang, T.Y.; Hsieh, Y.J.; Lu, P.L.; Lin, L.; Wang, L.C.; Wang, H.Y.; Tsai, T.H.; Shih, C.J.; Tseng, S.P. In vitro and in vivo assessments of inspired Ag/80S bioactive nanocomposites against carbapenem-resistant Klebsiella pneumoniae. Mater. Sci. Eng. C-Mater. Biol. Appl. 2021, 125, 112093.
- Biao, L.H.; Tan, S.N.; Wang, Y.L.; Guo, X.M.; Fu, Y.J.; Xu, F.J.; Zu, Y.G.; Liu, Z.G. Synthesis, characterization and antibacterial study on the chitosan-functionalized Ag nanoparticles. Mater. Sci. Eng. C-Mater. Biol. Appl. 2017, 76, 73–80.
- Sarker, S.R.; Polash, S.A.; Boath, J.; Kandjani, A.E.; Poddar, A.; Dekiwadia, C.; Shukla, R.; Sabri, Y.; Bhargava, S.K. Functionalization of elongated tetrahexahedral Au nanoparticles and their antimicrobial activity assay. ACS Appl. Mater. Interfaces 2019, 11, 13450–13459.
- Franco, D.; Calabrese, G.; Petralia, S.; Neri, G.; Corsaro, C.; Forte, L.; Squarzoni, S.; Guglielmino, S.; Traina, F.; Fazio, E.; et al. Antimicrobial effect and cytotoxic evaluation of Mg-doped hydroxyapatite functionalized with Au-nano rods. Molecules 2021, 26, 1099.
- Jayaramudu, T.; Varaprasad, K.; Pyarasani, R.D.; Reddy, K.K.; Akbari-Fakhrabadi, A.; Carrasco-Sanchez, V.; Amalraj, J. Hydroxypropyl methylcellulose-copper nanoparticle and its nanocomposite hydrogel films for antibacterial application. Carbohydr. Polym. 2021, 254, 117302.
- Vergara-Llanos, D.; Koning, T.; Pavicic, M.F.; Bello-Toledo, H.; Diaz-Gomez, A.; Jaramillo, A.; Melendrez-Castro, M.; Ehrenfeld, P.; Sanchez-Sanhueza, G. Antibacterial and cytotoxic evaluation of copper and zinc oxide nanoparticles as a potential disinfectant material of connections in implant provisional abutments: An in-vitro study. Arch. Oral Biol. 2021, 122, 105031.
- Prakash, V.; Kumari, A.; Kaur, H.; Kumar, M.; Gupta, S.; Bala, R. Green Synthesis, Characterization and antimicrobial activities of copper nanoparticles from the rhizomes extract of picrorhiza kurroa. Pharm. Nanotechnol. 2021, 9, 298–306.
- Lin, Z.J.; Liu, L.Z.; Wang, W.; Jia, L.; Shen, Y.Q.; Zhang, X.M.; Ge, D.T.; Shi, W.; Sun, Y.A. The role and mechanism of polydopamine and cuttlefish ink melanin carrying copper ion nanoparticles in antibacterial properties and promoting wound healing. Biomater. Sci. 2021, 9, 5951–5964.
- Fang, G.; Li, W.F.; Shen, X.M.; Perez-Aguilar, J.M.; Chong, Y.; Gao, X.F.; Chai, Z.F.; Chen, C.Y.; Ge, C.C.; Zhou, R.H. Differential Pd-nanocrystal facets demonstrate distinct antibacterial activity against Gram-positive and Gram-negative bacteria. Nat. Commun. 2018, 9, 129.
- Stankic, S.; Suman, S.; Haque, F.; Vidic, J. Pure and multi metal oxide nanoparticles: Synthesis, antibacterial and cytotoxic properties. J. Nanobiotechnol. 2016, 14, 129.
- Kong, H.J.; Chu, Q.; Fang, C.; Cao, G.D.; Han, G.R.; Li, X. Cu-Ferrocene-Functionalized CaO2 Nanoparticles to Enable Tumor-Specific Synergistic Therapy with GSH Depletion and Calcium Overload. Adv. Sci. 2021, 8, 2100241.
- Khan, A.U.; Arooj, A.; Tahir, K.; Ibrahim, M.M.; Jevtovic, V.; Al-Abdulkarim, H.A.; Saleh, E.A.M.; Al-Shehri, H.S.; Amin, M.A.; Li, B.S. Facile fabrication of novel Ag2S-ZnO/GO nanocomposite with its enhanced photocatalytic and biological applications. J. Mol. Struct. 2022, 1251, 131991.
- Peng, H.; Rossetto, D.; Mansy, S.S.; Jordan, M.C.; Roos, K.P.; Chen, I.A. Treatment of wound infections in a mouse model using Zn2+-releasing phage bound to gold nanorods. ACS Nano 2022, 16, 4756–4774.
- Fontecha-Umana, F.; Rios-Castillo, A.G.; Ripolles-Avila, C.; Rodriguez-Jerez, J.J. Antimicrobial activity and prevention of bacterial biofilm formation of silver and zinc oxide nanoparticle-containing polyester surfaces at various concentrations for use. Foods 2020, 9, 442.
- Saha, R.K.; Debanath, M.K.; Paul, B.; Medhi, S.; Saikia, E. Antibacterial and nonlinear dynamical analysis of flower and hexagon-shaped ZnO microstructures. Sci. Rep. 2020, 10, 2598.
- Tong, T.Z.; Wilke, C.M.; Wu, J.S.; Binh, C.T.T.; Kelly, J.J.; Gaillard, J.F.; Gray, K.A. Combined toxicity of nano-ZnO and nano-TiO2: From Single- to Multinanomaterial Systems. Environ. Sci. Technol. 2015, 49, 8113–8123.
- Hebeish, A.A.; Abdelhady, M.M.; Youssef, A.M. TiO2 nanowire and TiO2 nanowire doped Ag-PVP nanocomposite for antimicrobial and self-cleaning cotton textile. Carbohydr. Polym. 2013, 91, 549–559.
- Ng, A.M.C.; Chan, C.M.N.; Guo, M.Y.; Leung, Y.H.; Djurisic, A.B.; Hu, X.; Chan, W.K.; Leung, F.C.C.; Tong, S.Y. Antibacterial and photocatalytic activity of TiO2 and ZnO nanomaterials in phosphate buffer and saline solution. Appl. Microbiol. Biotechnol. 2013, 97, 5565–5573.
- Mori, Y.; Tagawa, T.; Fujita, M.; Kuno, T.; Suzuki, S.; Matsui, T.; Ishihara, M. Simple and environmentally friendly preparation and size control of silver nanoparticles using an inhomogeneous system with silver-containing glass powder. J. Nanopart. Res. 2011, 13, 2799–2806.
- Nasrollahzadeh, M.; Sajadi, S.M.; Khalaj, M. Green synthesis of copper nanoparticles using aqueous extract of the leaves of Euphorbia esula L and their catalytic activity for ligand-free Ullmann-coupling reaction and reduction of 4-nitrophenol. RSC Adv. 2014, 4, 47313–47318.
- Din, M.; Arshad, F.; Hussain, Z.; Mukhtar, M. Green adeptness in the synthesis and stabilization of copper nanoparticles: Catalytic, antibacterial, cytotoxicity, and antioxidant activities. Nanoscale Res. Lett. 2017, 12, 638.
- Sondi, I.; Salopek-Sondi, B. Silver nanoparticles as antimicrobial agent: A case study on E-coli as a model for Gram-negative bacteria. J. Colloid Interface Sci. 2004, 275, 177–182.
- Wang, L.N.; Muhammed, M. Synthesis of zinc oxide nanoparticles with controlled morphology. J. Mater. Chem. 1999, 9, 2871–2878.
- Harandi, F.N.; Khorasani, A.C.; Shojaosadati, S.A.; Hashemi-Najafabadi, S. Living Lactobacillus-ZnO nanoparticles hybrids as antimicrobial and antibiofilm coatings for wound dressing application. Mater. Sci. Eng. C-Mater. Biol. Appl. 2021, 130, 112457.
- Fan, J.N.; Cheng, Y.Q.; Sun, M.T. Functionalized gold nanoparticles: Synthesis, properties and biomedical applications. Chem. Rec. 2020, 20, 1474–1504.
- van der Meer, S.B.; Hadrovic, I.; Meiners, A.; Loza, K.; Heggen, M.; Knauer, S.K.; Bayer, P.; Schrader, T.; Beuck, C.; Epple, M. New tools to probe the protein purface: Ultrasmall gold nanoparticles carry amino acid binders. J. Phys. Chem. B 2021, 125, 115–127.
- Friedman, N.; Dagan, A.; Elia, J.; Merims, S.; Benny, O. Physical properties of gold nanoparticles affect skin penetration via hair follicles. Nanomed.-NBM 2021, 36, 102414.
- Li, J.; Zhang, W.G.; Sun, J.B. Enhanced NO2 detection using hierarchical porous ZnO nanoflowers modified with graphene. Ceram. Int. 2016, 42, 9851–9857.
More
