You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Camila Xu and Version 1 by Eduardo Bossone.
Acute heart failure (AHF) is defined as a new onset or recurrence of HF symptoms and signs requiring emergency therapeutic interventions. It may occur as the first manifestation of HF, or more frequently as an acute decompensation of chronic HF.
- acute heart failure
- biomarkers
- cardiac ultrasound
- computer tomography
1. Introduction
Acute heart failure (AHF) is defined as a new onset or recurrence of HF symptoms and signs requiring emergency therapeutic interventions [1,2][1][2]. It may occur as the first manifestation of HF, or more frequently as an acute decompensation of chronic HF [3].
Different AHF classification criteria have been proposed, mainly reflecting the clinical heterogeneity of the syndrome [e.g., hemodynamic status (wet/dry–warm/cold) or according to clinical scenario (decompensated heart failure, acute right heart failure, acute pulmonary edema, cardiogenic shock)] [3] (Table 1).
Table 1.
Classification of AHF.
| Acutely Decompensated Heart Failure | Acute Pulmonary Oedema | Isolated Right Ventricular Failure | Cardiogenic Shock |
|---|---|---|---|
| Description |
3.2. In Hospital
3.2.1. Triage
AHF patients admitted to the emergency department (ED) with mild symptoms and signs of congestion, no renal dysfunction, negative troponin values, and very low neuropeptide (NP) levels can be discharged directly home after a small dose of diuretics and adjustments of oral therapy as needed. They should be referred to their physician with the advice to be clinically followed by the HF multidisciplinary outpatient clinic [17].
On the other hand, hemodynamically unstable patients should be admitted to the cardiology ward or ICU. In this regard, admission ICU criteria include RR > 25, SpO2 < 90%, use of accessory muscles for breathing, SBP < 90 mmHg, need for intubation (or already intubated), or signs of hypoperfusion [oliguria, cold peripheries, altered mental status, lactate > 2 mmol/L, metabolic acidosis, and venous oxygen saturation (SvO2) < 65%] [17,18][17][18] (Figure 1).
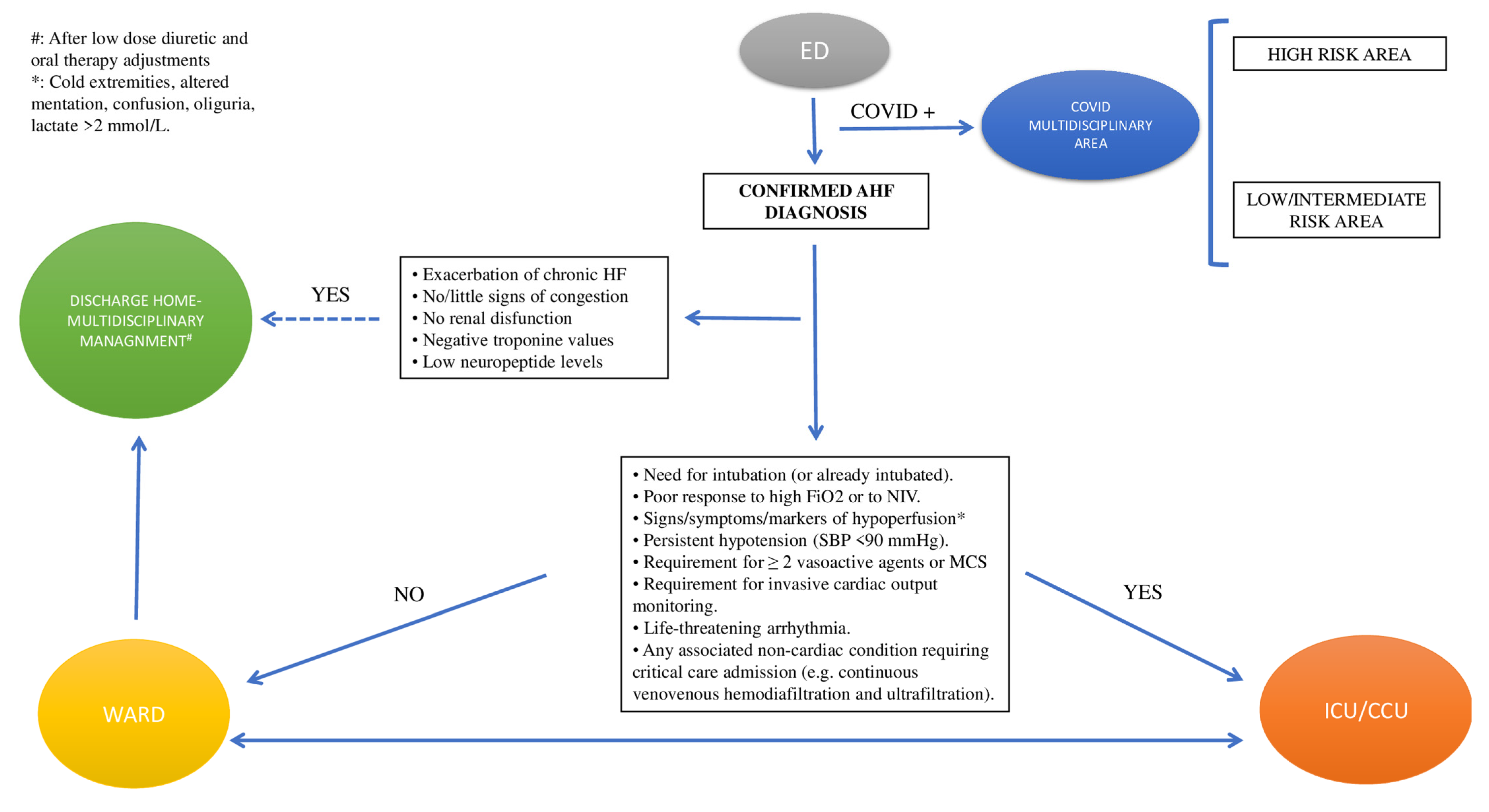
Figure 1. Triage. Abbreviations: AHF: acute heart failure; ED: emergency department; ICU/CCU: intensive cardiology unit/critical care unit; MCS: mechanical circulatory support; NIV: non-invasive ventilation; SBP: systolic blood pressure.
3.2.2. Diagnostic Workup (Figure 2)
Step 1
- a.
-
Search for reversible causes (Table 2)
Management starts with the search for specific causes of AHF. These include acute coronary syndromes (ACS), hypertensive emergency, rapid arrhythmias or severe bradycardia/conduction disturbances, acute mechanical causes (i.e., acute valve regurgitation), acute pulmonary embolism (PE), infections, and tamponade (CHAMPIT). Dietary and fluid restriction and medication noncompliance should also be ascertained at this time.
After exclusion of these conditions, which need to be treated/corrected urgently, management of AHF differs according to clinical presentation [3,17][3][17].
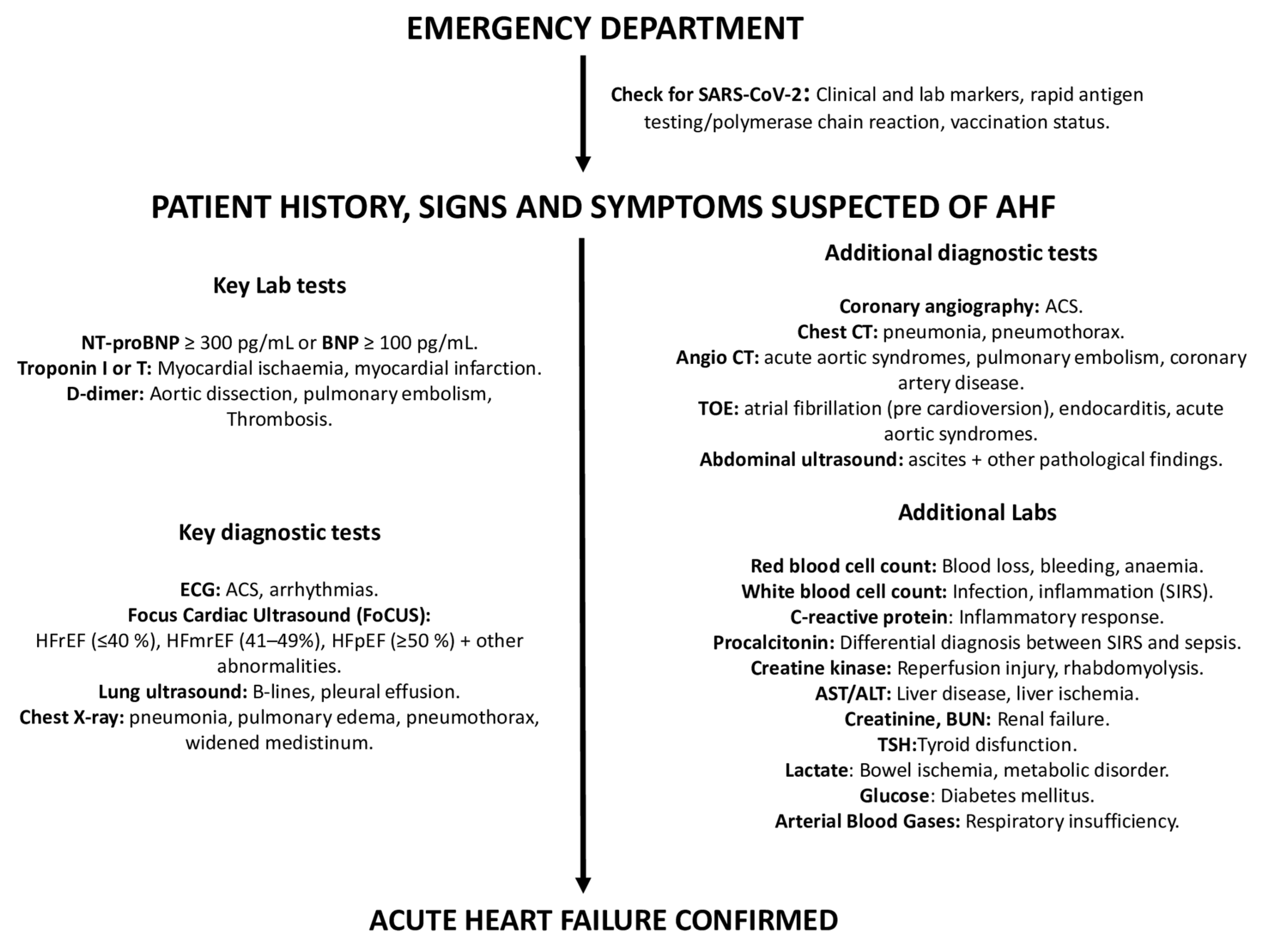
Figure 2. Diagnostic workup of AHF. Abbreviations: ACS: acute coronary syndrome; ALT: alanine aminotransferase; AST: aspartate aminotransferase; BUN: blood urea nitrogen, BNP: brain natriuretic peptide; CT: computed tomography; ECG: electrocardiogram; TOE: transesophageal echocardiogram; TSH: thyroid-stimulating hormone.
Table 2.
Triggers of AHF.
| Triggers | Lab Test | Invasive/Non-Invasive Test | Notes |
|---|---|---|---|
| Progressive fluid retention in patients with history of HF | Lung congestion and acute respiratory failure | ||
| ACS [19 |
| Reaction Severity | Symptoms | Recommendation | ||||
|---|---|---|---|---|---|---|
| ,20][19][20] | RV dysfunction and/or pre-capillary pulmonary hypertension | hs-cTn (I or T) |
| Severe cardiac dysfunction with marked hypotension (SBP < 90 mmHg) despite adequate LV filling pressure | ||
|
||||||
| Mild | Limited urticaria, pruritus, or skin edema; mild nasopharyngeal symptoms such as sneezing, rhinorrhea, or nasal congestion |
|
Do not require premedication | |||
| Onset | Gradual (days) | Rapid (hours) | Gradual/rapid | Gradual/rapid | ||
| Arrhythmias [21,22,23][21][22][23] | Electrolytes, TFTs |
|
||||
| Moderate | Generalized erythema, urticaria, pruritus, or edema Hoarseness or throat tightness with or without mild hypoxia; wheezing with mild hypoxia |
Premedication is recommended Prednisone—50 mg by mouth at 13 h, 7 h, and 1 h before contrast media injection OR Methylprednisolone—32 mg by mouth 12 h and 2 h before contrast media injection PLUS |
|
Main clinical presentation | ||
| Acute Myocarditis [24 | Wet and warm (rarely wet and cold) | ] | hs-cTn (I or T), PCR, ESR, WBC countWet and warm (rarely wet and cold) |
| Wet and cold | Wet and cold |
| Heart rate | ↑ | ↑ | Usually ↓ | ↑ | ||
| SBP | Variable | Variable | ↓ | ↓ | ||
| Cardiac index | Variable | Variable | ↓ | ↓ | ||
| Hypoperfusion | +/− | +/− | + | + | ||
| PCWP | ↑↑ | ↑↑↑ | ↓ | ↑↑ | ||
| Main treatment |
Diuretics Inotropic agents/vasopressors (If peripheral hypoperfusion/hypotension) Short-term MCS or RRT if needed |
O2 (CPAP/NIV) Diuretics Vasodilators Inotropic agents/vasopressors (If peripheral hypoperfusion/hypotension) Short-term MCS or RRT if needed |
Diuretics for congestion Inotropic agents/vasopressors (If peripheral hypoperfusion/hypotension) Short-term MCS or RRT if needed |
Inotropic agents/vasopressors Short-term MCS or RRT if needed |
Abbreviations: CPAP: continuous positive airway pressure; HF: heart failure; LV: left ventricle; MCS: mechanical circulatory support; NIV: non-invasive ventilation; PCWP: pulmonary capillary wedge pressure; RRT: renal replacement therapy; RV: right ventricle; SBP: systolic blood pressure; ↑: increase; ↓: decrease. Modified from “McDonagh T.A.; et al.; 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure” [3].
AHF is the most frequent cause of unplanned hospital admissions in patients >65 years of age and is associated with poor outcomes, with in-hospital and 1-year mortality rates of ~10% and ~30%, respectively, with 90-day readmission rates ~20–30% [4]. Moreover, it imposes a significant financial burden to health systems, with the total medical cost of annual median hospitalizations estimated at USD ~16,000 per patient [5,6][5][6].
2. Epidemiology
The mean age of patients presenting with AHF ranges between 70 and 73 years. About half of patients are male. The majority (65–75%) have a known history of HF. At presentation, most of them have normal or increased blood pressure, while patients presenting with hypotension are generally less than ≤8%, including patients with cardiogenic shock (CS) that represent less than ≤1–2% of cases [7].
Patients presenting with AHF often suffer from several other conditions besides HF. Comorbid states are roughly divided into cardiovascular and non-cardiovascular states. The cardiovascular history usually comprises arterial hypertension (HTN) (~70% of patients), coronary artery disease (CAD) (~50–60%), and atrial fibrillation (AF) (~30–40%) [8,9][8][9].
Non-cardiovascular comorbidities include diabetes mellitus (DM) (~40%), renal dysfunction (~20–30%), chronic obstructive pulmonary disease (COPD) (~20–30%), and anemia (~15–30%) [7,10,11][7][10][11].
A significant number of AHF patients (~35–40%) do not have reduced left ventricle ejection fraction (LVEF) [5,12][5][12]. In this regard, patients with preserved LVEF are usually older (mean age of 75 years) and more frequently female (~60% of patients). Furthermore, they are less affected by CAD but suffer HTN and DM more frequently [13].
3. Management
3.1. Pre-Hospital
AHF patients should immediately (‘time-to-treatment’ concept) receive appropriate therapy and be rapidly transferred to the nearest hospital, preferably to a site with an intensive cardiology unit (CCU/ICU) [3,14][3][14].
In the pre-hospital setting, AHF patients benefit from non-invasive monitoring, including heart and respiratory rate (HR and RR), BP, pulse oximetry (SpO2), and continuous electrocardiogram (ECG) [3,15][3][15].
Oxygen therapy should be administered if SpO2 < 90%. In patients with respiratory distress, non-invasive ventilation (NIV) should be implemented [3,16]
| Diphenhydramine—50 mg intravenously, intramuscularly, or by mouth 1 h before contrast medium | ||||
| Severe | Severe edema, including facial and laryngeal edema, anaphylaxis, hypoxia | Consider alternative tests. If the test is necessary premedication is recommended Prednisone—50 mg by mouth at 13 h, 7 h, and 1 h before contrast media injection OR Methylprednisolone—32 mg by mouth 12 h and 2 h before contrast media injection PLUS Diphenhydramine—50 mg intravenously, intramuscularly, or by mouth 1 h before contrast medium |
|
|
| Endocarditis [25] | ESR, CRP, blood culture, autoimmunity testing in selected cases |
|
Patients presenting with severe heart failure or cardiogenic shock should be referred early and managed in a reference center with immediate surgical facilities. | |
| Acute aortic syndromes [26] | D-dimer |
|
D-dimer is highly sensitive to rule out classical AAD within the first 6 h of symptom onset in low–moderate-risk patients. | |
| Mechanical cause (free wall rupture, ventricular septal defect, acute mitral regurgitation, cardiac tamponade) [19,20][19][20] | hs-cTn (I or T), D-dimer | TTE | Prompt intervention/surgery is needed; transfer to Hub center. | |
| Pulmonary embolism [27] | D-dimer, hs-cTn, ABG |
|
If hemodynamically unstable, transfer to ICU. | |
| Hypertension emergency [28] | FBC, creatinine, electrolytes, LDH, haptoglobin, hs-cTn, pregnancy test in women of child-bearing age |
|
Patients with severe hypertension associated with AHF require an urgent reduction of BP with IV drug administration. | |
| Pneumonia [29] | FBC, ESR, CRP, PCT |
|
Admission to an ICU for patients with hypotension requiring vasopressors or respiratory failure requiring mechanical ventilation. | |
| COPD exacerbation or asthma [30] | ABG, PCR, PCT |
|
Admission to an ICU for patients with hypotension requiring vasopressors or respiratory failure requiring mechanical ventilation. | |
| Thyroid dysfunction [31] | TFTs |
|
Management of myxedema coma and thyroid storm requires both medical and supportive therapies and should be treated in an ICU setting. | |
| Anemia [32] | FBC | - | Urgent RBC transfusion needed. |
Abbreviations: ABG: arterial blood gases; ALS: advanced life support; CABG: coronary artery bypass graft surgery; CCT: cardiac computer tomography; CRP: C-reactive protein; CT: computer tomography; CTA: computed tomography angiography; CTPA: CT pulmonary angiogram; ECG: electrocardiogram; ESR: erythrocyte sedimentation rate; FBC: full blood count; hs-Tn: high-sensitive troponins; ICD: implantable cardioverter defibrillator; ICU: intensive care unit; MRI: magnetic resonance imaging; PCI: percutaneous coronary intervention; PCT: procalcitonin; RBC: red blood cells; TFTs: thyroid function tests; TOE: transesophageal echocardiogram; TTE: transthoracic echocardiogram, SMVT: sustained monomorphic ventricular tachycardia.
- b.
At the time of hospital admission, it is advisable to:
- -
-
Search for clinical and laboratory clues suggesting COVID-19 infection;
- -
-
Perform SARS-CoV-2 rapid antigen testing/polymerase chain reaction;
- -
-
Check for COVID-19 vaccination status.
- c.
-
Assess presenting symptoms and signs
The most common symptoms (reflecting pulmonary and/or systemic congestion) include dyspnea during exercise or at rest, orthopnea, fatigue, and reduced exercise tolerance. Clinical signs usually include peripheral oedema, jugular vein distension, the presence of a third heart sound and pulmonary rales [2].
Symptoms and signs such as cold and clammy skin, altered mental status, and oliguria indicate peripheral hypoperfusion—impending CS [2].
Step 2
- a.
-
Lab tests
Neuropeptides
Cardiovascular biomarkers play a crucial role in the diagnostic–prognostic process of AHF. Upon presentation to the ED, plasma NP levels (BNP, NT-proBNP, or MR-proANP) should be measured (point-of-care assay) in all patients with acute dyspnea. Due to the strong link with hemodynamic intracardiac stress, they may help to differentiate between cardiac and non-cardiac causes of acute dyspnea [35,36][35][36].
Cut-offs for AHF are BNP < 100 pg/mL, NT-proBNP < 300 pg/mL, and MR-proANP < 120 pg/mL, with normal NP concentrations making the diagnosis of AHF extremely unlikely.
However, there are many causes of elevated NP levels—both cardiovascular (CV) and non-CV—that might reduce their diagnostic accuracy. These causes include AF, increasing age, and acute or chronic kidney disease. Conversely, NP concentrations may be disproportionately low in obese patients, in patients with pre-left ventricle causes of HF (i.e., mitral stenosis and acute mitral regurgitation), or pericardial diseases.
Troponin
In addition to ACS, elevated high-sensitivity troponin I/T (hsTn I/T) levels may be observed in most non-ACS AHF patients and are associated with worse in-hospital and post-discharge outcomes [39].
Others
Further lab tests (i.e., BUN (or urea), creatinine, electrolytes, glucose, complete blood count, procalcitonin, PCR, and D-dimer) may be useful to detect and/or to confirm clinically suspected comorbidities and/or end-organ damage [15].
SpO2/arterial blood gas (ABG)
SpO2 should be measured routinely at the time of AHF patient presentation and continuous monitoring may be needed in the first hours or days.
Routine ABG is not needed. Specific indications for ABG are: respiratory distress [defined as acute increase in the work of breathing or significant tachypnea (RR > 25 breaths/min)], documented hypoxemia (SpO2 < 90%) not responsive to supplemental oxygen, and evidence of acidosis or elevated lactate levels. In the case of respiratory failure, ABG may show PaO2 < 60 mmHg, PaCO2 > 45 mmHg or PaO2/FiO2 < 300 mmHg. Of note, venous sample might acceptably indicate pH and CO2 [15].
- b.
-
ECG
Routine admission ECG is recommended since it can exclude ACS and arrhythmias. In this regard, careful attention should be paid to ECG changes suggestive of myocardial ischemia. Tachyarrhythmias [i.e., AF (present in 20% to 30% patients), ventricular tachycardia] or bradyarrhythmias (i.e., advanced atrio-ventricular blocks) are also a common trigger for AHF [3,21,22][3][21][22].
- c.
-
Chest X-ray
Chest X-ray may reveal lung congestion and/or pleural effusion. Furthermore, it may identify non-cardiac-disease causes of the patient’s symptoms (i.e., pneumonia, pneumothorax, widened mediastinum).
- d.
-
Transthoracic echocardiography (TTE)
TTE represents the single most useful imaging technique to investigate AHF etiology and to guide related therapeutic interventions.
A “Focus Cardiac Ultrasound” (FoCUS), followed by comprehensive TTE exam, is recommended in all patients to assess LV global systolic (reduced vs. preserved EF) and diastolic function, regional wall abnormalities, valvular heart (stenosis and/or regurgitations) and pericardial disease. In addition, it is of paramount importance to evaluate right heart structure and function, as well as pulmonary pressures, as these are major prognostic determinants [40].
As a note, an E:E’ ratio greater than 15 predicts a pulmonary arterial wedge pressure (PAWP) greater than 15 mm Hg, and has been demonstrated to be accurate in the ED and intensive care settings [2,41][2][41] (Figure 3).
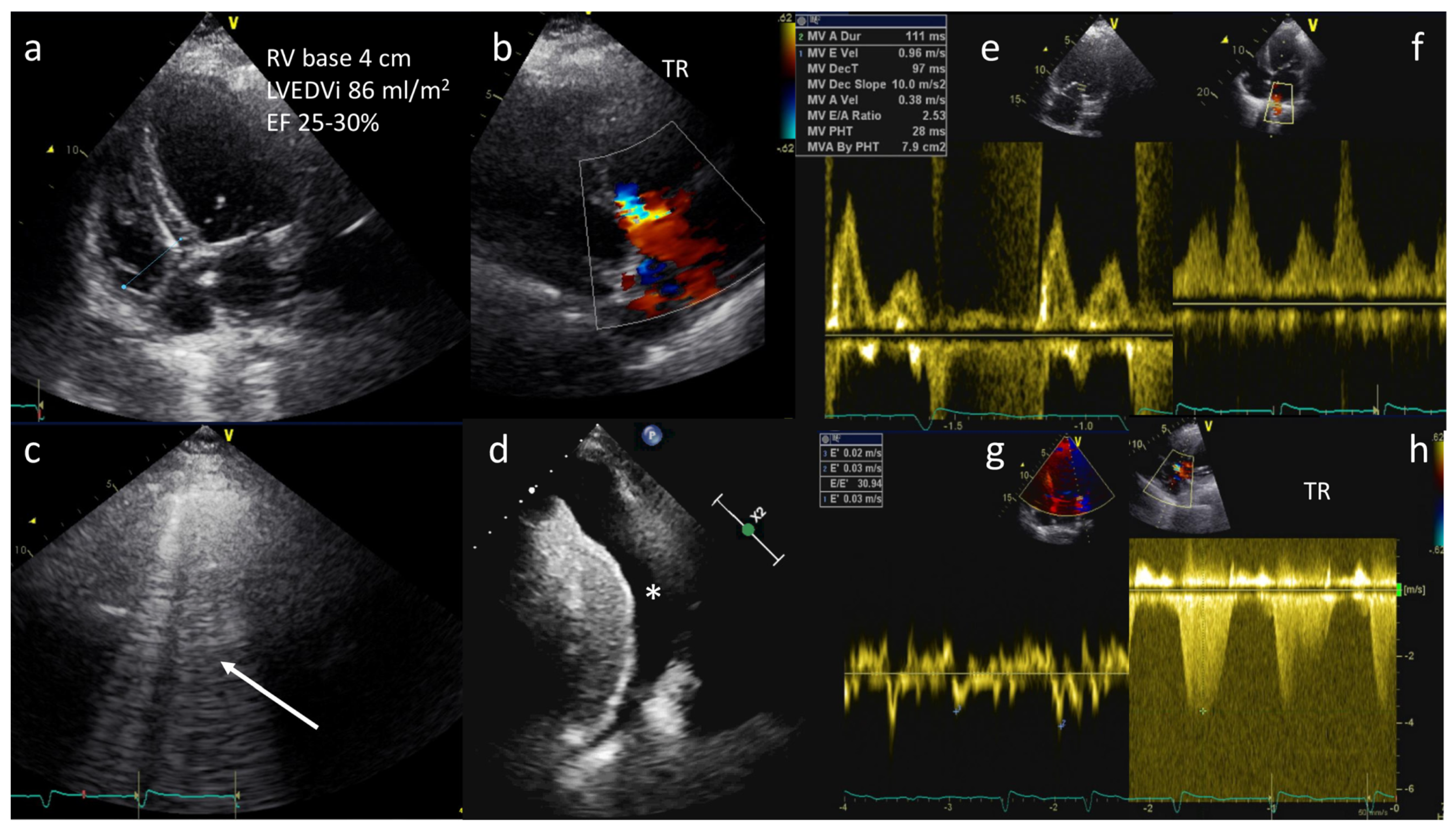
Figure 3. A 68-year-old female with history of dilated cardiomyopathy was admitted for shortness of breath, fatigue, and low-extremity edema. A diagnosis of acute pulmonary edema was made. TTE showed severe LV dilation (LVEDVi 86 mL/m2), severe reduction in ejection fraction (EF 25–30%), mildly dilated right ventricle (basal diameter 4 cm) (a), and moderate tricuspid regurgitation (TR) (b). Lung ultrasound showing B-lines in all sites explored (arrow) and pleural effusion (*) (c,d). Diastolic dysfunction with increased left ventricular end-diastolic pressures (LVEDP) and PAWP (E/A: 2.5, E/E’: 30 and S < D on pulmonary veins) (e–g) and estimated pulmonary artery pressure of 70 mmHg (h).
- e.
-
Lung ultrasound (LUS)
LUS has emerged as a valuable modality to detect and monitor pulmonary congestion in patients with AHF in a low-cost, portable, real-time, and radiation-free manner.
It outperforms the diagnostic accuracy of the chest radiograph in the detection of pleural water (pleural effusion) and lung water (pulmonary congestion as multiple B-lines) [42].
B-lines are well defined (laser-like), hyperechoic, vertical comet-tail artifacts that arise strictly from the pleural line, move in sync with lung sliding and spread to the edge of the screen without fading and erasing A lines. The number of lines is proportional to the severity of congestion and identifies the cardiogenic origin of dyspnea with 85% sensitivity and 92% specificity [43].
The B profile is useful to track dynamic changes in pulmonary congestion in responses to treatment, and its persistence at pre-discharge or in clinically stable outpatients with heart failure is predictive of heart failure hospitalization or death [44].
The amount of pleural effusion can be scored as trivial (<2 mm), small (2 to 15 mm), moderate (15 to 25 mm), or large (>25 mm). Furthermore, LUS represents a guide to thoracentesis in patients with AHF and at least moderate pleural effusion [45].
As a note, the evaluation of “lung sliding” (a horizontal, to-and-fro movement, beginning at the pleural line and synchronous with respiration) is helpful in the differential diagnosis of several parenchymal lung diseases that are present as comorbidities in HF or as causes of dyspnea suspected to be cardiac in origin. For instance, “lung sliding” disappears in pneumothorax and it is reduced or abolished in the case of pneumonia, acute respiratory distress syndrome (ARDS), or pleural adhesions [43].
- f.
-
Abdominal ultrasound (AUS)
AUS can be useful for measurement of the inferior vena cava (IVC) diameter as an indirect measure of right atrial pressures (IVC < 21 mm that collapses >50% suggests normal right atrial pressure) [46].
In HF patients, an increased IVC diameter might detect abnormal intravascular volume even prior to any change in symptoms or body weight, and in turn monitor the response to diuretics. AUS can also detect ascites and abdominal aortic aneurysm [46].
- g.
-
Transesophageal echocardiogram (TEE)
TEE may be performed in suspected endocarditis and acute aortic syndromes (AAS). Furthermore, it may be useful to better define heart valve abnormalities and to detect intracardiac shunt and thrombi. Absolute contraindications include: unrepaired tracheoesophageal fistula, esophageal obstruction/stricture, perforated hollow viscus, active gastric/esophageal bleeding, poor airway control, severe respiratory depression, and uncooperative, unsedated patient [48].
Step 3. Additional Non-Invasive and Invasive Tests
- a.
-
High-resolution chest computed tomography (Chest HR-CT)
Chest HR-CT should be considered when pulmonary parenchymal component is suspected among patients presenting with AHF.
CT can also identify signs of pulmonary edema, such as interlobular septal thickening, fissural thickening, peribronchovascular thickening, perihilar or bat-wing appearance of oedema, increased artery-to-bronchus ratio, pleural effusion, and cardiac enlargement in more advanced HF [49,50][49][50].
Furthermore, high-resolution CT provides an effective modality to evaluate patients with suspected COVID-19.
- b.
-
Chest CT angiography (CTA)
CTA can be used as a one-step imaging modality (dual rule-out strategy) to exclude PE or AAS. It can be performed with most CT equipment. Furthermore, with state-of-the-art CT equipment, synchronizing image acquisition with the cardiac cycle, it is possible to perform the so-called Triple Rule-Out strategy (TRO). This protocol allows the heart and the coronary arteries to be imaged, allowing the exclusion of ACS in a clinical context where this diagnosis might not be straightforward. The main drawbacks of CTA are the administration of iodinated contrast agent, which may cause acute kidney injury or allergic reactions, even though the amount of contrast material currently required to perform the scan is quite low compared to in the past (i.e., using state-of-the-art CT technology, 50 mL). Furthermore, the use of ionizing radiation should be avoided in younger patients, especially women [51].
Recent CT technology also allows the performance of a full anatomical and functional assessment of cardiac and thoracic structures. Hence, a patient undergoing this kind of assessment will have all heart chamber volumes and functionality assessed, the presence of thrombosis within the cardiac chambers ruled out, the superior and inferior vena cava assessed for patency and distention, the pulmonary artery evaluated for dilatation, and so forth. When COVID-19 is assessed in the context of a TRO protocol, it is referred to as Quadruple Rule-Out [52]. When other causes for the acute settings are included in the evaluation, it can be referred to as Quintuple Rule-Out. Because of this flexibility and wide range of rule-in/rule-out capabilities and its relatively easy access, CT is already, and will become, an increasingly central tool in all acute clinical settings (Figure 4 and Figure 5).
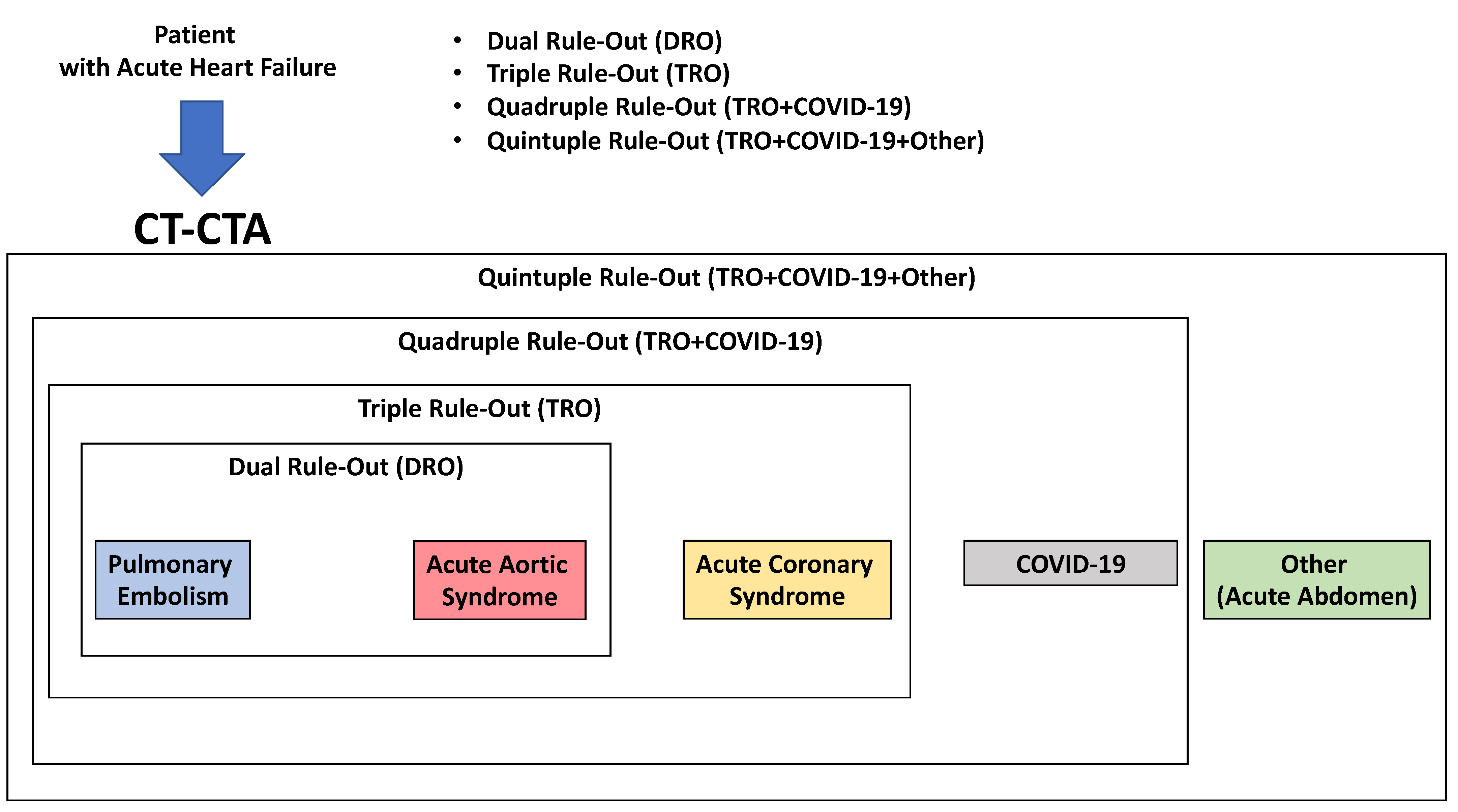
Figure 4.
Use of CT in acute heart failure.
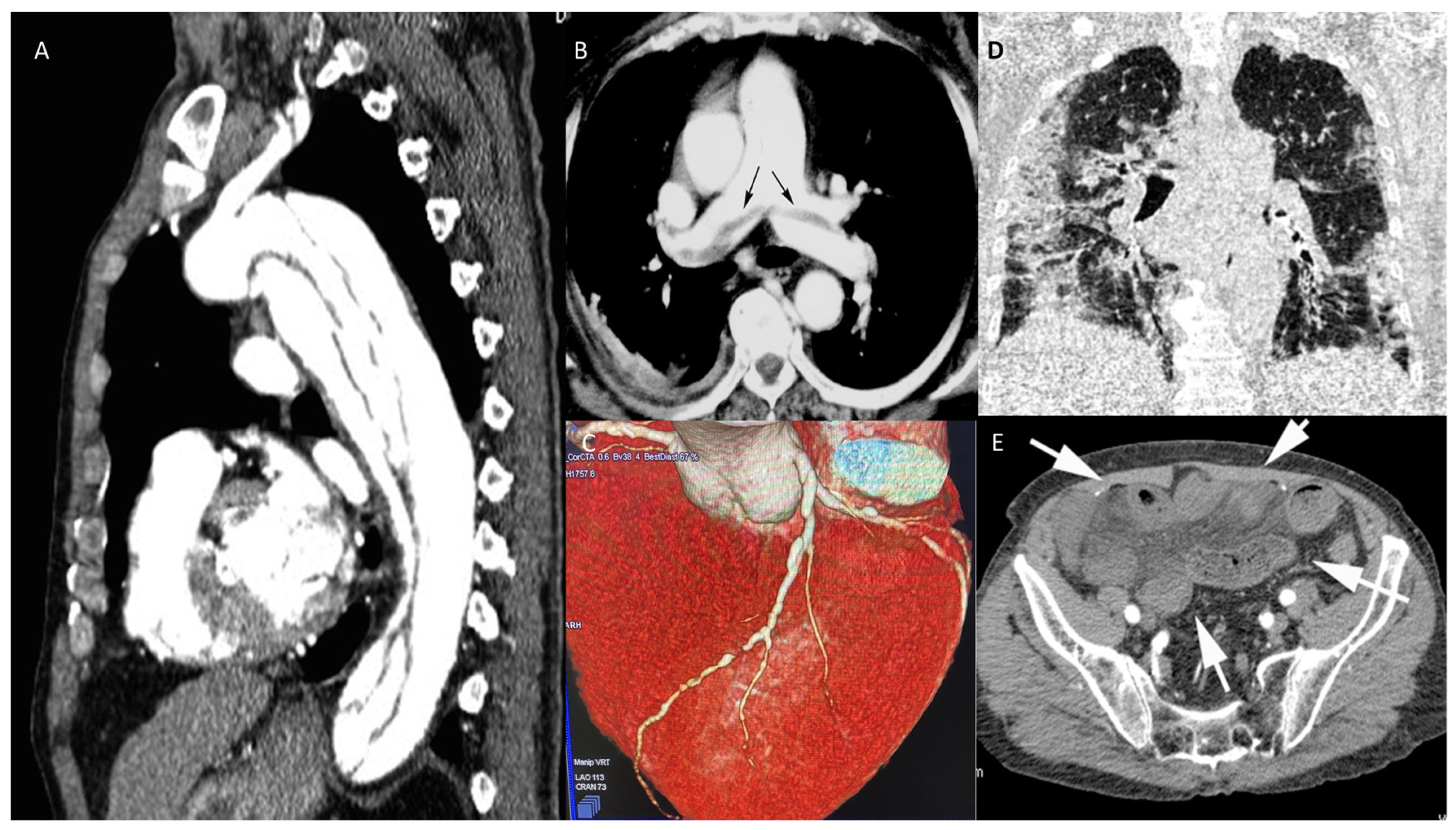
Figure 5. CT in different scenarios. Type B thoracic aortic dissection: post-contrast sagittal CT reconstruction of the aorta demonstrates a medio-intimal flap that begins below left subclavian arterial origin and extends up to diaphragmatic hiatus (A). Pulmonary thromboembolism: post-contrast axial CT reconstruction depicts linear contrast defects inside the lumen of main pulmonary arteries (arrows) due to thromboembolism (B). Volume rendering post-contrast CT of the left descending coronary artery depicts a brief stenosis of the medium segment (C). Coronal unenhanced chest CT shows ground glass opacities of the lungs, especially on the left side, due to interstitial COVID pneumonia (D). Post-contrast axial CT image of the pelvis demonstrates ileal loops ischemia with a stratified appearance of the ileal loop’s wall (arrows) due to intramural edema and low submucosal enhancement associated with mesenterial free fluid. (E).
CIN (contrast induced nephropathy) remains one of the most serious complications of iodinated contrast medium (CM). It is defined as a ≥25% increase in serum creatinine from the baseline value, or an absolute increase of at least 0.5 mg/dL (44.2 µmol/L), 48–72 h after the administration of radiographic contrast media that is not attributable to other causes [53].
Pre-existing renal impairment represents the most important risk factor for CIN. The baseline renal function of patients undergoing contrast studies is best assessed with calculations of glomerular filtration rate (GFR), such as the MDRD or Cockcroft–Gault formulae in adults [53].
Patients at high risk of developing CIN should be identified early and prophylactic measures implemented before the procedure (Table 3).
Table 3.
Prevention of contrast-induced nephropathy.
| GFR ≥ 60 mL/min |
|---|
| Extremely low risk for CIN: specific prophylaxis or follow up not required |
| GFR < 60 mL/min (Moderate–Severe Kidney Disease) |
|
Abbreviations: CIN: contrast-induced nephropathy; CM: contrast medium; LVEF: left ventricular ejection fraction; NYHA: New York Heart Association. Modified from “Neumann FJ; et al. 2018 ESC/EACTS Guidelines on myocardial revascularization” [53].
The frequency of allergic-like adverse events related to the intravascular administration of iodinated CM is low and has decreased considerably since the use of nonionic low-osmolality contrast media. However, the majority of adverse side effects to CM are mild non-life-threatening events that usually require only observation, reassurance, and/or supportive measures [54]. Severe reactions (i.e., bronchospasm, laryngeal edema, anaphylaxis) occur rarely and are unpredictable. A frequently recommended premedication oral regimen for elective examinations is shown in Table 4.
Table 4.
Premedication protocols to avoid allergic reactions.
- c.
-
Coronary angiography
References
- Bozkurt, B.; Coats, A.J.S.; Tsutsui, H.; Abdelhamid, C.M.; Adamopoulos, S.; Albert, N.; Anker, S.D.; Atherton, J.; Böhm, M.; Butler, J.; et al. Universal definition and classification of heart failure: A report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure: Endorsed by the Canadian Heart Failure Society, Heart Failure Association of India, Cardiac Society of Australia and New Zealand, and Chinese Heart Failure Association. Eur. J. Heart Fail. 2021, 23, 352–380.
- Felker, G.M.; Teerlink, J.R. Diagnosis and Management of Acute Heart Failure. In Braunwald’s Heart Disease a Textbook of Cardiovascular Medicine, 11th ed.; Zipes, D.P., Libby, P., Bonow, R., Mann, L.D., Tomaselli, G.F., Eds.; Elsevier: Philadelphia, PA, USA, 2019; pp. 462–489.
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726.
- Arrigo, M.; Jessup, M.; Mullens, W.; Reza, N.; Shah, A.M.; Sliwa, K.; Mebazaa, A. Acute heart failure. Nat. Rev. Dis. Primer 2020, 6, 16.
- Nieminen, M.S.; Brutsaert, D.; Dickstein, K.; Drexler, H.; Follath, F.; Harjola, V.-P.; Hochadel, M.; Komajda, M.; Lassus, J.; Lopez-Sendon, J.L.; et al. EuroHeart Failure Survey II (EHFS II): A survey on hospitalized acute heart failure patients: Description of population. Eur. Heart J. 2006, 27, 2725–2736.
- Urbich, M.; Globe, G.; Pantiri, K.; Heisen, M.; Bennison, C.; Wirtz, H.S.; Di Tanna, G.L. A Systematic Review of Medical Costs Associated with Heart Failure in the USA (2014–2020). PharmacoEconomics 2020, 38, 1219–1236.
- Farmakis, D.; Filippatos, G. Acute heart failure: Epidemiology, classification, and pathophysiology. In The ESC Textbook of Intensive and Acute Cardiovascular Care, 3rd ed.; Tubaro, M., Vranckx, P., Bonnefoy, E., Price, S., Vrints, C., Eds.; Oxford University Press: Oxford, UK, 2021; pp. 603–616.
- Di Palo, K.E.; Barone, N.J. Hypertension and Heart Failure. Heart Fail. Clin. 2020, 16, 99–106.
- Kim, I.-C. Atrial Fibrillation and Heart Failure with Preserved Ejection Fraction. Heart Fail. Clin. 2021, 17, 377–386.
- Pellicori, P.; Cleland, J.G.F.; Clark, A.L. Chronic Obstructive Pulmonary Disease and Heart Failure. Heart Fail. Clin. 2020, 16, 33–44.
- Ananthram, M.G.; Gottlieb, S.S. Renal Dysfunction and Heart Failure with Preserved Ejection Fraction. Heart Fail. Clin. 2021, 17, 357–367.
- Sugimoto, T. Acute Decompensated Heart Failure in Patients with Heart Failure with Preserved Ejection Fraction. Heart Fail. Clin. 2020, 16, 201–209.
- Fonarow, G.C.; Stough, W.G.; Abraham, W.T.; Albert, N.M.; Gheorghiade, M.; Greenberg, B.H.; O’Connor, C.M.; Sun, J.L.; Yancy, C.W.; Young, J.B. Characteristics, Treatments, and Outcomes of Patients With Preserved Systolic Function Hospitalized for Heart Failure. J. Am. Coll. Cardiol. 2007, 50, 768–777.
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e895–e1032.
- Mebazaa, A.; Yilmaz, M.B.; Levy, P.; Ponikowski, P.; Peacock, W.F.; Laribi, S.; Ristic, A.D.; Lambrinou, E.; Masip, J.; Riley, J.P.; et al. Recommendations on pre-hospital & early hospital management of acute heart failure: A consensus paper from the Heart Failure Association of the European Society of Cardiology, the European Society of Emergency Medicine and the Society of Academic Emergenc: Recommendations on pre-hospital & early hospital management of acute heart failure. Eur. J. Heart Fail. 2015, 17, 544–558.
- Masip, J.; Gayà, M.; Páez, J.; Betbesé, A.; Vecilla, F.; Manresa, R.; Ruíz, P. Pulsioximetría en el diagnóstico de insuficiencia cardiaca aguda. Rev. Esp. Cardiol. 2012, 65, 879–884.
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.-P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200.
- Weng, C.-L. Meta-analysis: Noninvasive Ventilation in Acute Cardiogenic Pulmonary Edema. Ann. Intern. Med. 2010, 152, 590.
- Collet, J.-P.; Thiele, H.; Barbato, E.; Barthélémy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Rev. Esp. Cardiol. Engl. Ed. 2021, 74, 544.
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur. Heart J. 2018, 39, 119–177.
- Zeppenfeld, K.; Tfelt-Hansen, J.; de Riva, M.; Winkel, B.G.; Behr, E.R.; Blom, N.A.; Charron, P.; Corrado, D.; Dagres, N.; de Chillou, C.; et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur. Heart J. 2022, 43, 3997–4126.
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.-A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2021, 42, 373–498.
- Glikson, M.; Nielsen, J.C.; Kronborg, M.B.; Michowitz, Y.; Auricchio, A.; Barbash, I.M.; Barrabés, J.A.; Boriani, G.; Braunschweig, F.; Brignole, M.; et al. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Eur. Heart J. 2021, 42, 3427–3520.
- Tschöpe, C.; Ammirati, E.; Bozkurt, B.; Caforio, A.L.P.; Cooper, L.T.; Felix, S.B.; Hare, J.M.; Heidecker, B.; Heymans, S.; Hübner, N.; et al. Myocarditis and inflammatory cardiomyopathy: Current evidence and future directions. Nat. Rev. Cardiol. 2021, 18, 169–193.
- Habib, G.; Lancellotti, P.; Antunes, M.J.; Bongiorni, M.G.; Casalta, J.-P.; Del Zotti, F.; Dulgheru, R.; El Khoury, G.; Erba, P.A.; Iung, B.; et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC)Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur. Heart J. 2015, 36, 3075–3128.
- 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adultThe Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur. Heart J. 2014, 35, 2873–2926.
- Konstantinides, S.V.; Meyer, G.; Becattini, C.; Bueno, H.; Geersing, G.-J.; Harjola, V.-P.; Huisman, M.V.; Humbert, M.; Jennings, C.S.; Jiménez, D.; et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur. Heart J. 2020, 41, 543–603.
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104.
- Jobs, A.; Simon, R.; de Waha, S.; Rogacev, K.; Katalinic, A.; Babaev, V.; Thiele, H. Pneumonia and inflammation in acute decompensated heart failure: A registry-based analysis of 1939 patients. Eur. Heart J. Acute Cardiovasc. Care 2018, 7, 362–370.
- Halpin, D.M.G.; Criner, G.J.; Papi, A.; Singh, D.; Anzueto, A.; Martinez, F.J.; Agusti, A.A.; Vogelmeier, C.F. Global Initiative for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease. The 2020 GOLD Science Committee Report on COVID-19 and Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2021, 203, 24–36.
- Leung, A.M. Thyroid Emergencies. J. Infus. Nurs. 2016, 39, 281–286.
- Sirbu, O. Anemia in heart failure—From guidelines to controversies and challenges. Anatol. J. Cardiol. 2018, 20, 52.
- Rosano, G.; Jankowska, E.A.; Ray, R.; Metra, M.; Abdelhamid, M.; Adamopoulos, S.; Anker, S.D.; Bayes-Genis, A.; Belenkov, Y.; Ben Gal, T.; et al. COVID-19 vaccination in patients with heart failure: A position paper of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2021, 23, 1806–1818.
- Bartoletti, M.; Azap, O.; Barac, A.; Bussini, L.; Ergonul, O.; Krause, R.; Paño-Pardo, J.R.; Power, N.R.; Sibani, M.; Szabo, B.G.; et al. ESCMID COVID-19 living guidelines: Drug treatment and clinical management. Clin. Microbiol. Infect. 2022, 28, 222–238.
- Núñez, J.; de la Espriella, R.; Rossignol, P.; Voors, A.A.; Mullens, W.; Metra, M.; Chioncel, O.; Januzzi, J.L.; Mueller, C.; Richards, A.M.; et al. Congestion in heart failure: A circulating biomarker-based perspective. A review from the Biomarkers Working Group of the Heart Failure Association, European Society of Cardiology. Eur. J. Heart Fail. 2022, 24, 1751–1766.
- Salzano, A.; D’Assante, R.; Israr, M.Z.; Eltayeb, M.; D’Agostino, A.; Bernieh, D.; De Luca, M.; Rega, S.; Ranieri, B.; Mauro, C.; et al. Biomarkers in Heart Failure. Heart Fail. Clin. 2021, 17, 223–243.
- Mueller, C.; McDonald, K.; de Boer, R.A.; Maisel, A.; Cleland, J.G.F.; Kozhuharov, N.; Coats, A.J.S.; Metra, M.; Mebazaa, A.; Ruschitzka, F.; et al. Heart Failure Association of the European Society of Cardiology practical guidance on the use of natriuretic peptide concentrations. Eur. J. Heart Fail. 2019, 21, 715–731.
- Hollenberg, S.M.; Warner Stevenson, L.; Ahmad, T.; Amin, V.J.; Bozkurt, B.; Butler, J.; Davis, L.L.; Drazner, M.H.; Kirkpatrick, J.N.; Peterson, P.N.; et al. 2019 ACC Expert Consensus Decision Pathway on Risk Assessment, Management, and Clinical Trajectory of Patients Hospitalized With Heart Failure. J. Am. Coll. Cardiol. 2019, 74, 1966–2011.
- Harjola, V.-P.; Mullens, W.; Banaszewski, M.; Bauersachs, J.; Brunner-La Rocca, H.-P.; Chioncel, O.; Collins, S.P.; Doehner, W.; Filippatos, G.S.; Flammer, A.J.; et al. Organ dysfunction, injury and failure in acute heart failure: From pathophysiology to diagnosis and management. A review on behalf of the Acute Heart Failure Committee of the Heart Failure Association (HFA) of the European Society of Cardiology (ESC): Organ dysfunction and failure in AHF. Eur. J. Heart Fail. 2017, 19, 821–836.
- Via, G.; Hussain, A.; Wells, M.; Reardon, R.; ElBarbary, M.; Noble, V.E.; Tsung, J.W.; Neskovic, A.N.; Price, S.; Oren-Grinberg, A.; et al. International Evidence-Based Recommendations for Focused Cardiac Ultrasound. J. Am. Soc. Echocardiogr. 2014, 27, 683.e1–683.e33.
- Mitchell, C.; Rahko, P.S.; Blauwet, L.A.; Canaday, B.; Finstuen, J.A.; Foster, M.C.; Horton, K.; Ogunyankin, K.O.; Palma, R.A.; Velazquez, E.J. Guidelines for Performing a Comprehensive Transthoracic Echocardiographic Examination in Adults: Recommendations from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2019, 32, 1–64.
- Gargani, L. Ultrasound of the Lungs. Heart Fail. Clin. 2019, 15, 297–303.
- Picano, E.; Scali, M.C.; Ciampi, Q.; Lichtenstein, D. Lung Ultrasound for the Cardiologist. JACC Cardiovasc. Imaging 2018, 11, 1692–1705.
- Lichtenstein, D.A.; Mezière, G.A. Relevance of Lung Ultrasound in the Diagnosis of Acute Respiratory Failure*: The BLUE Protocol. Chest 2008, 134, 117–125.
- Coiro, S.; Rossignol, P.; Ambrosio, G.; Carluccio, E.; Alunni, G.; Murrone, A.; Tritto, I.; Zannad, F.; Girerd, N. Prognostic value of residual pulmonary congestion at discharge assessed by lung ultrasound imaging in heart failure: Prognostic value of B-lines after discharge from HF hospitalisation. Eur. J. Heart Fail. 2015, 17, 1172–1181.
- Pellicori, P.; Platz, E.; Dauw, J.; Maaten, J.M.; Martens, P.; Pivetta, E.; Cleland, J.G.F.; McMurray, J.J.V.; Mullens, W.; Solomon, S.D.; et al. Ultrasound imaging of congestion in heart failure: Examinations beyond the heart. Eur. J. Heart Fail. 2021, 23, 703–712.
- Iida, N.; Seo, Y.; Sai, S.; Machino-Ohtsuka, T.; Yamamoto, M.; Ishizu, T.; Kawakami, Y.; Aonuma, K. Clinical Implications of Intrarenal Hemodynamic Evaluation by Doppler Ultrasonography in Heart Failure. JACC Heart Fail. 2016, 4, 674–682.
- Puchalski, M.D.; Lui, G.K.; Miller-Hance, W.C.; Brook, M.M.; Young, L.T.; Bhat, A.; Roberson, D.A.; Mercer-Rosa, L.; Miller, O.I.; Parra, D.A.; et al. Guidelines for Performing a Comprehensive Transesophageal Echocardiographic. J. Am. Soc. Echocardiogr. 2019, 32, 173–215.
- Suciadi, L.P.; William, Y.; Jorizal, P.; Tarigan, V.N.; Santoso, A.H.; Henrina, J.; Tedjasukmana, F.; Kristanti, N.M. Comparing Lung CT in COVID-19 Pneumonia and Acute Heart Failure: An Imaging Conundrum. Cureus 2021, 13, e15120.
- Bernheim, A.; Mei, X.; Huang, M.; Yang, Y.; Fayad, Z.A.; Zhang, N.; Diao, K.; Lin, B.; Zhu, X.; Li, K.; et al. Chest CT Findings in Coronavirus Disease-19 (COVID-19): Relationship to Duration of Infection. Radiology 2020, 295, 200463.
- Mangalat, D.; Kalogeropoulos, A.; Georgiopoulou, V.; Stillman, A.; Butler, J. Value of cardiac CT in patients with heart failure. Curr. Cardiovasc. Imaging Rep. 2009, 2, 410–417.
- Pontone, G.; Baggiano, A.; Conte, E.; Teruzzi, G.; Cosentino, N.; Campodonico, J.; Rabbat, M.G.; Assanelli, E.; Palmisano, A.; Esposito, A.; et al. “Quadruple Rule-Out” With Computed Tomography in a COVID-19 Patient With Equivocal Acute Coronary Syndrome Presentation. JACC Cardiovasc. Imaging 2020, 13, 1854–1856.
- Neumann, F.-J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.-P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 2019, 40, 87–165.
- Andreucci, M.; Solomon, R.; Tasanarong, A. Side Effects of Radiographic Contrast Media: Pathogenesis, Risk Factors, and Prevention. BioMed Res. Int. 2014, 2014, 741018.
More
