Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Vidyasrilekha Sanapalli and Version 2 by Catherine Yang.
Fused pyridines are reported to display various pharmacological activities, such as antipyretic, analgesic, antiprotozoal, antibacterial, antitumor, antifungal, anti-inflammatory, and antiapoptotic. They are widely used in the field of medicinal chemistry. Imidazopyridines (IZPs) are crucial classes of fused heterocycles that are expansively reported on in the literature. Evidence suggests that IZPs, as fused scaffolds, possess more diverse profiles than individual imidazole and pyridine moieties. Bacterial infections and antibacterial resistance are ever-growing risks in the 21st century. Only one IZP, i.e., rifaximin, is available on the market as an antibiotic
- imidazopyridine
- synthetic approaches
- antibacterial activity
1. Introduction
Fused pyridines are an outstanding class of heterocycles with a diverse pharmacological profile which researchers have explored extensively [1][2][3][4][5][1,2,3,4,5]. Among them, imidazopyridine (IZP), i.e., imidazole fused with pyridine ring, comprises an important class of pharmacologically active nitrogen-containing fused heterocycles [6][7][8][9][6,7,8,9]. All these derivatives exhibit a wide range of pharmacological activities, such as antipyretic, analgesic, anxiolytic, antiprotozoal, antibacterial, antitumor, antifungal, anti-inflammatory, and antiapoptotic activities [10][11][12][13][14][15][10,11,12,13,14,15]. Moreover, several drugs which are available on the market, like necopidem and saripidem (anti-anxiolytic) [16], olprinone (acute heart failure) [17], zolpidem (insomnia) [18], zolimidine (peptic ulcer) [19], alpidem (anxiolytic) [18], and rifaximin (antibiotic) [20] possess IZP moieties.
Consequently, there is a continuous effort to conceive novel strategies to develop imidazopyridine with different substituents at various positions on the imidazole and pyridine moieties (Figure 1) and to evaluate the biological activities of the resulting compounds. In this rentry, the researchers cview, we concisely present the important synthetic methodologies of imidazopyridines reported to date, along with their antibacterial activities against wild and resistant pathogens.
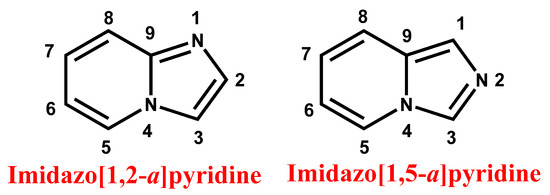
Figure 1.
General structure and nomenclature of imidazopyridine.
2. Strategies for the Synthesis of Imidazopyridines
Several approaches, e.g., multicomponent, condensation, oxidative coupling, amino-oxygenation, tandem reaction, hydroamination, etc., have been put forward for synthesizing this privileged scaffold. These approaches have been consequently optimized to produce IZP and its derivatives with high yield and purity. The literature describes some important synthetic routes, which are summarized in Figure 2.
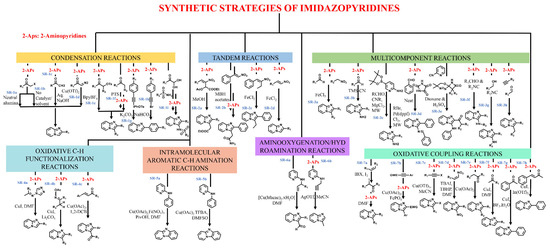
Figure 2.
Synthetic strategies for the preparation of imidazopyridines.
The (SR)-1a synthetic route is a traditional strategy involving a simple condensation reaction of 2-aminopyridines with α-haloketone in the presence of neutral alumina, as proposed by Sahu et al., 2005 [21]. Zhu et al., 2009 [22], later optimized this process. They reported the synthesis (SR-1b) of IZP with α-haloketone and 2-aminopyridine in the absence of a catalyst and in solvent-free conditions at 60 °C. Stasyuk et al., 2012 [23], reported the synthesis (SR-1c) of IZP from ketones via the in situ production of α-iodoketone. A library of compounds was synthesized via Ortoleva-King reaction followed by ring closure. The authors then studied the optoelectronic properties of these compounds. IZP with 2-hydroxyphenyl at position 2 established an excited-state intramolecular proton transfer (ESIPT), displaying strong emission bands in the blue region.
Further, α-diazoketone is just as crucial as α-haloketone for synthesizing IZPs (SR-1d), as reported by Yadav et al., 2007 [24]. IZPs were obtained with good selectivity and yields from the 2-aminopyridines and aliphatic/aromatic α-diazoketone in the presence of Cu(OTf)2. This reaction mechanism involved imine formation and nitrogen insertion as critical synthetic steps. Xie et al., 2004 [25], synthesized IZPs from the reaction of α-tosyloxyketones with 2-aminopyridines in the presence of an ionic liquid, i.e., BPyBF4 (SR-1e). In general, the use of organic solvents as a reaction medium requires much time and controlled temperature. To avoid these limitations, ionic solvents have been applied.
Ueno and Tang, 2004 [26], synthesized IZPs from alcohol and ketone via the in situ generation of α-sulfonyloxyketones (oxidative conversion) in the presence of macroporous polystyrene sulfonic acid and (diacetoxyiodo)benzene in the first step. The second step was carried out with 2-aminopyridine in the presence of potassium carbonate and acetonitrile (SR-1f). Liu et al., 2004 [27], developed a robust and simple synthetic method for the synthesis of IZPs from alkynyl(phenyl)iodonium salts with 2-aminopyridine in the presence of potassium carbonate and chloroform via [3][3][3,3]-sigmatropic rearrangement followed by intramolecular cyclization (SR-1g). In addition, Wu et al., 2011 [28], synthesized 3-arylIZPs with 1-bromo-2-pheylacetylenes/1,1-dibromo-2-phenylethenes and 2-aminopyridines in the presence of catalyst-free cascade reaction (SR-1h) and utilized only sodium bicarbonate as a base for the transformation. Yu et al., 2014 [29], reported a facile one-pot synthesis of N-(imidazo [1,2-a]pyridin-3-yl)sulfonamides using 2-aminopyridines, sulfonamides, and arylglyoxal hydrates in the presence of zinc chloride (SR-1i).
Tandem reactions have also been employed for the synthesis of IZPs. Nair et al., 2021 [30], synthesized IZPs from a 2-aminopyridine reaction with Morita-Baylis-Hillman (MBH) acetates of nitroalkenes in methanol. This reaction involved Michael’s addition of 2-aminopyridine with MBH acetates (SR-2a & 2b). Further, Yan et al., 2012 [31], synthesized 3-methyl-2-arylimidazo [1,2-a]pyridine derivatives via Fe(II)-catalyzed tandem coupling of 2-methylnitroolefins and 2-aminopyridines (SR-2c). Santra et al., 2013 [32], also developed a facile synthesis method for 3-unsubstituted IZPs from nitroolefins and 2-aminopyridine in the presence of ferric chloride (SR-2d).
Nevertheless, employing a multi-component strategy has also been found to be effective for synthesizing IZP and its derivatives. Yan et al., 2014 [33], described a one-pot, three-component approach for synthesizing IZPs using aldehyde, nitroalkane, and 2-aminopyridine in the presence of iron as a catalyst (SR-3a). Further, Schwerkoske et al., 2005 [34], used a three-component approach for IZP via the reaction of trimethylsilyl cyanide, aldehyde, and 2-aminopyridine in the presence of scandium triflate under microwave irradiation (SR-3b). DiMauro et al., 2007 [35], developed a multi-component microwave-assisted one-pot cyclization/Suzuki coupling reaction to synthesize 2,6-disubstituted-3-amino-IZPs from isonitriles, aldehydes, bromo derivatives, and 2-aminopyridines (SR-3c).
Another study by Adib et al., 2008 [36], established an efficient multi-component synthesis method for 3-amino-2-aryl IZPs from imidazolidine-2,4,5-trione, 2-aminopyridines and benzaldehydes under solvent-free conditions (SR-3d). Shao et al., 2011 [37], reported a one-pot reaction of β-lactam carbenes with 2-pyridyl isonitriles followed by acidic hydrolysis in 1,4-dioxane, resulting in 2-carbonyl-3(pyridylamino)imidazo-[1,2-a]pyridines with good quality and high yield (SR-3e).
In addition, Khan et al., 2012 [38], proposed the Ugi reaction for the synthesis of IZPs, employing aromatic aldehyde, aromatic amidine, and isocyanide in the presence of bromodimethylsulfonium bromide as a catalyst at room temperature (SR-3f). Ramesha et al., 2013 [39], developed an efficient synthesis method of IZPs from a variety of alcohols using polyphosphonic anhydride (SR-3g) as a catalyst. Another copper-catalyzed multi-component approach was followed by Chernyak et al., 2010 [40], for synthesizing IZP from 2-aminopyridines, aldehydes, and terminal alkynes (SR-3h).
An oxidative C-H functionalization strategy was also utilized for the synthesis of IZPs from N-(alkylidene)-4H-1,2,4-triazole-4-amines and pyridines in the presence of a copper catalyst in DMF, as reported by Yu et al., 2013 (SR-4a) [41]. A later study by Huang et al., 2013 [42], synthesized IZPs through the oxidative cyclization of pyridines with ketone oxime esters in the presence of copper-iodide as a catalyst (SR-4b). Another methodology for the generation of 3-aroyl IZPs through oxidative coupling between 2-aminopyridines and chalcones in the presence of copper as catalyst (SR-4c) was reported by Monir et al., 2014 [43].
The literature also proposes the synthesis of fused IZPs through intramolecular aromatic C-H amination. Wang et al., 2010 [44], synthesized pyrido [1,2-a]benzimidazole in the presence of copper and iron catalysts through direct intramolecular aromatic C-H amination (SR-5a). A study by Masters et al., 2011 [45], described the facile synthesis of pyrido [1,2-a]benzimidazole via copper-catalyzed amination (SR-5b).
Aminooxygenation and hydroamination are the other two effective strategies for the synthesis of IZPs. Wang et al., 2011 [46], developed an elegant intramolecular dehydrogenative aminooxygenation reaction for the synthesis of IZPs comprising a formyl group from the acyclic precursors in the presence of copper in DMF (SR-6a). Chioua et al., 2013 [47] developed a regioselective synthesis technique for 3-methyl IZPs through silver-mediated cycloisomerization of N-(prop-2-yn-1-yl)pyridine-2-amines using acetonitrile as a solvent (SR-6b).
Another strategy, i.e., oxidative coupling, was proposed by Donohoe et al., 2012 (SR-7a) [48] for the synthesis of IZPs through the formation of in situ α-iodoketones which react with available alkenes and 2-aminopyridine. Further, Zeng et al., 2012 [49], reported a facile and robust synthesis method for IZPs through an oxidative coupling reaction of alkyne with aminopyridine in the presence of copper/iron catalysts (SR-7b). A study by Gao et al., 2013 [50], described a one-pot oxidative coupling methodology for the generation of 2-haloimidazo [1,2-a]pyridines from 2-aminopyridines and haloalkynes in the presence of copper triflate (SR-7c).
The oxidative coupling reaction uses 2-aminopyridine and nitroalkenes, which are good Michael acceptors, for the synthesis of 3-unsubstituted IZPs in the presence of Lewis acid. Yan et al., 2014 [51], developed a modified metal-free strategy employing TBHP as the oxidant and a TBAI catalyst in DMF (SR-7d). Bagdi et al., 2013 [52], established the synthesis of IZP and its derivatives using 2-aminopyridine and aryl ketones through C-H functionalization in the presence of a copper catalyst (SR-7e). At the same time, Chandra Mohan et al., 2013 [53], developed a synthesis method for IZP employing a CuI catalyst in a DMF solvent (SR-7f). Later, Cai et al., 2013 [54], demonstrated the synthesis of heteroaromatic IZPs in the presence of a copper iodide or boron trifluoride etherate catalyst (SR-7g). Another methodology was developed by Zhang et al., 2013 [55], for the synthesis of functionalized IZPs from aliphatic, aromatic, or unsaturated ketones in the presence of an In(OTf)3 catalyst (SR-7h).
In addition to these synthetic strategies, eco-friendly and straightforward procedures, like photochemical methods, have been applied for the synthesis of IZPs to overcome challenges like environmental issues and dependence upon non-renewable sources. Photochemical methods offer many advantages over traditional heating strategies, notably using visible light and benign organic catalysts and solvents. Photocatalytic reactions have been applied with metallic or metal-free catalysts for the synthesis and functionalization of IZPs. Tran et al., 2022, reviewed the literature on recent advancements in the photochemical synthesis of IZPs [56].
3. Antibacterial Profile of Imidazopyridines
IZP is one of the most important scaffolds amongst various fused heterocyclic systems. It possess a broad range of pharmacological activities. Although significant research has been carried out against bacterial infections, only one drug, rifaximin, is available as an antibiotic on the market. There is considerable evidence to support the antibacterial activity of IZP. This may pave the way for the development of antibiotics to overcome resistance. An evaluation of the antibacterial qualities of IZPs suggested the involvement of various pathways in their mechanism of action. IZPs can target several enzymes associated with the synthesis of cell wall/peptidoglycan, protein, folic acid, DNA, or RNA, to eradicate infections. Investigations have exemplified various substituents on IZP rings which afford significant antibacterial activity. Once again, IZP is a multitargeted scaffold.
