Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Lindsay Dong and Version 1 by Arunkumar Palaniappan.
The techniques involved in culturing cells are critical as results are based on cellular response to drugs, cellular cues, external stimuli, and human physiology. In order to establish in vitro cultures, cells are either isolated from normal or diseased tissue and allowed to grow in two or three dimensions. Two-dimensional (2D) cell culture methods involve the proliferation of cells on flat rigid surfaces resulting in a monolayer culture, while in three-dimensional (3D) cell cultures, the additional dimension provides a more accurate representation of the tissue milieu.
- in vitro models
- 2D and 3D cell cultures
- 3D tissue models
- 3D bioprinting
1. Introduction
In vitro two-dimensional (2D) cell culture methods are a widely used tool for understanding biological functions such as cellular interaction, mechanisms of disease initiation and progression, production of proteins, cellular biology, and, more recently, the development of engineered tissue mimics. In a 2D environment, cells are grown as a monolayer over a flat plastic surface, where they adhere and spread. However, the simplicity of this model makes the depiction and simulation of complex tissue structures challenging. Two-dimensional monolayer cultures have been used for decades to study the cellular responses to biochemical and biophysical cues. These systems do not always mimic human physiological conditions despite providing significant advancements in the understanding of cellular behavior [1], thereby resulting in non-predictive results.
In recent years, the paradigm has shifted towards three-dimensional (3D) cell cultures. Increasing research-based evidence suggests that 3D tissue models are a better option for mimicking complex tissue or organ architecture (cell–cell and cell–matrix interactions) and physiology [2]. These models are gaining importance from basic research to advanced application-based research such as drug testing/screening and other translational purposes. In human tissue, cells are encapsulated within extracellular matrix (ECM) proteins in a 3D environment [3]. The ECM function under defined biophysical and biochemical signals, which regulate cellular functions such as proliferation, adhesion, migration, differentiation, and morphogenesis and maintain homeostasis [4]. Hence, different 3D models are evolving with the combination of cells and proteins to recapitulate native organs and the cellular microenvironment. This aids in understanding the various organs and tissue functions under a controlled laboratory setting and offer the possibility to generate organ-specific and personalized drug testing platforms [5].
The bioengineering and designing of complex biomimetic tissue for model systems involve considering several design characteristics and parameters. A 3D tissue model system can be generated through the fabrication of spheroids and organoids; however, while being able to provide a 3D microenvironment, a critical challenge with these systems is the lack of vasculature, which is essential in providing oxygen and nutrients while removing metabolic waste from cells. Alternatively, a scaffold that mimics the ECM is generated via techniques such as 3D bioprinting, electrospinning, and solvent casting/particulate leaching (SCPL) to create porous structures that house the cells, growth factors, vasculature, and transcription factors. The choice of biomaterial to generate the ECM is critical. There are a variety of natural and synthetic biomaterials available, with each having its own benefits and limitations. There has been an increased interest in the combination of biomaterials to generate hybrid biomaterials, which enhance the structural and biological properties of biomaterials.
2. Types of 3D Tissue Models
2.1. Anchorage Independent (Non-Scaffold Based) 3D Tissue Models
2.1.1. Spheroids
Spheroids are perfectly spherical cellular aggregates in suspension generated from primary cell types and cell lines. The term was coined in 1970 by Sutherland et al. when the group dissociated Chinese Hamster V79 lung cells which formed spherical aggregates [6]. There are various techniques involved in the fabrication of spheroid, including the hanging drop technique, microwell hanging drop technique, liquid overlay technique, microwell array from micropatterned agarose wells, rotating wall vessel, and magnetic levitation (Figure 21A(i–vi)) [7][8]. Microfluidic technology and 3D bioprinting have also been utilized in the generation of spheroids [9][10][11]. The most common applications of spheroids are in creating tumor models, stem cell research, tissue engineering, and transplantation therapy. The key advantages of using this method as a 3D tissue model are that it facilitates cell–cell and cell–matrix interactions providing a physiochemical environment similar to in vivo while maintaining intrinsic phenotypic properties and improving the viability and proliferation of cells [8].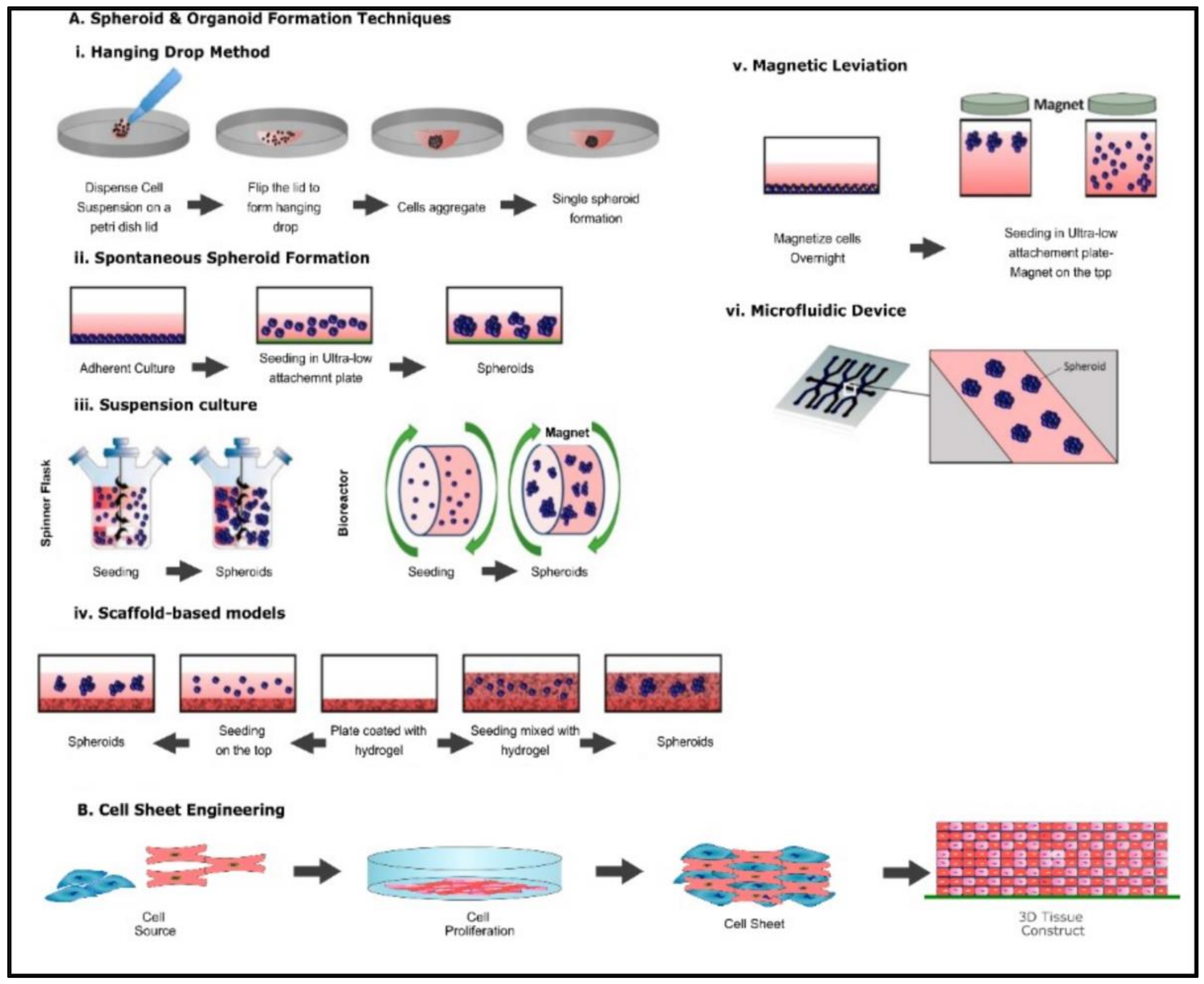
Figure 2. Common fabrication techniques used for the creation of spheroids, organoids, and cell sheet (A) (i) Hanging drop method (ii) Spontaneous spheroid formation (iii) suspension culture (iv) ECM method (v) Magnetic levitation method, (vi) Microfluidic device method. Altered and reproduced with permission from [12] under Open Access CC BY 4.0. MDPI (B) Schematic representation of cell sheet engineering.
2.1.2. Organoids
Organoids are 3D self-aggregating assemblies containing multiple cell types arranged spatially, such as cells in a tissue, recapitulating cellular and molecular stages in early organ development [13][14]. They have been used as tissue models to explore mechanisms of organ development. Organoids are increasingly being used in medical research, specifically in preclinical studies and in 3D tissue models, to study cellular interactions and drug-toxicology, pharmacology, and microbiology [13]. The 3D architectural and functional similarities to the tissue of origin make organoids an excellent model for studying complex cell–cell interactions and tissue development. The fabrication of organoid models is similar to the processes involved in the generation of spheroids (Figure 21A(i–vi)) [12][15]. However, the key difference is that in organoid formation, pluripotent stem cells and embryonic stem cells are given specific signaling cues that act as instructions to form 3D organoids of a variety of tissues [15]. Organoids have been employed in the generation of optical cups, liver, brain, lung, and heart [16][17][18][19][20].2.1.3. Cell Sheet Engineering
Cell sheet engineering is a form of tissue engineering methodology that does not require a scaffold. In this method, cells are grown in vitro by placing a single-type cell on a stimuli-sensitive polymer (Figure 21B). In a culture environment suitable for cell growth, cells are grown till a three-dimensional cell sheet is generated. By inducing a stimulus such as heat, the polymer becomes hydrophilic, enabling the detachment of the cell sheet from the polymer base [21]. Cell sheet engineering has applications among various organs such as the heart, cornea, bladder, liver, and bone. The key advantage of using cell sheet engineering is the ability to co-culture cells and generate a vasculature network. For example, Sakaguchi et al. observed that endothelial cells within cell sheets spontaneously form blood vessel networks as in vivo capillaries [22].2.2. Anchorage Dependent (Scaffold Based) 3D Tissue Models
3D tissue models offer the versatility of generating mini-organs that mimic in vivo physiology of a specific tissue. However, these models do not completely recapitulate the characteristics of the tissue. Spheroids and organoids have major drawbacks, such as poor mechanical strength and closed 3D geometry. This results in decreased oxygen and nutrients delivery to the center and hampers the use of conventional assays and instrumentation for screening studies such as nutrient and oxygen transport, absorption kinetics of drugs, and cell–cell interactions [23][24]. The paradigm of tissue engineering involves the conglomeration of living cells within bioartificial support to generate a 3D living structure with mechanical, structural, and functional properties equivalent to human tissue [25]. While the generation of artificial constructs is primarily for regenerative purposes, artificial tissues are being developed to replace reliance on animal models, which are dissimilar to human physiology and do not provide accurate predictions for human tissue responses. The conventional methods, from the perspective of tissue engineering for regenerative purposes, rely on the generation of support structures that act as a temporary scaffold to aid tissue regeneration while gradually degrading and being replaced by autologous tissues [26]. However, from the perspective of modeling, tissue replication should be designed to recapitulate the specific conditions being mimicked. This process is extremely complex due to several factors involved in the mimicking of tissue. Specifically, each tissue exhibits varying features such as porosity, ECM composition, cell phenotypes, and signaling pathways [27]. Ergo, the fundamental elements to consider in the designing of artificial tissue are the material for scaffolds, the cell source, the chemical stimuli, and the method for generating the correct tissue architecture.2.2.1. Solvent Casting Particulate Leaching (SCPL)
SCPL is a popular technique used in the fabrication of highly porous polymer scaffolds for hard tissues such as bone and teeth. In this method, a salt that is insoluble in the polymer is admixed in a polymer solution followed by an evaporation process to remove the solvent, resulting in a salt-polymer composite. The composite matrix is then submerged in water to leach out the salt resulting in a highly porous structure [28]. Through this method, 50–90% porosity is achieved [29]. A key advantage of this method is the relative ease and low cost associated with the fabrication of highly porous and tunable pore size that enables the migration of cells within the scaffolds [30]. Similar processes that are employed in the generation of highly porous structures are freeze-drying [31][32], thermal-induced phase separation (TIPS) [33], and gas foaming [34].2.2.2. Electrospinning
The term is derived from electrostatic spinning and is a method that utilizes a high-voltage electric field to draw charged threads of ultrafine nanometric scale fibers from polymer melts or solutions [35][36]. The technique is complicated and involves a process where a charged droplet of polymer in a liquid phase under high voltage results in an electrostatic repulsion counteracting surface tension and elongation of the droplet to a critical point of liquid stream eruption termed a Taylor cone [36].2.2.3. Bioprinting
3D bioprinting is the layer-by-layer deposition of cell-laden biomaterials in 3D space based on a predetermined geometry. Complex geometries and shapes are designed through computer-aided design (CAD) software or geometries extracted from medical images. The main modalities of 3D bioprinting are based on the delivery system of the cell-laden biomaterials termed bio-inks and include extrusion-based (extrusion can be achieved via pneumatic, piston, or screw), inkjet (thermal or piezoelectric), and laser-assisted [37]. Three-dimensional bioprinting is a rapidly evolving technology employed to print a variety of tissue structures of various organs, and the frontier of 3D bioprinting is the printing of a complete artificial whole organ, which was most recently achieved by Mirdamadi et al. [38] using a novel technique termed Freeform Reversible Embedding of Suspended Hydrogels (FRESH). The scholars modified an extrusion-based bioprinter and embedded alginate in a support bath comprised of gelatin microparticles suspended in a calcium chloride solution [38]. The core principle is that the gelatin microparticles act as a support bath with multiple crosslinking strategies to gel the different types of hydrogels while providing support for embedded hydrogels that would normally collapse in conventional additive manufacturing processes as they are being printed [39].2.2.4. Organ-on-a-Chip
The process of developing novel drugs and medical interventions requires the use of in vitro modeling, followed by animal studies, to test the safety and efficacy of newly developed drugs before testing on humans. However, animal models do not provide accurate predictions for human responses. Clinical trials are time-consuming and not cost-effective in the long run. Most novel drugs fail in clinical trials, and therefore, there is a need to develop a system or model that mimics human physiology, remains cost-efficient, and has the capability to provide accurate data. In contrast to biological approaches to generate 3D tissue models, organ-on-a-chip (OOC) systems are used to recapitulate tissue and organ structure by leveraging microfluidic physics along with microfabrication engineering techniques and biomaterials to create micro-physiological systems that model tissue structure and disease conditions. Research into the development of microfluidic channels to study signal pathways, drug responses, and tissue functions is ongoing [40].3. Biomaterials for 3D Tissue Modelling
Advances in research have led to the development of improved 3D tissue models for in vitro studies. Cells in nature reside in a molecular matrix composed of protein, glycosaminoglycan, and glycoconjugate, termed the extracellular matrix (ECM). The ECM provides physical scaffolding, biochemical cues, and mechanical stability to cells and is necessary for morphogenesis and homeostasis [41]. The engineering of ECM that mimics native tissue matrix begins with the identification of a biomaterial that is critical in the formation of a scaffold. The choice of biomaterial is dependent on the tissue being modeled. Biomaterials are based on three categories (a) Polymers, (b) metallic, and (c) ceramics. Factors that influence the choice of materials are the type of tissue being mimicked, structural integrity, adequate mechanical environment, bioactivity, biocompatibility, and biodegradability [41]. The biomaterial should provide structural support for cellular attachment, growth, proliferation, and migration while consisting of adequate mechanical properties and an environment native tissue matrix provide to cells. Materials should be bioactive and biocompatible to provide bioactive cues and growth factors while reducing the risk of immunological response in the presence of an artificial scaffold. Additionally, the scaffold or matrix should act as a support structure facilitating correct localization and retention at the site of tissue damage [42]. While biodegradability is key for the formation of the vascular network and allows for patients’ own ECM to replace the scaffold and degrade over time without any cytotoxic effects [43], this factor is organ-specific. For example, in regenerative medicine for hard tissues such as bone or teeth, materials are engineered from metallic or ceramic biomaterials to reduce the rate of biodegradability. Table 31 provides a summary of the various biomaterials and their pros and cons.Table 31.
List of Biomaterials, both natural and synthetic employed in tissue engineering and their advantages and disadvantages.
| Biomaterial | Type | Pros | Cons | Ref | ||
|---|---|---|---|---|---|---|
| Collagen | Natural | High biocompatibility, biodegradable, high cell adhesion, and cell remodeling. Has high printability, is biocompatible, low immunogenicity | Poor mechanical properties, unpredictable degradation in vivo, high thrombogenic potential | [44] | ||
| Gelatin | Natural | Cheap, biocompatible, easy to modify, good proliferation, biodegradable | Brittleness, low mechanical properties, fast degradation | [45] | ||
| Chitosan | Natural | Biocompatible, biodegradable, high cell proliferation | Lower mechanical properties, immunogenic | [46] | ||
| Fibrin | Natural | High cell adhesion and viability, quick gelation and good cell migration, and vascularization | low printability, biocompatibility, low mechanical strength | [47] | ||
| Hyaluronic Acid | Natural | Biocompatible, biodegradable, high cell proliferation and viability, high printability | low mechanical strength | [48] | ||
| Alginate | Natural | Biocompatible, biodegradable, sustained release, adoptable mechanical strength with cell growth, rapid gelation | low cell adhesion | [49] | ||
| Pectin | Natural | Cheap, biocompatible, can be modified, plant derived, good cell proliferation, biodegradable | Poor mechanical properties, Slower gelation time | [50] | ||
| Decellularized | ECM | Natural | Keeps vasculature network intact | Variation caused by different decellularization methods, | [51] | |
| Starch | Natural | Cheap, biocompatible, versatile rheology, | Poor mechanical properties, slower gelation time, needs high temperature (70–90 °C) to gelatinize, at higher temperatures, phase separation between composite materials may occur | [52] | ||
| Fucoidan | Natural | Good bioactive properties, biocompatible, biodegradable, used to enhance properties of other natural biomaterials | Does not gel on its own, crosslinking strategies need to be optimized, high synthesis cost | [53] | ||
| Silk Fibroin | Natural | Biocompatible, good mechanical properties | High cost of production, | [54] | ||
| Hydroxyapatite | Natural/Synthetic Synthesis | Bioactive, biocompatible, hydrophilic, | brittleness, low tensile strength and fracture toughness | [55][56] | ||
| Polycaprolactone (PCL) | Synthetic | Moderate mechanical strength. Biocompatible | Slow degradation, lower cell adhesion/aggregation, hydrophobic, inflammation due to acid degradation products | [57] | ||
| Poly Lactic-co-Glycolic Acid (PLGA) | Synthetic | Biocompatible, biodegradable, immunogenic | Brittle and relatively hard, lower cell adhesion/aggregation, inflammation due to acid degradation products | [58] | ||
| Poly(itaconate-co-citrate-cooctanediol) (PICO) | Synthetic | Biocompatible, biodegradable, cheap, good mechanical properties, fast crosslinking, non-cytotoxic to cells | UV cross linking | [59] | ||
| Poly (ethylene glycol) | (PEG) | Synthetic | Biocompatible, biodegradable, can be modified with various functional groups | Moderate mechanical strength, low printability, difficulty in scalability, Lower cell adhesion | [49] | |
| Polyphosphazenes | Synthetic | Biocompatible, good mechanical properties, slow degradation (hard tissues) | Slow degradation (soft tissues) | [54][60] | ||
| Polyurethanes | Synthetic | Good mechanical properties, good rheological properties | Poor degradability, copolymerization is required | [61] | ||
| Polyanhydrides | Synthetic | Good flexibility, controllable degradation rates | Weak mechanical properties | [61] | ||
| Poly(propelene-fumarate) | Synthetic | Good processability, good ductility, biocompatibility, easily forms covalent polymer networks | Challenging to handle the material due to high viscosity, increased cytotoxicity and acute inflammation, variation in molecular weight between crosslinking agents | [62][63] | ||
| Metals | Synthetic | Biocompatible with good mechanical properties, low degradability (Tissue dependent) | Subject to oxidation, low degradability (Tissue dependent), may be cytotoxic due to release of free metal ions | [64] | ||
| Ceramics | Synthetic | Osteoinductive and osteoconductive in bioactive ceramics, low toxicity, biocompatible, angiogenetic potential, | High brittleness, weak, low bioactivity | [64] |
References
- Duval, K.; Grover, H.; Han, L.; Mou, Y.; Pegoraro, A.; Fredberg, J.; Chen, Z. Modeling physiological events in 2D vs. 3D cell culture. Physiology 2017, 32, 266–277.
- Edmondson, R.; Broglie, J.; Adcock, A.; Yang, L. Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. Assay Drug Dev. Technol. 2014, 32, 266–277.
- Langhans, S. Three-dimensional in vitro cell culture models in drug discovery and drug repositioning. Front. Pharmacol. 2018, 9, 6.
- Kusindarta, D.; Wihadmadyatami, H. The Role of Extracellular Matrix in Tissue Regeneration. In Tissue Regeneration; InTech: Singapore, 2018.
- Gibot, L. 3D tissue models to bridge the gap between cell culture and tissue in assessing electroporation. In Handbook of Electroporation; Springer: Cham, Germany, 2017.
- Sutherland, R.; McCredie, J.; Inch, W. Growth of multicell spheroids in tissue culture as a model of nodular carcinomas. J. Natl. Cancer Inst. 1971, 46, 113–120.
- Fennema, E.; Rivron, N.; Rouwkema, J.; van Blitterswijk, C.; De Boer, J. Spheroid culture as a tool for creating 3D complex tissues. Trends Biotechnol. 2013, 31, 108–115.
- Ryu, N.; Lee, S.; Park, H. Spheroid Culture System Methods and Applications for Mesenchymal Stem Cells. Cells 2019, 8, 1620.
- Lim, W.; Park, S. A microfluidic spheroid culture device with a concentration gradient generator for high-throughput screening of drug efficacy. Molecules 2018, 23, 3355.
- Utama, R.; Atapattu, L.; O’Mahony, A.; Fife, C.; Baek, J.; Allard, T.; O’Mahony, K.; Ribeiro, J.; Gaus, K.; Kavallaris, M.; et al. A 3D Bioprinter Specifically Designed for the High-Throughput Production of Matrix-Embedded Multicellular Spheroids. IScience 2020, 23, 101621.
- Moshksayan, K.; Kashaninejad, N.; Warkiani, M.; Lock, J.; Moghadas, H.; Firoozabadi, B.; Saidi, M.; Nguyen, N. Spheroids-on-a-chip: Recent advances and design considerations in microfluidic platforms for spheroid formation and culture. Sens. Actuators B Chem. 2018, 263, 151–176.
- Hoarau-Véchot, J.; Rafii, A.; Touboul, C.; Pasquier, J. Halfway between 2D and animal models: Are 3D cultures the ideal tool to study cancer-microenvironment interactions? Int. J. Mol. Sci. 2018, 19, 181.
- Davies, J. Organoids and mini-organs: Introduction, history, and potential. In Organoids and Mini-Organs; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 3–23.
- Augustyniak; Bertero, A.; Coccini, T.; Baderna, D.; Buzanska, L.; Caloni, F. Organoids are promising tools for species-specific in vitro toxicological studies. J. Appl. Toxicol. 2019, 39, 1610–1622.
- Velasco, V.; Shariati, S.; Esfandyarpour, R. Microtechnology-based methods for organoid models. Microsyst Nanoeng. 2020, 6, 1–13.
- Eiraku, M.; Takata, N.; Ishibashi, H.; Kawada, M.; Sakakura, E.; Okuda, S.; Sekiguchi, K.; Adachi, T.; Sasai, Y. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature 2011, 472, 51–56.
- Takebe, T.; Sekine, K.; Enomura, M.; Koike, H.; Kimura, M.; Ogaeri, T.; Zhang, R.; Ueno, Y.; Zheng, Y.; Koike, N.; et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature 2013, 499, 481–484.
- Lancaster, M.; Knoblich, J. Generation of cerebral organoids from human pluripotent stem cells. Nat. Protoc. 2014, 9, 2329–2340.
- Miller, A.; Dye, B.; Ferrer-Torres, D.; Hill, D.; Overeem, A.; Shea, L.; Spence, J. Generation of lung organoids from human pluripotent stem cells in vitro. Nat. Protoc. 2019, 14, 518–540.
- Richards, D.; Li, Y.; Kerr, C.; Yao, J.; Beeson, G.; Coyle, R.; Chen, X.; Jia, J.; Damon, B.; Wilson, R.; et al. Human cardiac organoids for the modelling of myocardial infarction and drug cardiotoxicity. Nat. Biomed. Eng. 2020, 4, 446–462.
- Hong, J.; Yeo, M.; Yang, G.; Kim, G. Cell-electrospinning and its application for tissue engineering. Int. J. Mol. Sci. 2019, 20, 6208.
- Townsend-Nicholson, A.; Jayasinghe, S. Cell electrospinning: A unique biotechnique for encapsulating living organisms for generating active biological microthreads/scaffolds. Biomacromolecules 2006, 7, 3364–3369.
- Lin, R.; Chang, H. Recent advances in three-dimensional multicellular spheroid culture for biomedical research. Biotechnol. J. 2008, 3, 1172–1184.
- Wilson, S.; Tocchi, A.; Holly, M.; Parks, W.; Smith, J. A small intestinal organoid model of non-invasive enteric pathogen-epithelial cell interactions. Mucosal Immunol. 2015, 8, 352–361.
- Kim, M.; Evans, D. Tissue Engineering: The Future of Stem Cells. Top. Tissue Eng. 2005, 2, 1–21.
- Eltom, A.; Zhong, G.; Muhammad, A. Scaffold Techniques and Designs in Tissue Engineering Functions and Purposes: A Review. Adv. Mater. Sci. Eng. 2019, 2019, 1–13.
- Caddeo, S.; Boffito, M.; Sartori, S. Tissue engineering approaches in the design of healthy and pathological in vitro tissue models. Front. Bioeng. Biotechnol. 2017, 5, 40.
- Sola, A.; Bertacchini, J.; D’Avella, D.; Anselmi, L.; Maraldi, T.; Marmiroli, S.; Messori, M. Development of solvent-casting particulate leaching (SCPL) polymer scaffolds as improved three-dimensional supports to mimic the bone marrow niche. Mater. Sci. Eng. C 2019, 96, 153–165.
- Li, Z.; Xie, M.B.; Li, Y.; Ma, Y.; Li, J.; Dai, F. Recent progress in tissue engineering and regenerative medicine. J. Biomater. Tissue Eng. 2016, 6, 755–766.
- Sanz-Herrera, J.; García-Aznar, J.; Doblaré, M. On scaffold designing for bone regeneration: A computational multiscale approach. Acta Biomater. 2009, 5, 219–229.
- Brougham, C.; Levingstone, T.; Shen, N.; Cooney, G.; Jockenhoevel, S.; Flanagan, T.; O’Brien, F. Freeze-Drying as a Novel Biofabrication Method for Achieving a Controlled Microarchitecture within Large, Complex Natural Biomaterial Scaffolds. Adv. Healthc. Mater. 2017, 6, 1700598.
- Anandan, D.; Stella, S.M.; Nambiraj, N.A.; Vijayalakshmi, U.; Jaiswal, A. Development of mechanically compliant 3D composite scaffolds for bone tissue engineering applications. J. Biomed. Mater. Res. Part A 2018, 106, 3267–3274.
- Martínez-Pérez, C.A.; Olivas-Armendariz, I.; Castro-Carmona, J.S.; García-Casillas, P.E. Scaffolds for Tissue Engineering Via Thermally Induced Phase Separation. In Advances in Regenerative Medicine; IntechOpen: Singapore, 2011.
- Dehghani, F.; Annabi, N. Engineering porous scaffolds using gas-based techniques. Curr. Opin. Biotechnol. 2011, 22, 661–666.
- Zhong, W. Nanofibres for Medical Textiles. In Advances in Smart Medical Textiles: Treatments and Health Monitoring; Woodhead Publishing: Southen, UK, 2016.
- Zheng, Y. Fabrication on bioinspired surfaces. In Bioinspired Design of Materials Surfaces; Elsevier: Amsterdam, The Netherlands, 2019.
- Li, J.; Chen, M.; Fan, X.; Zhou, H. Recent advances in bioprinting techniques: Approaches, applications and future prospects. J. Transl. Med. 2016, 14, 1–15.
- Mirdamadi, E.; Tashman, J.; Shiwarski, D.; Palchesko, R.; Feinberg, A. FRESH 3D bioprinting a full-size model of the human heart. ACS Biomater. Sci. Eng. 2020, 6, 6453–6459.
- Lee, A.; Hudson, A.; Shiwarski, D.; Tashman, J.; Hinton, T.; Yerneni, S.; Bliley, J.; Campbell, P.; Feinberg, A. 3D bioprinting of collagen to rebuild components of the human heart. Science 2019, 365, 482–487.
- Aziz, A.; Geng, C.; Fu, M.; Yu, X.; Qin, K.; Liu, B. The role of microfluidics for organ on chip simulations. Bioengineering 2017, 4, 39.
- Kim, Y.; Ko, H.; Kwon, I.; Shin, K. Extracellular matrix revisited: Roles in tissue engineering. Int. Neurourol. J. 2016, 20, S23–S29.
- NIH Stem Cell, NIH Stem Cell Information Home Page—Stem Cell Basics, In Stem Cell Information. Available online: https://stemcells.nih.gov/ (accessed on 31 December 2022).
- Doss, M.; Sachinidis, A. Current Challenges of iPSC-Based Disease Modeling and Therapeutic Implications. Cells 2019, 8, 403.
- Ricklefs, M.; Korossis, S.; Haverich, A.; Schilling, T. Polymeric Scaffolds for Bioartificial Cardiovascular Prostheses. In Scaffolds in Tissue Engineering—Materials, Technologies and Clinical Applications; IntechOpen: Singapore, 2017.
- Wang, X.; Ao, Q.; Tian, X.; Fan, J.; Tong, H.; Hou, W.; Bai, S. Gelatin-based hydrogels for organ 3D bioprinting. Polymers 2017, 9, 401.
- Xu, B.; Li, Y.; Deng, B.; Liu, X.; Wang, L.; Zhu, Q. Chitosan hydrogel improves mesenchymal stem cell transplant survival and cardiac function following myocardial infarction in rats. Exp. Ther. Med. 2017, 13, 588–594.
- Wang, Z.; Lee, S.; Cheng, H.; Yoo, J.; Atala, A. 3D bioprinted functional and contractile cardiac tissue constructs. Acta Biomater. 2018, 70, 48–56.
- Gaetani, R.; Doevendans, P.; Metz, C.; Alblas, J.; Messina, E.; Giacomello, A.; Sluijter, J. Cardiac tissue engineering using tissue printing technology and human cardiac progenitor cells. Biomaterials 2012, 33, 1782–1790.
- Maiullari, F.; Costantini, M.; Milan, M.; Pace, V.; Chirivì, M.; Maiullari, S.; Rainer, A.; Baci, D.; Marei, H.; Seliktar, D.; et al. A multi-cellular 3D bioprinting approach for vascularized heart tissue engineering based on HUVECs and iPSC-derived cardiomyocytes. Sci. Rep. 2018, 8, 13532.
- Mehrali, M.; Thakur, A.; Kadumudi, F.; Pierchala, M.; Cordova, J.; Shahbazi, M.; Mehrali, M.; Pennisi, C.; Orive, G.; Gaharwar, A.; et al. Pectin Methacrylate (PEMA) and Gelatin-Based Hydrogels for Cell Delivery: Converting Waste Materials into Biomaterials. ACS Appl. Mater. Interfaces 2019, 11, 12283–12297.
- Kc, P.; Hong, Y.; Zhang, G. Cardiac tissue-derived extracellular matrix scaffolds for myocardial repair: Advantages and challenges. Regen. Biomater. 2019, 6, 185–199.
- Dong, D.; Li, J.; Cui, M.; Wang, J.; Zhou, Y.; Luo, L.; Wei, Y.; Ye, L.; Sun, H.; Yao, F. In Situ “clickable” Zwitterionic Starch-Based Hydrogel for 3D Cell Encapsulation. ACS Appl. Mater. Interfaces 2016, 8, 4442–4455.
- Reys, L.; Silva, S.; Da Costa, D.S.; Oliveira, N.; Mano, J.; Reis, R.; Silva, T. Fucoidan Hydrogels Photo-Cross-Linked with Visible Radiation As Matrices for Cell Culture. ACS Biomater. Sci. Eng. 2016, 2, 1151–1161.
- Chen, Q.; Zhu, C.; Thouas, G.A. Progress and challenges in biomaterials used for bone tissue engineering: Bioactive glasses and elastomeric composites. Prog. Biomater. 2012, 1, 2.
- Family, R.; Solati-Hashjin, M.; Nik, S.; Nemati, A. Surface modification for titanium implants by hydroxyapatite nanocomposite. Casp. J. Intern. Med. 2012, 3, 460.
- Lee, H.; Byun, S.; Cho, S.; Yang, B. Past, present, and future of regeneration therapy in oral and periodontal tissue: A review. Appl. Sci. 2019, 9, 1046.
- Ho, C.; Mishra, A.; Lin, P.; Ng, S.; Yeong, W.; Kim, Y.; Yoon, Y. 3D Printed Polycaprolactone Carbon Nanotube Composite Scaffolds for Cardiac Tissue Engineering. Macromol. Biosci. 2017, 17, 1600250.
- Mironov, A.V.; Grigoryev, A.; Krotova, L.; Skaletsky, N.; Popov, V.; Sevastianov, V. 3D printing of PLGA scaffolds for tissue engineering. J. Biomed. Mater. Res. A 2017, 105, 104–109.
- Savoji, H.; Huyer, L.D.; Mohammadi, M.; Lai, B.L.; Rafatian, N.; Bannerman, D.; Shoaib, M.; Bobicki, E.; Ramachandran, A.; Radisic, M. 3D Printing of Vascular Tubes Using Bioelastomer Prepolymers by Freeform Reversible Embedding. ACS Biomater. Sci. Eng. 2020, 6, 1333–1343.
- Ulery, B.; Nair, L.; Laurencin, C. Biomedical applications of biodegradable polymers. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 832–864.
- Kunduru, K.; Basu, A.; Domb, A. Biodegradable Polymers: Medical Applications. In Encyclopedia of Polymer Science and Technology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016.
- Kasper, F.; Tanahashi, K.; Fisher, J.; Mikos, A. Synthesis of poly(propylene fumarate). Nat. Protoc. 2009, 4, 518–525.
- Kinard, L.; Kasper, F.; Mikos, A. Synthesis of oligo(Poly(ethylene glycol) fumarate). Nat. Protoc. 2012, 7, 1219–1227.
- Bahraminasab, M.; Sahari, B.; Edwards, K.; Farahmand, F.; Arumugam, M. Aseptic loosening of femoral components—Materials engineering and design considerations. Mater. Des. 2012, 44, 155–163.
More
