Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Dean Liu and Version 1 by Nadezhda Kudryasheva.
Mechanisms of low-dose responses of bacteria can be considered at the biochemical, chemical, and physico-chemical levels. In this section, we review the radiation effects on enzymatic reactions, chemical low-molecular components of these reactions, and content of reactive oxygen species, respectively.
- low-dose
- radionuclides
- radiotoxicity
- radiation hormesis
- bioassay
1. Changes in the Rates of Intracellular Enzymatic Processes under Exposure to Radionuclides
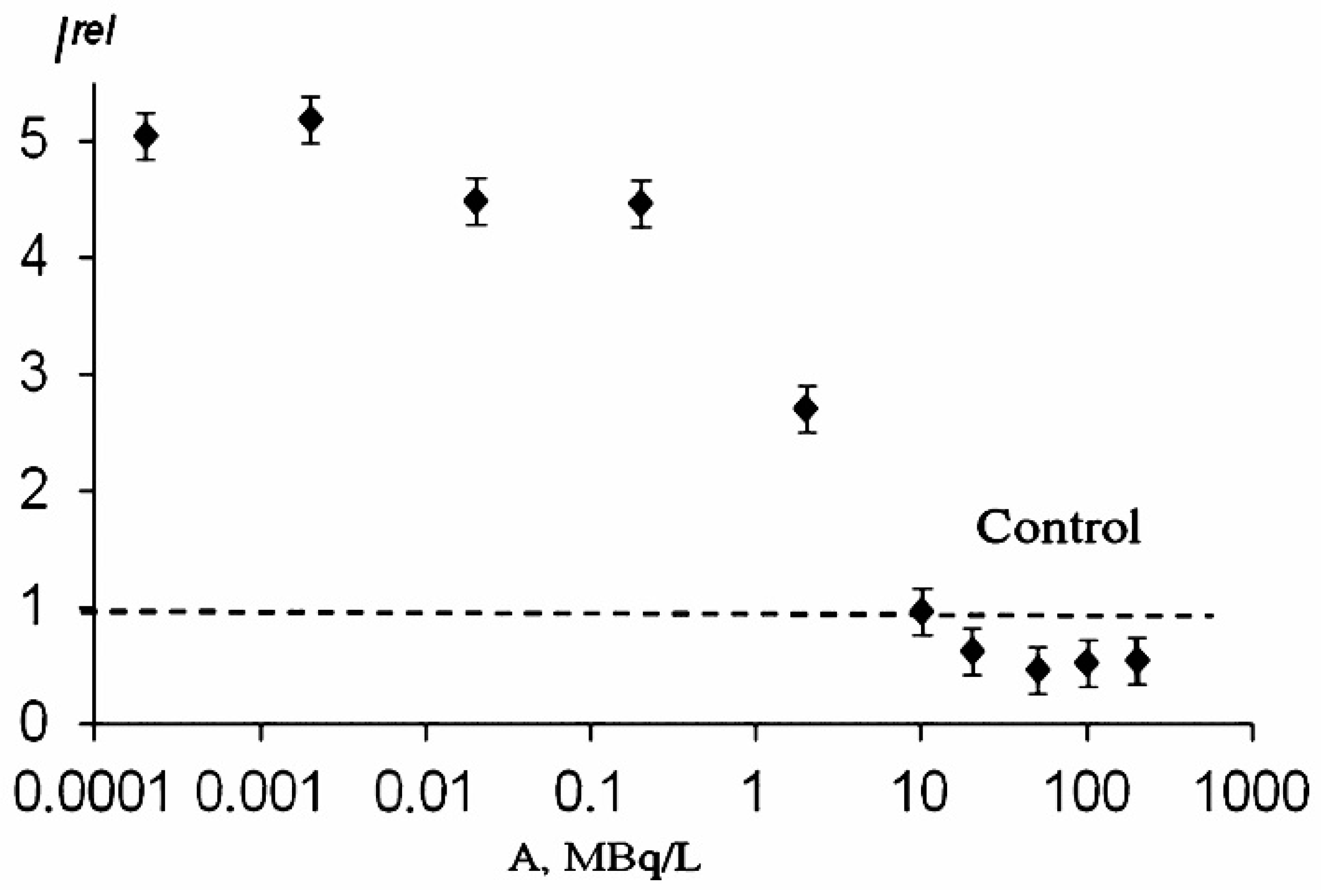
The effects of alpha- and beta-emitting radionuclides (americium-241 and tritium) on the bioluminescence system of coupled enzyme reactions catalyzed by bacterial luciferase and NADH:FMN-oxidoreductase (see in Introduction) were studied in [31,33,80][1][2][3]. Bioluminescence activation and inhibition were observed. A monotonic dependence on the concentration of tritiated water is evident from Figure 61. However, the authors [78][4] did not find similar monotonic dependence in a wide concentration range of tritiated water, and bioluminescence activation was only registered within the range of tritium radioactivity concentration of 0.005–200 Mbq/L.
Similar to the luminous bacterial cells, a low-concentration increase in the bioluminescence intensity was observed in the enzymatic system under low-concentration exposure to thorium-232 [96][6], thus revealing the hormetic phenomenon in the enzymatic assay system in thorium solutions.
2. Consumption of An Intracecllular Reducer, NADH
NADH is an organic intracellular reducer; it can be considered an indicator of the reduction activity in enzymatic and cellular systems, which is involved into complex metabolic processes in organisms.
The rates of NADH oxidation were studied in solutions of the components of the bioluminescent enzyme system: the enzyme preparation and FMN [94][7]. The data obtained in this study are shown in Table 1. The rates of NADH oxidation were determined in the presence and absence of thorium-232.
Table 1.
Rates of NADH oxidation (V) in the solutions of different composition. The wavelength of optical density registration was 340 nm. The concentration of Th(NO
3
)
4,
was 10
| Number of Solutions | Components of Solutions | V∙10 | 8 | , M | |||
|---|---|---|---|---|---|---|---|
| without Th | with Th | ||||||
| 1 | NADH | 2.43 | 4.05 | ||||
| 2 | NADH + enzyme preparation | 4.05 | 6.07 | ||||
| 3 | NADH + FMN | 14.20 | 20.60 | ||||
| 4 | NADH + FMN + enzyme preparation | 16.20 | 26.70 | ||||
An increase in the rates of NADH oxidation in the solutions exposed to thorium-232 can be found in all the samples (1–4, Table 1), with this increase being equal to 1.5–1.7. This result demonstrates that thorium-232 increases the efficiency of the reduction process involving enzymes and biologically important molecules (FMN and NADH). Hence, thorium can both (1) increase the bioluminescence intensity by accelerating the enzymatic processes and (2) decrease the bioluminescence intensity by accelerating the non-enzymatic processes and removing low-molecular components out of active enzymatic centers. The balance between these two processes depends on the peculiarities of the enzyme environment and these should be taken into consideration while explaining the activation or inhibition effects of radionuclides.
3. Active Role of Reactive Oxygen Species
Molecular mechanisms of the radionuclide bioeffects are conventionally attributed to reactive oxygen species (ROS) which are generated in water bodies in the presence of dissolved molecular oxygen [31,88,103,104][1][8][9][10]. On the other hand, ROS are native products of metabolic oxidative processes in living organisms [105,106,107][11][12][13]. It was demonstrated that luminous marine bacteria naturally increase the ROS content in aquatic media, and intensify the ROS production upon the addition of tritium [78,97][4][14].
Chemically, ROS are products of the partial reduction of oxygen; the ROS group includes hydroxyl radicals (OH•), hydrogen peroxide (H2O2), superoxide anion (O2•−), etc. [102][15].
According to modern approaches, ROS are able to produce both damaging and signal bioeffects [108,109][16][17]; they regulate vital functions, such as cellular protective or apoptosis responses [103][9]. ROS are responsible for migration, proliferation, and differentiation [110,111][18][19]; they are known as stimulators of cell division [112,113][20][21] and cell death—apoptosis, necrosis, and autophagy [114,115][22][23]. The signal function of ROS is now being discussed [112,116,117,118][20][24][25][26]. It should be noted that ROS can serve both as inter- and intra-cellular messengers [119,120,121][27][28][29]. Both reactive oxygen and nitrogen species [122][30] released by cells can serve as signal particles which initiate the radiation-induced ‘bystander effect’ [123,124][31][32]. It is stated in [125,126,127][33][34][35] that ROS are responsible for both inhibiting (toxic) and activating bioeffects. It is noted in the papers mentioned before that the lack of ROS can suppress biological functions, similarly to the excess of ROS, but only the latter is widely and conventionally stated and discussed in biomedical literature. The reason for both effects is the disturbance of the ROS balance in bacterial suspensions.
Rozhko et al. [79][36] explained the decrease in the ROS content in bacterial suspensions in tritiated water by consumption of ROS in the bacterial bioluminescence reaction followed by the formation of a reaction intermediate–peroxide flavin derivative [21,128][37][38]. An increase in the ROS content was also observed in this study, which was explained by the intensification of complex metabolic processes in bacterial cells under radioactive exposure to tritiated water, similarly to the explanation presented in [79][36]. Direct correlations between the time-dependences of the ROS content and the bacterial bioluminescence intensity were found in the studies by Rozhko [79[14][36],97], presenting the basis for the explanation of the bioluminescence activation or inhibition under exposure to the radionuclide.
The luminescent marine bacteria naturally increased the ROS content in aqueous media, and additionally increased the ROS production up to 300% in the presence of tritium [79][36]. Hašler et al. [129][39] confirmed that tritiated water can stimulate the ROS production in another type of bacteria, Pseudendoclonium basilense, a bacterial strain from standing water. The 300% activation of luminescence of marine bacteria by tritiated water was attributed to the ‘bystander effect’ [79][36]. The result was explained by the ‘trigger’ effect of tritium decay products, and by the signaling function of ROS.
The time-course of the ROS content in the bacterial suspension in the presence of the alpha-emitting radionuclide thorium-232 is presented in Figure 2 as curve 2, according to [96][6]. A moderate decrease in the ROS content (compared to the nonradioactive control sample) with a tendency to its restoration was found. Negative correlations between the ROS content and the bioluminescence intensity were found in this study, thus demonstrating inverse relations between the bacterial physiological functions and the ROS concentration in the environment. It was concluded that the consumption of ROS contributes to the bioluminescence activation under low-dose exposure to thorium-232. It is assumed that the role of ROS should be taken into consideration in studying molecular mechanisms of the ‘hormesis’ approach [53,130,131,132][40][41][42][43].
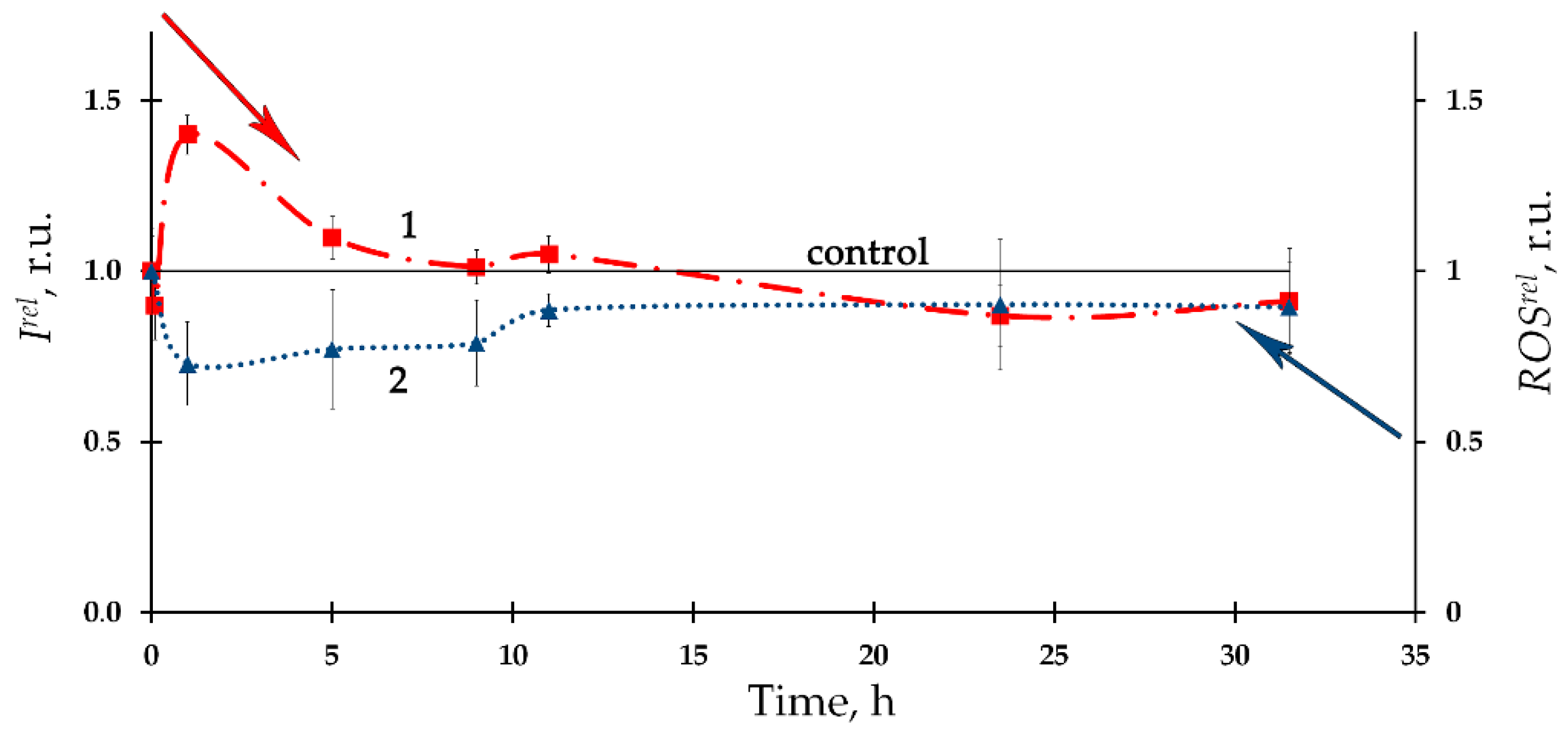
Hence, one should indicate the differences in the correlations for the effects of the alpha and beta emitting radionuclides (americium-241 and tritium, respectively): correlation coefficients between the time-dependences of the bioluminescence intensity and the ROS content differed in their sign. This fact reflects the complexity of the ROS-dependent processes occurring in the biological systems under exposure to radionuclides of different types.
4. Repair of DNA Damage
The first hypothetical mechanism of the hormesis phenomenon is based on repairing DNA damage [75,133,134][44][45][46]. The involvement of non-genetic mechanisms into low-dose chronic radioactive effects in luminous bacteria was proved earlier by Rozhko et al. [99,100][47][48] with the reference to the activation of membrane processes as a result of ionization of water media in radioactive solutions [7,135][49][50].
References
- Selivanova, M.A.; Mogilnaya, O.A.; Badun, G.A.; Vydryakova, G.A.; Kuznetsov, A.M.; Kudryasheva, N.S. Effect of Tritium on Luminous Marine Bacteria and Enzyme Reactions. J. Environ. Radioact. 2013, 120, 19–25.
- Alexandrova, M.; Rozhko, T.; Vydryakova, G.; Kudryasheva, N. Effect of Americium-241 on Luminous Bacteria. Role of Peroxides. J. Environ. Radioact. 2011, 102, 407–411.
- Selivanova, M.A.; Rozhko, T.V.; Devyatlovskaya, A.N.; Kudryasheva, N.S. Comparison of Chronic Low-Dose Effects of Alpha- and Beta-Emitting Radionuclides on Marine Bacteria. Cent. Eur. J. Biol. 2014, 9, 951–959.
- Rozhko, T.V.; Nemtseva, E.V.; Gardt, M.V.; Raikov, A.V.; Lisitsa, A.E.; Badun, G.A.; Kudryasheva, N.S. Enzymatic Responses to Low-Intensity Radiation of Tritium. Int. J. Mol. Sci. 2020, 21, 8464.
- Kudryasheva, N.S.; Kovel, E.S. Monitoring of Low-Intensity Exposures via Luminescent Bioassays of Different Complexity: Cells, Enzyme Reactions, and Fluorescent Proteins. Int. J. Mol. Sci. 2019, 20, 4451.
- Kolesnik, O.V.; Rozhko, T.V.; Lapina, M.A.; Solovyev, V.S.; Sachkova, A.S.; Kudryasheva, N.S. Development of Cellular and Enzymatic Bioluminescent Assay Systems to Study Low-Dose Effects of Thorium. Bioengineering 2021, 8, 194.
- Strumińska-Parulska, D.; Falandysz, J. A Review of the Occurrence of Alpha-Emitting Radionuclides in Wild Mushrooms. Int. J. Environ. Res. Public Health 2020, 17, 8220.
- Smith, R.W.; Wang, J.; Schültke, E.; Seymour, C.B.; Bräuer-Krisch, E.; Laissue, J.A.; Blattmann, H.; Mothersill, C.E. Proteomic Changes in the Rat Brain Induced by Homogenous Irradiation and by the Bystander Effect Resulting from High Energy Synchrotron X-Ray Microbeams. Int. J. Radiat. Biol. 2013, 89, 118–127.
- Matsumoto, H.; HamadaA, N.; Takahashi, A.; Kobayashi, Y.; Ohinishi, T. Vanguards of Paradigm Shift in Radiation Biology: Radiation-Induced Adaptive and Bystander Responses. J. Radiat. Res. 2007, 48, 97–106.
- Azzam, E.I.; Jay-Gerin, J.-P.; Pain, D. Ionizing Radiation-Induced Metabolic Oxidative Stress and Prolonged Cell Injury. Cancer Lett. 2012, 327, 48–60.
- Brynildsen, M.P.; Winkler, J.A.; Spina, C.S.; MacDonald, I.C.; Collins, J.J. Potentiating Antibacterial Activity by Predictably Enhancing Endogenous Microbial ROS Production. Nat. Biotechnol. 2013, 31, 160–165.
- Ezraty, B.; Gennaris, A.; Barras, F.; Collet, J.-F. Oxidative Stress, Protein Damage and Repair in Bacteria. Nat. Rev. Microbiol. 2017, 15, 385–396.
- Abdal Dayem, A.; Hossain, M.; Lee, S.; Kim, K.; Saha, S.; Yang, G.-M.; Choi, H.; Cho, S.-G. The Role of Reactive Oxygen Species (ROS) in the Biological Activities of Metallic Nanoparticles. Int. J. Mol. Sci. 2017, 18, 120.
- Rozhko, T.V.; Kolesnik, O.V.; Badun, G.A.; Stom, D.I.; Kudryasheva, N.S. Humic Substances Mitigate the Impact of Tritium on Luminous Marine Bacteria. Involvement of Reactive Oxygen Species. Int. J. Mol. Sci. 2020, 21, 6783.
- Ernawati; Suryadi, H.; Mun’im, A. Effect of Gamma Irradiation on the Caffeoylquinic Acid Derivatives Content, Antioxidant Activity, and Microbial Contamination of Pluchea Indica Leaves. Heliyon 2021, 7, e07825.
- Dickinson, B.C.; Chang, C.J. Chemistry and Biology of Reactive Oxygen Species in Signaling or Stress Responses. Nat. Chem. Biol. 2011, 7, 504–511.
- Zakhvataev, V.E. Stress-Induced Bystander Signaling as a Possible Factor Contributing to Neuronal Excitability and Seizure Generation/Epileptogenesis. Med. Hypotheses 2016, 90, 57–62.
- Griendling, K.K.; Touyz, R.M.; Zweier, J.L.; Dikalov, S.; Chilian, W.; Chen, Y.-R.; Harrison, D.G.; Bhatnagar, A. Measurement of Reactive Oxygen Species, Reactive Nitrogen Species, and Redox-Dependent Signaling in the Cardiovascular System. Circ. Res. 2016, 119, 39–75.
- Suzen, S.; Gurer-Orhan, H.; Saso, L. Detection of Reactive Oxygen and Nitrogen Species by Electron Paramagnetic Resonance (EPR) Technique. Molecules 2017, 22, 181.
- Proctor, P. Electron-Transfer Factors in Psychosis and Dyskinesia. Physiol. Chem. Phys. 1972, 4, 349–360.
- Nadeev, A.D.; Goncharov, N.V. Reactive Oxygen Species in the Cells of Cardiovascular System. Complex Issues Cardiovasc. Dis. 2015, 4, 80–94.
- Aprioku, J.S. Pharmacology of Free Radicals and the Impact of Reactive Oxygen Species on the Testis. J. Reprod. Infertil. 2013, 14, 158–172.
- Imlay, J.A. The Molecular Mechanisms and Physiological Consequences of Oxidative Stress: Lessons from a Model Bacterium. Nat. Rev. Microbiol. 2013, 11, 443–454.
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does It Benefit Cancer Cells? Trends Biochem. Sci. 2016, 41, 211–218.
- Moloney, J.N.; Cotter, T.G. ROS Signalling in the Biology of Cancer. Semin. Cell Dev. Biol. 2018, 80, 50–64.
- Fetoni, A.R.; Paciello, F.; Rolesi, R.; Paludetti, G.; Troiani, D. Targeting Dysregulation of Redox Homeostasis in Noise-Induced Hearing Loss: Oxidative Stress and ROS Signaling. Free Radic. Biol. Med. 2019, 135, 46–59.
- Hancock, J.T.; Desikan, R.; Neill, S.J. Role of Reactive Oxygen Species in Cell Signalling Pathways. Biochem. Soc. Trans. 2001, 29, 345.
- Kashmiri, Z.N.; Mankar, S.A. Free Radicals and Oxidative Stress in Bacteria. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 34–40.
- Srinivas, U.S.; Tan, B.W.Q.; Vellayappan, B.A.; Jeyasekharan, A.D. ROS and the DNA Damage Response in Cancer. Redox Biol. 2019, 25, 101084.
- Luzina, E.L.; Popov, A. V Synthesis and Anticancer Activity of N-Bis(Trifluoromethyl)Alkyl-N′-Thiazolyl and N-Bis(Trifluoromethyl)Alkyl-N′-Benzothiazolyl Ureas. Eur. J. Med. Chem. 2009, 44, 4944–4953.
- Jella, K.K.; Moriarty, R.; McClean, B.; Byrne, H.J.; Lyng, F.M. Reactive Oxygen Species and Nitric Oxide Signaling in Bystander Cells. PLoS ONE 2018, 13, 17.
- Sokolov, M.; Neumann, R. Changes in Gene Expression as One of the Key Mechanisms Involved in Radiation-induced Bystander Effect (Review). Biomed. Rep. 2018, 9, 99–111.
- Sushko, E.S.; Vnukova, N.G.; Churilov, G.N.; Kudryasheva, N.S. Endohedral Gd-Containing Fullerenol: Toxicity, Antioxidant Activity, and Regulation of Reactive Oxygen Species in Cellular and Enzymatic Systems. Int. J. Mol. Sci. 2022, 23, 5152.
- Kovel, E.S.; Kicheeva, A.G.; Vnukova, N.G.; Churilov, G.N.; Stepin, E.A.; Kudryasheva, N.S. Toxicity and Antioxidant Activity of Fullerenol C60,70 with Low Number of Oxygen Substituents. Int. J. Mol. Sci. 2021, 22, 6382.
- Kovel, E.; Sachkova, A.; Vnukova, N.; Churilov, G.; Knyazeva, E.; Kudryasheva, N. Antioxidant Activity and Toxicity of Fullerenols via Bioluminescence Signaling: Role of Oxygen Substituents. Int. J. Mol. Sci. 2019, 20, 2324.
- Rozhko, T.V.; Nogovitsyna, E.I.; Badun, G.A.; Lukyanchuk, A.N.; Kudryasheva, N.S. Reactive Oxygen Species and Low-Dose Effects of Tritium on Bacterial Cells. J. Environ. Radioact. 2019, 208–209, 106035.
- Nemtseva, E.V.; Kudryasheva, N.S. The Mechanism of Electronic Excitation in the Bacterial Bioluminescent Reaction. Russ. Chem. Rev. 2007, 76, 91–100.
- Wilson, T.; Hastings, J.W. Bioluminescence. Annu. Rev. Cell Dev. Biol. 1998, 14, 197–230.
- Hašler, P.; Ondřej, V.; Švécarová, M.; Sedlářová, M.; Vaidová, B.; Poulíčková, A. Tritium Influence on Morphology, Reactive Oxygen Species Production and Catalase Gene Expression in Pseudendoclonium basilense and Stigeoclonium nanum (Chlorophyta). Fottea 2017, 17, 127–135.
- Calabrese, E. Hormesis: Path and Progression to Significance. Int. J. Mol. Sci. 2018, 19, 2871.
- Ge, H.; Zhou, M.; Lv, D.; Wang, M.; Xie, D.; Yang, X.; Dong, C.; Li, S.; Lin, P. Novel Segmented Concentration Addition Method to Predict Mixture Hormesis of Chlortetracycline Hydrochloride and Oxytetracycline Hydrochloride to Aliivibrio fischeri. Int. J. Mol. Sci. 2020, 21, 481.
- Jargin, S. Hormesis and Radiation Safety Norms: Comments for an Update. Hum. Exp. Toxicol. 2018, 37, 1233–1243.
- Shibamoto, Y.; Nakamura, H. Overview of Biological, Epidemiological, and Clinical Evidence of Radiation Hormesis. Int. J. Mol. Sci. 2018, 19, 2387.
- Min, J.; Lee, C.W.; Gu, M.B. Gamma-Radiation Dose-Rate Effects on DNA Damage and Toxicity in Bacterial Cells. Radiat. Environ. Biophys. 2003, 42, 189–192.
- Burlakova, E.B.; Konradov, A.A.; Maltseva, E.L. Effects of Extremely Weak Chemical and Physical Stimuli on Biological Systems. Biophysics 2004, 49, 522–534.
- Kurvet, I.; Ivask, A.; Bondarenko, O.; Sihtmäe, M.; Kahru, A. LuxCDABE-Transformed Constitutively Bioluminescent Escherichia Coli for Toxicity Screening: Comparison with Naturally Luminous Vibrio Fischeri. Sensors 2011, 11, 7865–7878.
- Rozhko, T.V.; Badun, G.A.; Razzhivina, I.A.; Guseynov, O.A.; Guseynova, V.E.; Kudryasheva, N.S. On the Mechanism of Biological Activation by Tritium. J. Environ. Radioact. 2016, 157, 131–135.
- Rozhko, T.V.; Guseynov, O.A.; Guseynova, V.E.; Bondar, A.A.; Devyatlovskaya, A.N.; Kudryasheva, N.S. Is Bacterial Luminescence Response to Low-Dose Radiation Associated with Mutagenicity? J. Environ. Radioact. 2017, 177, 261–265.
- Belli, M.; Indovina, L. The Response of Living Organisms to Low Radiation Environment and Its Implications in Radiation Protection. Front. Public Health 2020, 8, 601711.
- Khan, A.U.H.; Blimkie, M.; Yang, D.S.; Serran, M.; Pack, T.; Wu, J.; Kang, J.Y.; Laakso, H.; Lee, S.H.; Le, Y. Effects of Chronic Low-Dose Internal Radiation on Immune-Stimulatory Responses in Mice. Int. J. Mol. Sci. 2021, 22, 7303.
More
