Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 3 by Conner Chen and Version 2 by Conner Chen.
Heavy metals are included as one of the main abiotic stresses which reduces plant development and productivity all over the world.
- heavy metal
- nutrient uptake
- sustainable agriculture
1. Introduction
Important technological progressions and modernizations have been made in current years in the field of crop growing to tackle the difficulties of sustainable manufacture and food safety [1]. Continuous agricultural innovations, such as the use of nanotechnology, are important to feed the rapidly growing world population. The term “Nanotechnology” was coined by Eric Drexler [2], and involves dealing with materials whose structures exhibit appreciably unique and enhanced chemical, physical, and biological possessions as a result of their particle size in the nanoscale [3]. Nanotechnology has great effects on the commercial implementation of nano minerals in the fields of information technology, engineering, medicine, food, and pigments, with pharmaceutical, biological, and electrical applications [4].
It is broadly understood that agricultural output needs to be increased to feed a global population which is carefully expected to be 2 billion in the next 30 years. The nanoparticles (NPs) are micro-elements that are extremely fine in nature with sizes ranging from 1–100 nm in at least two of their dimensions [5]. Garcia-Lopez et al. [6] reported that NPs play significant roles in plant growth, yield, and quality. They have existed in nature since the beginning of the Earth’s history in the form of ashes from volcanoes and woods fires, oxides of metals, and canals and oceans [7]. NPs possess unique catalytic properties [8], and remarkable progress has been noted during the past few years regarding their special features such as the high surface area to volume ratio and reactivity in comparison with bulk sized materials [9].
Potential NPs innovations have been employed in agriculture biotechnology, food safety, water and sewage treatment, biosensing, clinical diagnosis, and therapy application [10]. The input of NPs to enhance plant growth and productivity with the objective to increase quality and overall production of crops is presently being researched all over the world. NPs are of different types such as inorganic NPs, carbon-based NPs, dendrimers, and composites [11]. Carbon-based NPs include fullerenes and carbon nanotubes (single-walled and multi-walled), whereas inorganic NPs are broadly divided into metals (Au (gold), Ag (silver), and Al (aluminum), and metal oxides (ZnO, TiO2, Fe2O3, NiO, CoO, CeO2, etc.). Nanosized polymer networks built from branched units are considered dendrimers, while composite NPs are made by combining one type of NPs with another or with larger materials. NPs have been already successfully applied in agriculture and in environmental applications. Several studies have revealed that NPs could improve plant seed germination, plant photosynthesis, and resistance to oxidative stress, as well as crop yield and quality [12][13][14][15][16][17][18][19][20].
Heavy metals are included as one of the main abiotic stresses which reduces plant development and productivity all over the world (Figure 1) [21]. At present, extensive anthropogenic activities relay negative effects on plant development by accumulating heavy metals (HMs), and an increase in their concentration is immensely dangerous because HMs are toxic and mostly non-degradable in nature [22][23]. In soil, HMs get accumulated after leaching or being released after the oxidation process, making them easier to uptake by plants and ultimately affecting public health via the food chain [24].
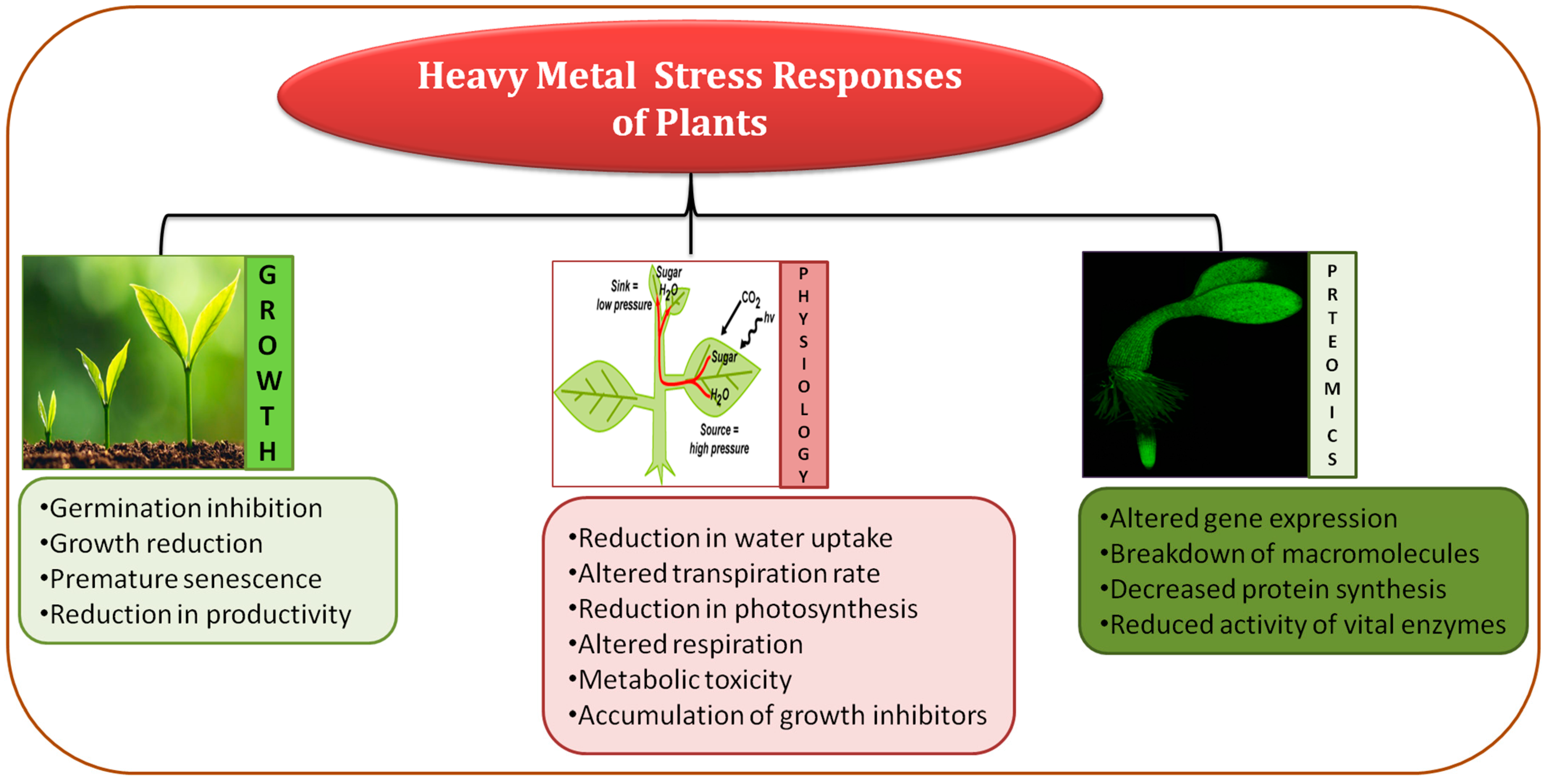
Figure 1. Diagrammatic representation of heavy metal effects on plant growth and development.
Accumulation of metals instigates direct or indirect harmful effects causing morpho-physiological abnormalities (decreased photosynthesis, plant growth, biomass, and yield), giving rise to the formation of ROS resulting in redox homeostasis disruption, damaging plasma membrane of cells, proteins, lipids, and nucleic acids, and causing disorder in metabolic activities that ultimately lead to reduced agricultural productivity [23][25][26]. Therefore, increasing plant tolerance against HM stress is a significant goal to diminish worsening food construction and to meet the order for increased food delivery for the increasing global population [27].
In order to reduce HM toxicity in plants, exogenous application of NPs has emerged as a cost-effective strategy [28], and various studies have confirmed their positive impacts on plant growth and development (Table 1). For example, in a study performed by Wang et al. [28], Fe3O4 NPs stimulated root growth of Cucumis sativus, Solanum lycopersicum L., Lactuca sativa, and Daucus carota in the presence of Cd. According to a recent study by Faizan et al. [29], the application of ZnO NPs and SA to rice plants reduced the toxicity of As. As-applied plants showed reduced plant growth, gas exchange indices, maximum quantum yield, and a reduction in protein content. As-stressed rice plants receive foliar fertigation (ZnO NPs and/or SA,) which reduces the oxidative stress as seen by lower synthesis the levels of ROS. Under the influence of ZnO NPs and salicylic acid (SA), the enzymatic activities of CAT, SOD, and POX, as well as the contents of proline and total soluble proteins, all improved, all of which play a crucial role to control various transcriptional pathways involved to tolerate oxidative stress.
NPs enhance resistance of plants against several toxic elements i.e., Cr, Cd, Fe, Al, and Mn [30][31][32]. NP application has also been known to reduce the uptake and translocation of Cd [33], Mn [34], and Pb [35] in plant tissues. Moreover, NPs play an important role to protect plants against environmental stresses by boosting antioxidant activity, accumulation of osmolytes, and synthesis of free amino acids and nutrient enhancements (Figure 2) [36]. Doubtlessly, a huge void of information regarding the valuable functions of NPs in the regulation of metal stress is yet to be filled.
2. Mechanism of Plant Growth Inhibition Induced by Heavy Metals
Among others, some HMs, i.e., Cd, Pb, Cr, As, Fe, Mn, and Al have been deemed the most effective metals. Below is a brief description of the impact of these HMs on plant growth, development, and yield.2.1. Cadmium (Cd)
Being one of the most notorious environmental pollutants, Cd causes significant adverse impacts on all organisms, affecting the health of plants and humans primarily via the food-chain through edible crops grown in Cd-polluted soil [37][38]. Geogenic (weathering of parent rocks and volcanic activity) and anthropogenic activities (irrigation with sewage, use of phosphate fertilizers and pesticides, manure application, fossil fuel combustion, mining, and smelting, as well as atmospheric precipitation) are the major Cd contributors in cultivable soils and fresh water reservoirs [39][40]. Owing to its potentially threatening demeanor (being readily absorbed, translocated, and accumulated in living cells, having a longer half-life and a non-biodegradable nature), it has escalated concerns among agriculturists and environmentalists [41]. Plants exposed to Cd toxicity have been reported to exhibit reduced biomass, photosynthesis, and productivity attributed to slower functioning at a morphological, physiological, and molecular level [42][43]. Saifullah et al. (2014) [44] elaborated that stomatal conductance and enzyme activities of plants can reduce post-Cd application. Moreover, the presence of Cd could enhance ROS generation and lead oxidative stress through enhanced H2O2 content, posing a threat to membrane integrity due to lipid peroxidation and overall damage to cellular proteins, nucleic acids, and lipids, which in extreme cases, could finally result in cell death [45].2.2. Lead (Pb)
Heavy metal pollutants such as Pb can enter the soil via several ways and induce a broad range of toxicity to living organisms [46]. This metal remains stable in soil for a long period of time, is difficult to dissociate, and with the help of food chain, it eventually accumulates in the cells of living organisms including human beings [47]. Pb impairs cell division, growth, and performance of plants as well as chlorophyll contents [48]. In particular, seed germination rate is strongly affected by Pb and may result from its interference with a plant’s protease and amylase enzymes [49]. At low concentrations, Pb potentially causes loss of cristae, mitochondrial swelling, vacuolization of endoplasmic reticulum, and bent, short, and stubby roots [50]. Photosynthetic machinery and nutrient allocation may also deteriorate after lead exposure followed by growth retardation and plant death [51].2.3. Chromium (Cr)
Cr was discovered by a French chemist Vauquelin and stands as the seventh most abundant element on the Earth’s crust [52]. The contamination of soil, sediments, and groundwater by this toxic metal may cause deleterious effects on plants and animals [53]. Cr is a non-essential element for plants; hence, they do not have any special mechanism for its uptake but due to its high solubility and oxidizing potential it becomes detrimental to their growth and development [54]. In nature, Cr is present in different oxidation states; among them trivalent (Cr3+) and hexavalent (Cr6+) are the most stable forms [55]. Cr6+ is considered to be more damaging than Cr3+ because of its association with O2 as chromate or dichromate; conversely, Cr3+ is associated with organic matter in soil [56]. Cr reduces seed germination by having effects on the activities of amylase and on the consequent sugars transport to the embryo axes; however, the activities of protease enzymes increase with its application, which may contribute to the decrease in seed germination, particularly of Cr-exposed seeds [57]. Barton et al. [58] demonstrated that the addition of Cr significantly inhibited the shoot growth in lucuma cultures. The leaf growth and size of spinach plants grown in 60 mg Cr kg−1 soil showed a marked decrease in growth rate and caused burning of leaf tips and/or margins [59]. In the study of Vajpayee et al. [60] on Vallisneria spiralis, Cr application in the nutrient medium considerably reduced biomass production and dry weight of the plant. The decrease in the dry matter is an indicator of Cr toxicity which decreases the size of the peripheral part of the Portulaca oleracea [61].2.4. Iron (Fe)
Fe is an important element for plants [62][63]. It plays a vital role as a co-factor for various enzymes that are involved in respiration, photosynthesis, chloroplast synthesis, nucleic acid synthesis, enzyme catalyzed processes, metal homeostasis, and maintaining the structural and functional integrity of proteins and chlorophyll [64][65]. Moreover, Fe assists in energy production within the plants. However, a growth medium having low pH or an excessive application of Fe can induce toxicity in most plant species [66]. Plants suffering from Fe deficiency may exhibit symptoms of interveinal chlorosis and stunted growth. Discoloration of the leaf is due to the plant crating enzymes to control free radicals that are present in elevated Fe condition. The phytotoxicity of Fe could be of two types, i.e., direct and indirect [67], where absorption of the metal and accumulation in the plants represents the direct type of Fe toxicity, while Fe plaque formation on the root surface (which affects nutrient absorption) falls under the second category [68].2.5. Manganese (Mn)
Mn is an important mineral nutrient for plants, and plays a pivotal role in several physio-biochemical processes. In crop plants, excessive Mn is disadvantageous and may significantly reduce fresh and dry biomass and photosynthetic activities, as well as cause biochemical disorders [69]. It is readily transported from root to shoot via transpiration stream; however, it is not readily remobilized thorough phloem to the other tissues or organs once it reaches the leaves [70] and its toxicity is a fairly general problem over other micronutrients. Elevated contents of Mn may result in necrotic brown patches on plants leaves and petioles, as well as on stems [71].References
- Kou, T.J.; Yu, W.W.; Lam, S.K.; Chen, D.L.; Hou, Y.P.; Li, Z.Y. Differential root responses in two cultivars of winter wheat (Triticum aestivum L.) to elevated ozone concentration under fully open-air field conditions. J. Agron. Crop Sci. 2018, 204, 325–332.
- Fortina, P.; Kricka, L.J.; Surrey, S.; Grodzinski, P. Nanotechnology, the promise and reality of new approaches to molecular recognition. Trends Biotechnol. 2005, 23, 168.
- Raje, K.; Ojha, S.; Mishra, A.; Munde, V.K.; Rawat, C.; Chaudhary, S.K. Impact of supplementation of mineral nano particles on growth performance and health status of animals, A review. J. Ento. Zool. Stud. 2018, 6, 1690–1694.
- Menazea, A.A.; Abdelghany, A.M.; Hakeem, N.A.; Osman, W.H.; Abd El-kader, F.H. Nd:YAG Nanosecond Laser Pulses for Precipitation Silver Nanoparticles in Silicate Glasses: AC Conductivity and Dielectric Studies. Silicon 2018, 12, 13–20.
- Afzal, S.; Singh, N.K. Effect of zinc and iron oxide nanoparticles on plant physiology, seed quality and microbial community structure in a rice-soil-microbial ecosystem. Environ. Pollut. 2022, 314, 120224.
- Garcia-Lopez, J.I.; Nino-Medina, G.; Olivares-Saenz, E.; Lira-Saldivar, R.; Barriga-Castro, E.D.; Vazquez-Alvardo, R.; Rodriguez-Salinas, P.A.; Zavala-Garcia, F. Foliar Application of Zinc Oxide Nanoparticles and Zinc Sulfate Boosts the Content of Bioactive Compounds in Habanero Peppers. Plants 2019, 8, 254.
- Bernhardt, E.S.; Colman, B.P.; Hochella, M.F.; Cardinale, B.J.; Nisbet, M.; Richardson, C.J.; Yin, L. An ecological perspective on nanomaterial impacts in the environment. J. Environ. Qual. 2010, 39, 1954–1965.
- Migowski, P.; Dupont, J. Catalytic applications of metal nanoparticles in imidazoliumionic liquids. Chemistry 2007, 13, 32–39.
- Afzal, S.; Aftab, T.; Singh, N.K. Impact of Zinc Oxide and Iron Oxide Nanoparticles on Uptake, Translocation, and Physiological Effects in Oryza sativa L. J. Plant Growth Regul. 2022, 41, 1445–1461.
- Cheng, Y.; Morshed, R.A.; Auffinger, B.; Tobias, A.L.; Lesniak, M.S. Multifunctional nanoparticles for brain tumor imaging and therapy. Adv. Drug Deliv. Rev. 2014, 66, 42–57.
- Srivastava, V.; Gusain, D.; Sharma, Y.C. A critical review on the toxicity of some widely engineered nanoparticles. Ind. Eng. Chem. Res. 2015, 54, 6209–6233.
- Raliya, R.; Tarafdar, J.C. ZnO nanoparticle biosynthesis and its effect on phosphorous-mobilizing enzyme secretion and gum contents in Clusterbean (Cyamopsis tetragonoloba L.). Agric. Res. 2013, 2, 48–57.
- Zafar, H.; Ali, A.; Ali, J.S.; Haq, I.U.; Zia, M. Effect of ZnO nanoparticles on Brassica nigra seedlings and stem explants growth dynamics and antioxidative response. Front. Plant Sci. 2016, 7, 535.
- Prasad, T.N.; Sudhakar, P.; Sreenivasulu, Y.; Latha, P.; Munaswamy, V.; Reddy, K.R.; Sreeprasad, T.S.; Sajanlal, P.R.; Pradeep, T. Effect of nanoscale zinc oxide particles on the germination.; growth and yield of peanut. J. Plant Nut. 2012, 35, 905–927.
- Furlani, P.R.; Clark, R.B. Screening sorghum for aluminium tolerance in nutrient solution. Agron. J. 1981, 73, 587–594.
- Lopez-Moreno, M.; de la Rosa, G.; Hernandez-Viezcas, J.; Castillo-Michel, H.; Botez, C.; Peralta-Videa, J.; Gardea-Torresdey, J. Evidence of the differential biotransformation and genotoxicity of ZnO and CeO nanoparticles on soybean (Glycine max) plants. Environ. Sci. Technol. 2010, 44, 7315–7320.
- Cao, F.; Liu, L.; Ibrahim, W.; Cai, Y. Alleviating effects of exogenous glutathione, glycinebetaine, brassinosteroids and salicylic acid on cadmium toxicity in rice seedlings (Oryza sativa). Agrotech 2013, 2, 107–112.
- Rico, C.M.; Majumdar, S.; Duarte-Gardea, M.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Interaction of nanoparticles with edible plants and their possible implications in the food chain. J. Agric. Food Chem. 2011, 59, 3485–3498.
- Djanaguiraman, M.; Belliraj, N.; Bossmann, S.H.; Prasad, P.V.V. High-temperature stress alleviation by selenium nanoparticle treatment in grain sorghum. ACS Omega 2018, 3, 2479–2491.
- Abd El-Aziz, M.E.; Morsi, S.M.M.; Salama, D.M.; Abdel-Aziz, M.S.; Abd Elwahed, M.S.; Shaaban, E.A.; Youssef, A.M. Preparation and characterization of chitosan/polyacrylicacid/copper nanocomposites and their impact on onion production. Int. J. Biol. Macromol. 2019, 123, 856–865.
- Ojuederie, O.B.; Olanrewaju, O.S.; Babalola, O.O. Plant Growth Promoting Rhizobacterial Mitigation of Drought Stress in Crop Plants, Implications for Sustainable Agriculture. Agronomy 2019, 9, 712.
- Sheng, J.J.; Wang, X.P.; Gong, P.; Tian, L.D.; Yao, T.D. Heavy metals of the Tibetan top soils Level source spatial distribution temporal variation and risk assessment. Environ. Sci. Pollut. Res. 2012, 19, 3362–3370.
- Shahid, M.; Khalid, S.; Abbas, G.; Shahid, N.; Nadeem, M.; Sabir, M. Heavy metal stress and crop productivity. In Crop Production and Global Environmental Issues; Hakeem, K.R., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 1–25.
- Hossain, M.B.; Jahiruddin, M.; Panaullah, G.M.; Loeppert, R.H.; Islam, M.R.; Duxbury, J.M. Spatial variability of arsenic concentration in soils and plants and its relationship with iron manganese and phosphorus. Environ. Pollut. 2008, 156, 739–744.
- Hassan, T.U.; Bano, A.; Naz, I. Alleviation of heavy metals toxicity by the application of plant growth promoting rhizobacteria and effects on wheat grown in saline sodic field. Int. J. Phytoremediation 2017, 19, 522–529.
- Khan, A.; Khan, A.L.; Muneer, S.; Kim, Y.H.; Al-Rawahi, A.; Al-Harrasi, A. Silicon and Salinity, Crosstalk in crop-mediated stress tolerance mechanisms. Front. Plant Sci. 2019, 10, 1429.
- Shahv, F.; Wu, W. Soil and crop management strategies to ensure higher crop productivity within sustainable environments. Sustainability 2019, 11, 1485.
- Wang, M.; Chen, L.; Chen, S.; Ma, Y. Alleviation of cadmium-induced root growth Farinhibition in crop seedlings by nanoparticles. Ecotoxicol. Environ. Saf. 2012, 79, 48–54.
- Faizan, M.; Sehar, S.; Rajput, V.D.; Faraz, A.; Afzal, S.; Minkina, T.; Sushkova, S.; Adil, M.F.; Yu, F.; Alatar, A.A.; et al. Modulation of cellular redox status and antioxidant defense system after synergistic application of zinc oxide nanoparticles and salicylic acid in rice (Oryza sativa) plant under arsenic stress. Plants 2021, 10, 2254.
- Shen, Y.; Tang, J.; Nie, Z.; Wang, Y.; Ren, Y.; Zuo, L. Preparation and application of magnetic Fe3O4 nanoparticles for wastewater purification. Sep. Purif. Technol. 2009, 68, 312–319.
- Skiba, E.; Wolf, W.M. Cerium Oxide Nanoparticles Affect Heavy Metals Uptake by Pea in a Divergent Way than Their Ionic and Bulk Counterparts. Water Air Soil Pollut. 2019, 230, 248.
- Zadeh, R.R.; Arvin, S.M.J.; Jamei, R.; Mozaffari, H.; Nejhad, F.R. Response of tomato plants to interaction effects of magnetic (Fe3O4) nanoparticles and cadmium stress. J. Plant Interact. 2019, 14, 474–481.
- Faraji, J.; Sepehri, A.; Salcedo-Reyes, J.C. Titanium dioxide nanoparticles and sodium nitroprusside alleviate the adverse effects of cadmium stress on germination and seedling growth of wheat (Triticum aestivum L.). Univ. Sci. 2018, 23, 61–87.
- Ragab, G.A.; Saad-Allah, K.M. Green synthesis of sulfur nanoparticles using Ocimum basilicum leaves and its prospective effect on manganese-stressed Helianthus annuus (L.) seedlings. Ecotoxicol. Environ. Saf. 2020, 191, 110242.
- Cai, F.; Wu, X.; Zhang, H.; Shen, X.; Zhang, M.; Chen, W.; Gao, Q.; White, J.C.; Tao, S.; Wang, X. Impact of TiO2 nanoparticles on lead uptake and bioaccumulation in rice (Oryza sativa L.). Nano Impact 2017, 5, 101–108.
- Farhangi-Abriz, S.; Torabian, S. Nano-silicon alters antioxidant activities of soybean seedlings under salt toxicity. Protoplasma 2018, 255, 953–962.
- Kabir, E.; Kumar, V.; Kim, K.H.; Yip, A.C.K.; Sohn, J.R. Environmental impacts of nanomaterials. J. Environ. Manag. 2018, 225, 261–271.
- Adil, M.F.; Sehar, S.; Chen, G.; Chen, Z.H.; Jilani, G.; Chaudhry, A.N.; Shamsi, I.H. Cadmium-zinc cross-talk delineates toxicity tolerance in rice via differential genes expression and physiological/ultrastructural adjustments. Ecotoxicol. Environ. Saf. 2020, 190, 110076.
- Ahmad, I.; Akhtar, M.J.; Zahir, Z.A.; Naveed, M.; Mitter, B.; Sessitsch, A. Cadmium-tolerant bacteria induce metal stress tolerance in cereals. Environ. Sci. Pollut. Res. Int. 2014, 21, 11054–11065.
- Adil, M.F.; Sehar, S.; Han, Z.; Lwalaba, J.L.W.; Jilani, G.; Zeng, F.; Chen, Z.H.; Shamsi, I.H. Zinc alleviates cadmium toxicity by modulating photosynthesis ROS homeostasis and ion flux kinetics in rice. Environ. Pollut. 2020, 265, 114979.
- Song, Y.; Jin, L.; Wang, X. Cadmium absorption and transportation pathways in plants. Int. J. Phytoremediation 2016, 19, 133–141.
- Baycu, G.; Gevrek-Kürüm, N.; Moustaka, J.; Csatári, I.; Rognes, S.E.; Moustakas, M. Cadmium-zinc accumulation and photosystem II responses of Noccaea caerulescens to Cd and Zn exposure. Environ. Sci. Pollut. Res. 2017, 24, 2840–2850.
- Qayyum, M.F.; Rehman, M.Z.; Ali, S.; Rizwan, M.; Naeem, A.; Maqsood, M.A.; Khalid, H.; Rinklebe, J.; Ok, Y.S. Residual effects of monoammonium phosphate.; gypsum and elemental sulfur on cadmium phytoavailability and translocation from soil to wheat in an effluent irrigated field. Chemosphere 2017, 174, 515–523.
- Saifullah, S.N.; Bibi, S.; Ahmad, M.; Ok, Y.S. Effectiveness of zinc application to minimize cadmium toxicity and accumulation in wheat (Triticum aestivum L.). Environ. Earth Sci. 2014, 71, 1663–1672.
- Vasiljeva, S.; Smirnova, G.; Basova, N.; Babarykin, D. Cadmium-Induced Oxidative Damage and Protective Action of Fractioned Red Beet (Beta vulgaris) Root Juice in Chickens. Agron. Res. 2018, 16, 1517–1526.
- Li, X.M.; Bu, N.; Li, Y.; Ma, L.; Xin, S.; Zhang, L. Growth photosynthesis and antioxidant responses of endophyte infected and non-infected rice under lead stress conditions. J. Hazard Mater. 2012, 213–214, 55–61.
- Jiao, W.; Chen, W.; Chang, A.C.; Page, A.L. Environmental risks of trace elements associated with long-term phosphate fertilizers applications, a review. Environ. Pollut. 2012, 168, 44–53.
- Maestri, E.; Marmiroli, M.; Visioli, G.; Marmiroli, N. Metal tolerance and hyperaccumulation, costs and trade-offs between traits and environment. Environ. Exp. Bot. 2010, 68, 1–13.
- Sengar, R.S.; Gautam, M.; Sengar, R.S.; Sengar, R.S.; Garg, S.K.; Sengar, K.; Chaudhary, R. Lead stress effects on physiobiochemical activities of higher plants. Rev. Environ. Contam. Toxicol. 2009, 196, 73–93.
- Jiang, W.; Liu, D. Pb-induced cellular defense system in the root meristematic cells of Allium sativum L. BMC Plant Biol. 2010, 10, 40.
- Islam, E.; Liu, D.; Li, T.; Yang, X.; Jin, X.; Mahmood, Q.; Tian, S.; Li, J. Effect of Pb toxicity on leaf growth physiology and ultrastructure in the two ecotypes of Elsholtzia argyi. J. Hazard. Mater. 2008, 154, 914–926.
- Mohan, D.; Pittman, C.U. Activated carbons and low cost adsorbents for remediation of tri-and hexavalent chromium from water. J. Hazard. Mater. 2006, 137, 762–811.
- Diwan, H.; Khan, I.; Ahmad, A.; Iqbal, M. Induction of phytochelatins and antioxidant defence system in Brassica juncea and Vigna radiata in response to chromium treatments. Plant Growth Regul. 2010, 61, 97–107.
- Shanker, A.K.; Cervantes, C.; Loza-Tavera, H.; Avudainayagam, S. Chromium toxicity in plants. Environ. Int. 2005, 31, 739–753.
- Augustynowicz, J.; Grosicki, M.; Hanus-Fajerska, E.; Lekka, M.; Waloszek, A.; Koloczek, H. Chromium (VI) bioremediation by aquatic macrophyte Callitriche cophocarpa Sendtn. Chemosphere 2010, 79, 1077–1083.
- Becquer, T.; Quantin, C.; Sicot, M.; Boudot, J.P. Chromium availability in ultramafic soils from New Caledonia. Sci. Total Environ. 2003, 301, 251–261.
- Zeid, I.M. Response of Phaseolus vulgaris to chromium and cobalt treatments. Biol. Plant 2001, 44, 111–115.
- Barton, L.L.; Johnson, G.V.; O’Nan, A.G.; Wagener, B.M. Inhibition of ferric chelate reductase in alfalfa roots by cobalt nickel chromium and copper. J. Plant Nutr. 2000, 23, 1833–1845.
- Sing, A.K. Effect of trivalent and hexavalent chromium on spinach (Spinacea oleracea L). Environ. Ecol. 2001, 19, 807–810.
- Vajpayee, P.; Rai, U.N.; Ali, M.B.; Tripathi, R.D.; Yadav, V.; Sinha, S. Chromium induced physiological changes in Vallisneria spiralis L and its role in phytoremediation of tannery effluent. Bull. Environ. Contam. Toxicol. 2001, 67, 246–256.
- Zurayk, R.; Sukkariyah, B.; Baalbaki, R. Common hydrophytes as bioindicators of nickel chromium and cadmium pollution. Water Air Soil Pollut. 2001, 127, 373–388.
- Celletti, S.; Lanz, M.; Bergamo, A.; Benedetti, V.; Basso, D.; Baratieri, M.; Cesco, S.; Mimmo, T. Evaluating the Aqueous Phase from Hydrothermal Carbonization of Cow Manure Digestate as Possible Fertilizer Solution for Plant Growth. Front. Plant Sci. 2022, 12, 1317.
- Muller, C.; Silveira, S.; Daloso, D.M.; Mendes, G.C.; Merchant, A.; Kuki, K.N.; Oliva, M.A.; Loureiro, M.E.; Almeida, A.M. Ecophysiological responses to excess iron in lowland and upland rice cultivars. Chemosphere 2017, 189, 123–133.
- Li, W.; Lan, P. The Understanding of the Plant Iron Deficiency Responses in Strategy I Plants and the Role of Ethylene in This Process by Omic Approaches. Front. Plant Sci. 2017, 8, 40.
- Bashir, K.; Rasheed, S.; Kobayashi, T.; Seki, M.; Nishizawa, N.K. Regulating Subcellular Metal Homeostasis, The Key to Crop Improvement. Front. Plant Sci. 2016, 7, 1192.
- Haider, F.U.; Liqun, C.; Coulter, J.A.; Cheema, S.A.; Wu, J.; Zhang, R.; Wenjun, M.; Farooq, M. Cadmium toxicity in plants: Impacts and remediation strategies. Ecotoxicol. Environ. Saf. 2021, 211, 111887.
- Araújo, I.P.S.; Costa, D.B.; De Moraes, R.J.B. Identification and Characterization of Particulate Matter Concentrations at Construction Jobsites. Sustainability 2014, 6, 7666–7688.
- Siqueira-Silva, A.I.; Rios, C.O.; Pereira, E.G. Iron toxicity resistance strategies in tropical grasses, The role of apoplastic radicular barriers. J. Environ. Sci. (China) 2019, 78, 257–266.
- Santos, E.F.; Kondo Santini, J.M.; Paixão, A.P.; Júnior, E.F.; Lavres, J.; Campos, M.; dos Reis, A.R. Physiological highlights of manganese toxicity symptoms in soybean plants, Mn toxicity responses. Plant Physiol. Biochem. 2017, 113, 6–19.
- Alejandro, S.; Holler, S.; Meier, B.; Peiter, E. Manganese in Plants: From Acquisition to subcellular allocation. Front. Plant Sci. 2020, 11, 300.
- Wu, S. Effect of manganese excess on the soybean plant cultivated under various growth conditions. J. Plant Nutr. 1994, 17, 991–1003.
More
