You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 1 by Ales Blinc and Version 2 by Rita Xu.
Classical risk factors play a major role in the initiation and development of atherosclerosis. Efforts have been made to identify biomarkers that indicate ongoing atherosclerosis. Among important circulating biomarkers associated with peripheral arterial disease (PAD) are inflammatory markers which are determined by the expression of different genes and epigenetic processes. Among these proinflammatory molecules, interleukin-6, C-reactive protein, several adhesion molecules, CD40 ligand, osteoprotegerin and others are associated with the presence and progression of PAD.
- peripheral arterial disease
- biomarkers
- inflammation
- prothrombotic
1. Introduction
Peripheral artery disease (PAD) is a common manifestations of atherosclerosis affecting more than 200 million people worldwide [1][2][1,2]. The presence of PAD represents a powerful and independent risk of cardiac and cerebral ischemic events [3]. It is well-established that hypertension, smoking, diabetes mellitus and hypercholesterolemia play a major role in the initiation and development of atherosclerosis and cardiovascular (CV) events [4]. However, the prognostic potency of each of these factors in atherogenesis differs in various arterial beds and estimation of risk for atherosclerotic CV events based only on classical risk factors is often insufficient [4]. In ancient times when classical risk factors were less prevalent, atherosclerosis was already widespread as evidenced by Egyptian mummies [5].
Plaque formation is influenced by the expression of different genes [4][6][7][4,6,7]. Oxidized low density lipoprotein (LDL) particles which accumulate in the intima strongly modulate inflammation-related gene expression through various signaling pathways [8]. Epigenetic processes are also involved in cytokine expression that strongly affects atherogenesis [7]. MicroRNAs (mi-RNAs) which are single-stranded, non-coding RNAs that modulate gene-expression at the posttranscriptional level, have emerged as promising novel biomarkers for different aspects PAD, including its detection, prediction of progression and revascularization outcomes [9].
Biomarkers refer to a broad category of quantifiable biological characteristics that predict CV events. Besides providing diagnostic and prognostic information, some biomarkers might also be helpful in assessing the efficacy of therapeutic interventions [10].
2. Inflammation and PAD
The role of inflammation in the atherogenic process was established as a basic pathophysiological process, which is responsible for the initiation, clinical manifestation and CV risk of atherosclerotic diseases [11]. The vascular inflammatory process is related to systemic inflammatory response and patients with preclinical or clinical atherosclerotic disease have increased circulatory inflammatory markers. Increased levels of inflammatory markers are associated with the development and progression of PAD, and the risk of developing cardiac and cerebrovascular ischemic events [12]. A number of cross-sectional and longitudinal studies demonstrated a close link between inflammation and PAD [13]. Inflammatory molecules are not simply markers of inflammation, they also play an active role in peripheral atherogenesis [14][15][14,15]. In a prospective case-control study in apparently healthy men enrolled in the Physicians Health Study (PHS) the relative risk of developing PAD increased significantly with each quartile of baseline C-reactive protein (CRP) and this increase was independent of other risk factors [16]. The PHS also showed that elevated levels of soluble intercellular adhesion molecule-1 (sICAM-1) are independently associated with the development of symptomatic PAD in men [17]. Similar findings were reported in the Women Health Study [18]. Among inflammatory biomarkers, the pro-inflammatory cytokine interleukin-6 (IL-6) was shown to be the strongest predictor of PAD and was independently associated with disease progression [13]. Circulating IL-6 levels significantly increase after exercise in patients with PAD, and higher IL-6 levels have been associated with lower functional capacity [19]. The Edinburg Artery Study also showed that interleukin-1 (IL-1) has an important predictive role in outcome of patients with PAD [20]. Interleukins, E-selectin and metalloproteinases predicted major events in patients with severe limb ischemia and allowed for the creation of a biomarker-model [21]. There are conflicting data on the role of interleukin-8 (IL-8). Some studies showed that PAD patients who were submitted to vascular surgical procedures had a higher production of IL-8 in polymorphonuclear leucocytes [22]. The anti-inflammatory interleukin-10 (IL-10), which is associated with reduced apoptosis of cells of the lipid core and thereby with the reduced risk of plaque rupture, has been related to reduced risk of developing atherosclerosis. However, the correlation between PAD and levels of IL-10 remains uncertain [23]. Several other inflammatory markers have been examined in a limited number of studies. In the Atherosclerotic Risk in Communities (ARIC) Study, monocyte chemoattractant protein-1 (MCP-1) was associated with the ankle-brachial index (ABI) [24]. Further, CD40 ligand was associated with an angiographic severity of PAD [25]. Serum osteoprotegerin was associated with a pathological ABI in a cohort study [26]. Wilson et al. reported that serum levels of of β2-microglobulin were higher in patient with PAD than in non-PAD patients and were independently associated with ABI [27].2.1. Inflammatory Markers as Mediators of Harmful Effects of Other Risk Factors for PAD
Risk factors of atherosclerosis often trigger their atherogenic effects through an inflammatory mechanism (Figure 1). Cigarette smoking and diabetes mellitus, the strongest predictors of developing PAD, promote oxidative stress, which directly and indirectly stimulates inflammatory pathways [28].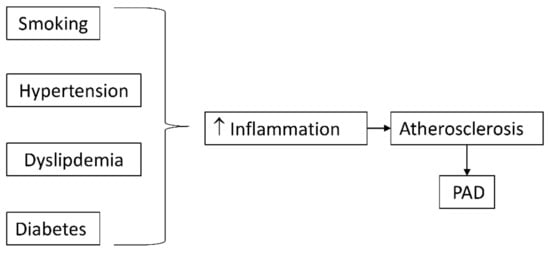
Figure 1. Classical risk factors for atherosclerosis promote inflammation which accelerates atherosclerosis and peripheral arterial disease (PAD).
