Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by jianhonng Huang and Version 2 by Catherine Yang.
Water and oxygen are the main reactants to produce Acid mine drainage (AMD), and the inhibition methods should consider controlling the availability of one or two components. Therefore, two strategies are adopted: (i) preventing oxygen from entering the tailings pile and thus reducing the rate of sulfide oxidation; and (ii) isolating the infiltration of external water and thus weakening the role of dissolved oxygen. According to different coverage principles, tailing cover systems can be divided into dry covers, wet covers, and organic covers.
- acid mine drainage
- tailing covering technology
- oxidation prevention
- sulfide tailings
1. Wet Covers
Wet covers forms a fully saturated water cover to prevent tailing oxidation caused by the advection transport and diffusion of oxygen [1][17], thus reducing the rate of tailing oxidation.
1.1. Flooded Cover
When sulfur-containing tailings are placed below the water surface, the low solubility (25 °C, 8.6 mg·L−1) and low effective diffusion coefficient (De = 2 × 10−9 m2·s−1) of oxygen in water can be utilized to create a natural anoxic environment, which can theoretically permanently inhibit the oxidation activity of tailings.
Unfortunately, the immersed sulfide tailings are not completely inert, and the oxidation reaction continued to occur at the tailing–water interface slowly. An investigation on a historical tailing pond (Savage River mine, located in northwest Tasmania, Australia) found that there was an oxidation zone of 0.05 m under the water surface of 1.5 m, and the acidic pH values were measured of the tailings pore water, and the heavy metals increased (Ni: 76–123 mg·kg−1, Zn: 45–54 mg·kg−1, Cu: 91–679 mg·kg−1) [2][22]. Under the action of wind and waves of shallow water overburden layer, turbulence and tailing re-suspension occurred at the air–water interface, which are important reasons for the oxidation of underwater tailings [3][23]. In addition, the increase in interstitial water acidity (pH, from 9.5 to 8.5) and SO42− production just under the interface also proves that the tailings gradually oxidized [4][24]. Research shows that the presence of a transitional oxidation front at approximately 0.3–0.6 m across the sub-aerial zone, with interlayer oxidation tailings containing pyrite enriched in Cu, Co and Zn, is observed. Fortunately, the low sulfur alteration intensity (SAI < 2/10) makes the combined organic and water cover effectively limiting, and the risk for AMD production was low [2][22] To tackle this problem, Mustafa et al. [5][25] weakened the influence of wind by optimizing the overlying depth, thus controlling the sediment re-suspension within an acceptable range.
Flooded cover is effective as an oxygen barrier, but it needs high economic expenditure, such as water supplement, dam construction, and maintenance. More importantly, it is not suitable for arid and semi-arid areas where annual evaporation is greater than precipitation, and water supplement will greatly increase the maintenance cost in the later stage [6][26]. In addition, the stability of dam is a consideration, especially in earthquake-prone areas.
1.2. Cover with Capillary Barrier Effects (CCBE)
CCBE is a typical multilayer cover mode, which consists of at least three layers of cover [7][27] (Figure 13). A capillary break layer (CBL) made of coarse-grained material is located at the bottom and serves as a support. The middle part is a moisture-retaining layer (MRL) made of fine particles, which has low saturated hydraulic conductivity (Ksat > 10−5 cm·s−1) and high water retention capacity to limit oxygen migration [8][28]. The surface layer is an evaporation protective layer (EPL) made of coarse particles, which plays a role in limiting evaporation and promoting horizontal drainage. When two kinds of particle layers with different particle sizes contact, the vertical flow between the two layers is often limited due to the difference in unsaturated hydraulic properties, resulting in a capillary barrier effect [9][10][29,30], which maintains high saturation in the layer with finer particle sizes (SR > 85%). Therefore, the substitution of various cheap materials (industrial waste, mining waste, natural materials, etc.) has become the research hotspot of CCBE.
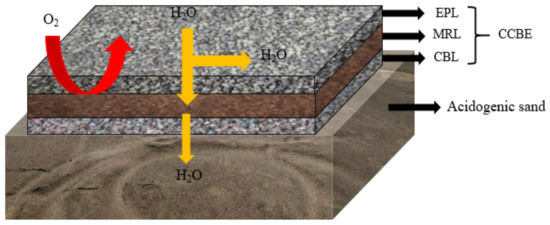
Figure 13.
Schematic diagram of cover with capillary barrier effects.
A large number of findings have confirmed the effectiveness of CCBE in limiting oxygen diffusion (about 99%) and AMD production [11][12][13][31,32,33]. Dagenais et al. [14][34] monitored the CCBE performance data for four consecutive years and found that the MRL remained saturated for many years, with oxygen flux as low as 10 g·m−2·a−1. Larochelle et al. [15][35] used acid-producing waste rock as CBL to conduct laboratory column experiments. They found that the saturation of MRL remained around 85–90%, and the pH remained close to neutral during the whole test period. A similar result was obtained by Molson et al. [13][33], wherein the O2 flux was only related to the porosity of the material when the MRL layer was close to saturation, and its thickness had relatively little influence. For example, in five pilot-scale field tests (four sites were constructed with CCBE over the tailings, a site was uncovered) of the Norebec-Manitou mine site, Canada, the finite volume model MIN3P was used to simulate the reduction of AMD discharge by using the cover with CCBE [13][33]. The monitored data after 1200 days showed that the pH remained neutral (about 6.5~7), the concentration of SO42− and Fe2+ decreased to 1700 mg·L−1 and 8 mg·L−1, respectively, and Cu2+ concentrations decreased to 0.001 mg·L−1. Overall, 1–7 orders of magnitude are reduced compared with the uncovered.
Generally, CCBE can effectively inhibit the generation of AMD. However, in the area where evaporation is greater than precipitation, it is easy to cause excessive desaturation of MRL layer and gradually lose the function as oxygen barrier. In addition, the CCBE has a disadvantage on long-term stability. According to the report of Pabst et al. [10][30], the inhibition efficiency of sulfur-containing tailings decreased significantly after being covered for 10 years. More importantly, CCBE is not being an economic option. The covering materials required for its construction are usually shipped from other locations because on-site alternatives such as non-acid-producing tailings are not readily available, which greatly increases construction costs [16][36]. Therefore, CCBE is not the optimal coverage option in terms of efficiency and economy.
1.3. Elevated Water Table (EWT)
The EWT method mainly relies on raising the groundwater level to keep the depth at the edge height of the capillary effect layer to increase the saturation of the tailings [17][18][19][37,38,39]. Hence, controlling the height of the water level is the key to the effectiveness of this technology, and it is also the main difference from flooded cover (Figure 24). In saturated or nearly saturated porous media, the effective diffusion coefficient of oxygen is very low, resulting in a lower oxygen flux that reduces the oxidation rate of sulfide minerals [20][40]. Therefore, this technology is usually coupled with a monolayer cover method. Bussière et al. [21][41] combined EWT with monolayer cover technique to significantly inhibit sulfide mineral oxidation. Related studies have confirmed this conclusion. For example, the combination of coarse-grained covering materials and EWT could increase penetration and limit water loss caused by evaporation [20][22][40,42]. The combination with fine-grained covering materials could promote moisture retention and prevent evapotranspirative demand, and no significant tailing oxidation is observed (<10−4 kg·(m2·a−1)−1) [23][24][43,44]. Some studies have shown that there is a correlation between the depth of groundwater level and the rate of oxygen consumption [25][45]. Ouangrawa et al. [17][37] demonstrated that oxygen diffusion and oxidation of sulfide minerals can be prevented by keeping the water table depth less than the air entry value (AVE) of tailings and keeping the tailings highly saturated (Sw ≥ 90%). In the simulated profiles experiment of SO42− and Fe(III), within column 1, peak SO42− and Fe(III) concentrations reached about 12,000 mg·L−1 and 2000 mg·L−1, respectively. However, as the EWT increased, within column 6, SO42− and Fe(III) concentrations were significantly lower, at 3300 mg·L−1 and 10 mg·L−1, respectively. Furthermore, the observed discharge pH became more neutral (pH~7–8) [18][38]. The decrease in SO42− and Fe(III) concentrations were limited by gypsum and ferrihydrite precipitation. Similar to flooded cover and CCBE technologies, EWT technique adopts the low effective diffusion coefficient of O2 in saturated and near-saturated media [20][26][19,40]. The transmission of O2 is the main factor limiting the oxidation of sulfide minerals.
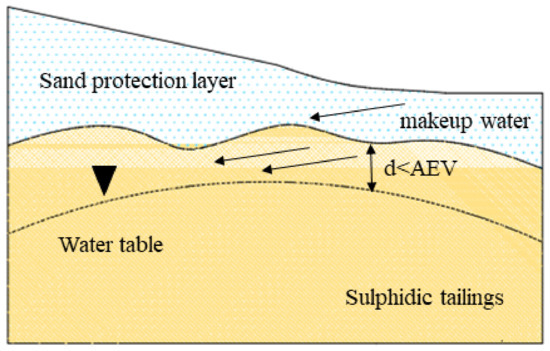
Figure 24. Schematic diagram of elevated water table coverage. (Note: the black triangle indicates the water level depth; the black dotted line indicates that the water level depth is less than the minimum value of AEV; and the black arrow indicates the flow direction of makeup water.
EWT technology is an effective auxiliary governance scheme, but its environmental and technical requirements are too high. The maintenance of high water level requires long-term funds investment, technical support, and personnel maintenance. The application of EWT technology can only be limited in humid areas with low evaporation, and artificial reconstruction will greatly increase the economic cost. This is a common problem with all wet cover techniques.
2. Dry Covers
Dry covers are low permeability preservative layers made of inorganic mineral materials. It can prevent oxygen and water from entering the tailings layer and reduce the oxidation rate of sulfide minerals [27][46]. The dry cover layer is composed of materials with different granulation characteristics, such as compacted clay, portland cement, fly ash, etc. [28][47].
2.1. Monolayer Cover
Monolayer cover is the simplest and most widely used method of tailing pond cover technology. It reduces the diffusion flux of oxygen and the accumulation of acid caused by oxidation by virtue of the low porosity and chemical properties of the surface covering material, such as limestone or phosphate minerals. In practical applications, monolayer cover usually refers to a mixture of two or more materials with different properties. In some studies, desulfurized tailings with non-acid producing activity were used as a single material covering layer, and the oxidation rate was reduced by 75~82% [29][21]. However, in the face of the huge storage of sulfide minerals, the production of AMD is still huge in sufficient oxidation time. Hence, the oxygen barrier performance of single layer covering is poor. Pabst et al. [30][48] put forward a similar view that a single material covering layer could not prevent the oxidation of sulfide minerals in the underlying tailings. TA tailings covered with CA produce a very acidic leachate (pH < 3) and, after 10 cycles, the pH was below 2.5. Meanwhile, the concentration of sulfate and iron reaches 40,000 mg·L−1 and 8000 mg·L−1, respectively. These condition inhibitions on AMD were limited. Therefore, more research tends to use a mixture of multiple materials. For example, the blending of tailings with waste rock [31][49], limestone [32][50], and neutralizing sludge [33][51] all showed better barrier performance as single-layer covering materials. Hakkou et al. [34][52] covered the surface of acid-producing tailings with 15% of alkaline phosphate waste mixed with coarse-size tailings and found that the acidity accumulation peaked at 3199 mg CaCO3·L−1 and stabilized at 280 mg CaCO3·L−1, and sulfate concentration caused by oxidation decreased significantly, from 4900 mg·L−1 to 480 mg·L−1, in a short time.
The availability of single-layer covering materials and the simplicity of technology implementation are favored by many researchers and mine managers. However, the performance of monolayer cover mainly depends on the thickness of the cover and the properties of mixed materials. Therefore, the increase in coverage thickness also means an increase in cost. In addition, the long-term stability of monolayer cover is also a concern. If the neutralization effect of surface alkalinity on the oxidation products of the lower layer is lost, the barrier effect will be greatly weakened. At the same time, the drying of the surface material can easily lead to the cracking of the covering layer. Dehydration cracks can provide preferential channels for air and water, making them lose their barrier function, providing a preferential channel for air and moisture [35][53]. Therefore, monolayer cover is more of a temporary protection measure.
2.2. Stabilization Cover
Stabilization treatment is mainly used to solidify sulfide minerals under the action of binders. The hard layer formed by solidifying can inhibit the reactivity of sulfide and the migration of harmful components, thereby eliminating the negative impacts on the receiving environment [36][55]. A commonly used binder is Portland cement. However, cement cannot be directly applied to high sulfur tailings because residual acid and sulfate salts inhibit its chemical stability [37][56]. In addition, cement is vulnerable to harmful effects caused by sulfate attack [38][39][57,58]. Therefore, as binders for stabilization cover processes, geopolymers have been recommended as a substitute [40][41][59,60]. Under the action of an alkali activator, solid silicoaluminate dissolves and releases Si and Al monomer and dimer. Then, polymerization occurs to form a hardened material with three-dimensional structure. Silicoaluminate is the main component of ore, so the reuse of tailings and waste rock has become an advantage [42][61].
Ahn et al. [37][56] used the polymer prepared by lime, tailings, and sodium silicate as the covering layer of sulfur-containing tailings. The TCLP results indicate that the leached amounts of heavy metals Cd, Fe, Mn, and Zn of GP tailings were significantly reduced by 99.3%, 92.9, 98.9, and 99.7%, respectively, with 10% S/S materials compared to the original tailings. The stability of heavy metals was attributed to the carbonate-bound phases, and sulfide minerals were surrounded by calcium silicate generated from sodium silicate, inhibiting further reaction. Ash fly is rich in SiO2 and Al2O3, which have the potential to prepare geopolymers. The research confirmed that the geopolymers prepared from fly ash have a great advantage in stabilizing Cr(NO3)3 [43][62]. In addition, the hard layer tends to be stable for a long time.
Stabilization cover is a relatively new method, which can physically weaken the water penetration and oxygen diffusion while solidifying and sequestration pollutants in a three-dimensional structure, thus improving the long-term availability of the covering layer. However, due to the complexity of the site environment of mine wasteland, such as runoff drainage with strong acidity and high sulfate concentration, the stability of cover should be seriously concerning, especially sulfate erosion and drying cracking.
2.3. Benign Material Cover
Two advantages make benign materials a competitive choice for cover layers. The first is the continuous alkaline release capacity, which reduces soluble metals and non-metals by consuming H+ to produce precipitation. The second is low permeability, which reduces the oxidation rate by physically reducing the oxygen flux. In recent years, industrial by-products and residues (such as green liquor slag, fly ash, cement kiln dust, red mud bauxite, etc.) have become common benign materials for inhibiting the generation of AMD due to their high neutralization potential [44][45][46][66,67,68]. The addition of these alkaline materials occurs during neutralizing reactions. the formation of secondary minerals (sulfate, carbonate, and hydroxide) can fix the dissolved metals through adsorption and co-precipitation [47][48][69,70]. A column experiment was filled with the mixture of pyrite and fly ash. The kinetic study confirmed that the pyrite oxidation rate was zero under alkaline pH when Fe(III) coating formed on the mineral surface [48][70]. Olds et al. [49][71] mixed granite powder and cement kiln ash at a volume of 4:1 as the overlay, effectively limiting the diffusion of oxygen with a permeability of only 10−7~10−6 m·s−1. Cement kiln ash had an alkalinity of about 650 kg CaCO3·t−1, which was dissolved and permeated slowly by rainfall to inhibit acid accumulation of the lower tailings [50][72]. The mixture of waste rock with pulp and alkaline by-products from steel mills caused trace element concentrations in the leaching solution to be less than 100 μg·L−1 and the pH to be close to neutral (pH~2) [44][66].
However, the continuous consumption of alkalinity limits the long-term performance of these kinds of materials. Industrial by-products usually contain more pollution impurities, which easily cause the release of toxic substances and increase the environmental burden. The release of heavy metals and the generation of neutralized sludge are environmental threats that need to be paid more attention to. Abreu et al. [51][73] found that, although red mud as a covering material has sufficient alkalinity for AMD generation inhibition, heavy metals accumulated at the red mud–tailing interface, causing secondary pollution along with the permeation and migration of leachate. Generally, the applications of industrial by-products have achieved remarkable results in the short-term validation, but the secondary pollution problems are inevitable, especially for the areas that need to be reclaimed after remediation, and the migration and enrichment of heavy metals should be strongly considered.
3. Organic Reactive Barriers (ORB)
The use of ORB to prevent oxygen penetration into the underlying tailings pond is an advanced technology in covering schemes as of recently. On the one hand, the physical advantages of the material itself ensure the physical barrier of external water and oxygen [52][74]. On the other hand, the metabolic reactions by internal microorganisms consume diffusive oxygen [53][9]. These two reasons cause oxygen depletion in the contact gap between the underlying sulfide minerals (Figure 37). Compared with other cover materials, organic cover materials have the advantages of low permeability, high cation exchange capacity and high alkalinity, thus limiting sulfide mineral oxidation and AMD generation [53][9]. Organic materials such as sawdust [54][75], straw, paper pulp and municipal waste compost [55][56][76,77] were used as organic cover layers, playing significant roles as oxygen barrier and acid-base modulator. However, the degradation of organic matter is rapid, and the long-term effectiveness of the covering needs a frequent supply of organic carbon-rich materials. Nason et al. [57][78] comfirmed that 20% of biological sludge was consumed within two years. At present, although the ORB cover has prominent oxygen barrier effects than single-layer cover in theory, there are more constraints in practice, such as the influence of temperature and pH on biological activity [58][79], the reduction of degradable substances on the treatment efficiency, and the impact of mine environment on microbial proliferation and variation.
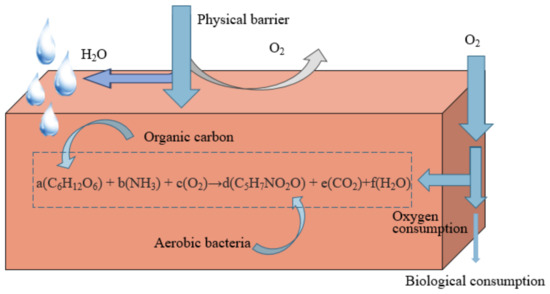
Figure 37.
Schematic diagram of oxygen consumption coverage considering microbial growth.
Furthermore, organic reactive materials can be used as a benign soil environment regulator. The decomposition process of organic covering can provide necessary nutrients (N, P, K, Ca, Mg, etc.) for plant growth, increase soil organic matter, and provide fertility for the soil environment. It can also create a suitable environment in the covering area to promote the proliferation of microorganisms. However, the use of organic rich waste can lead to risks. For example, organic cover may induce the reductive dissolution of secondary minerals, such as iron hydroxide, resulting in the release of toxic elements (As, Cd, Cu, Pb and Se). It may also promote the activity and proliferation of pro-oxidation-bacteria, resulting in adverse effects opposite to the oxygen barrier, and more attention should be paid to the environmental risks, especially for the use of sewage sludge. For example, NH4+ in sludge will lead to the formation of nitrate [58][79], leading to the oxidation of pyrite and promoting the generation of AMD. In addition, pathogenic microorganisms also have the risk of migration along with the surface runoff.
