A great number of functional genomics has shown that non-coding RNAs, especially miRNA and lncRNA, are involved in a diversity of developmental reproductive stages, from carpel formation and ovary development to the softening of the ripe/ripened fruit. Numerous genetic studies have also shown that miRNA and lncRNA regulation results in fruit development alteration, including organ pattern, fruit shape and size, as well as their developmental progress, such as miR159 involved in fruit set, miR160 associated with fruit shape, miR164 affecting locule number and miR156 regulating fruit softening. This layer of transcriptional control has been associated with ovule, seed and fruit development and fruit ripening, as well as stress responses, which are crucial developmental processes in breeding programs because of their relevance for crop production.
- miRNA
- long non-coding RNA
- stress response
- fruit
- agricultural traits
- CRISPR
- molecular breeding
1. Introduction
2. Functions of miRNAs and lncRNAs in Fruit Development
Fruit size and/or fruit number are crucial for improving yield and have a positive impact on consumer preference. It has been reported that the genes controlling tissue identity are involved in modulating fruit morphology, such as MADS-box genes, which are known to regulate floral organ identity, thereby regulating fruit development in Arabidopsis [8][18]. The regulatory module of miR172-AP2 has been highlighted in regulating fruit development in diverse plants. In Arabidopsis, miR172 promotes the silique fruit expansion process by the negative regulation of the activity of the APETALA2-like (AP2) gene [9][19], which would otherwise hinder the action of AGAMOUS (AG) and FRUITFUL (FUL) [10][20], two MADS-box transcription factors that are essential for ovary and silique growth [11][21]. miR172 has an adverse influence on fruit size in apples (Malus × domestica) through the negative regulation of AP2 that is required for hypanthium development into a pome fruit [12][22], resulting in small fruit size and an abnormal floral organ [13][23]. Another study in tomato, an ovary-derived fleshy fruit, revealed that the SlMIR172c and SlMIR172d loss-of-function mutant lines (slmir172c-dCR) resulted in abnormal flower organ and number identity [14][24]. These findings suggested a different role of miRNAs in dry and fleshy fruit. miRNAs regulate endogenous genes to impact development responses and even drive crop domestication; these results are consistent considering that silique is a true fruit deriving from ovary tissues, while the pome is a false fruit developing mainly from extra-carpellary tissues, such as sepals. MIR172 encodes highly similar miR172s, but exhibits differences in their distribution among fruit growth. These different biological functions and miRNA patterns in Arabidopsis, apple and tomato indicate the parallel evolution of the miRNA machinery in different fruit types. There are seven genes (SlMIR172a–g) that code for four unique species of miR172 (sly-miR172) in tomato [15][25], fifteen genes (mdm-MIR172a–o) in apple [16][26] and five genes (ath-miR172a–e) in Arabidopsis [17][27]. A recent study shows that the whole genome duplication (WGD) event of Populus trichocarpa stimulates the emergence of new miRNAs [18][28]. The number variety of miRNAs in different species may have resulted from the whole genome duplication event, thus contributing to the functional specialization of miRNAs and the functional importance of MIR genes. Many miRNAs are species-specific rather than conserved, which supports fruit type-specific divergence in miRNA evolution. Divergence in miRNAs or targets may have played important roles in horticultural crop domestication; for example, a loss-of-function mutation in MIR172p improved fruit size during apple domestication [12][22]. In addition, it has been observed that sly-MIR156a-c expressed in placenta, ovules and pre- and post-anthesis flowers in tomato [19][29], when overexpressing miR156a–c, resulted in the enhancement of vegetative development, a delay in flowering time, and a smaller number of fruits that presented ectopic leaf-like structures [20][15]. Moreover, the overexpression of tomato miR156 altered the expression of miR164, which is related to organ identity as well as carotenoid biosynthesis [20][15], suggesting that the miRNA–miRNA crosstalk and other molecular networks are also involved in fruit development. It has also been reported that miR156–miR172 pairs perform a negative correlation in flowering induction in A. thaliana, Nicotiana tabacum, D. glomerate and Oryza sativa [21][22][23][24][30,31,32,33], suggesting that miRNA–miRNA crosstalk plays an important role in the development of plant sexual organs. Furthermore, other miRNA regulation modules have also been identified in regulating fruit size and number (Table 1, Figure 1A,B). The sly-miR171a gene regulates hormone crosstalk between auxin and gibberellin in fruit size/weight by targeting two members of the GRAS family (SlGRAS24 and SlGRAS40) known as hormone regulators [25][34]. In this way, SlGRAS24 silencing results in GA3 and IAA accumulation, which leads to cell division and cell growth, and then floral initiation and seed number alteration [26][27][35,36]. Furthermore, sly-miR396a-3p/5p and sly-miR396b are mainly expressed in fruit, highlighting their potential role in fruit development. Knocking down miR396 by short tandem target mimic (STTM) showed an increase in fruit weight (66%), sepal size (153%), cell number (99%) and size (65%) [28][37], suggesting that the attenuation of miR396 results in the enhancement of some key performance indicators for fruit production. It has also been observed that knocking out miR164a by CRISPR/Cas9 to release the expression of NAM2/3 leads to decreased tomato fruit size [29][38]. The knocking down of miR1917 targeting an ethylene response gene CTR4 in tomato leads to bigger fruit [30][39]. It is reported that sly-miR159 is essential for fruit growth in Arabidopsis, and the mir159ab double mutant leads to small siliques [31][40], while its silencing results in larger fruits in tomato [32][41], suggesting fruits developed from the ovary may have evolved a different role of miRNAs in dry and fleshy fruit.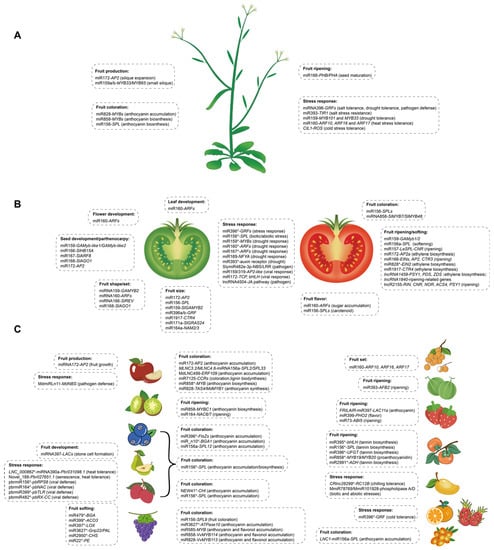
3. Functions of miRNAs and lncRNAs in Fruit Ripening
Fruit color variation is the most important agricultural trait of fruit ripening and chiefly affects the postharvest texture and consumers’ preferences. miRNAs have been extensively investigated in fruit development, and they also play an important role in fruit ripening. Interestingly, some miRNA regulations work in the same biological processes during fruit ripening. It has been found that the miR156-SPLs [58][59][60][65,66,67] and miR828/858-MYBs [61][62][63][64][65][68,69,70,71,72] modules are the conserved pathways to regulate fruit coloration in various fruit crops. For example, the miR156a-SPL12 module manipulates the accumulation of chlorophylls and anthocyanins during fruit ripening in blueberry, in which VcSPL12 interacts with VcMYBPA1 [59][66]. In pear, miR156-targeted SPLs interfere with the MYB-bHLH-WD40 complex in anthocyanin biosynthesis [66][73]. The transient overexpression of VvmiR156b/c/d in tomato promotes fruit coloring by repressing VvSPL9 transcription [67][74], suggesting that VvmiR156b/c/d-mediated VvSPL9 is involved in the formation of grape color. Similarly, in litchi (Litchi chinensis Sonn.), miR156a-targeted LcSPL1, interacting with LcMYB1, functions as a major cue in anthocyanin biosynthesis [60][67]. Moreover, the overexpression of miR156 promotes the accumulation of anthocyanins by targeting SPL9 in Arabidopsis, which negatively inhibits anthocyanin biosynthetic genes through the destabilization of an MYB-bHLH-WD40 transcriptional activation complex [58][65]. Another study reveals that long non-coding RNA MLNC3.2 and MLNC4.6 function as eTMs for miR156a and suppress the miR156a-mediated cleavage of SPL2-like and SPL33 during light-induced anthocyanin accumulation in apple fruit [68][75]. Similarly, the lncRNA LNC1-induces the downregulation of SPL9 through endogenous target mimics of miR156a, which leads to increased anthocyanin content in sea buckthorn (Hippophae rhamnoides Linn.) [69][76]. Moreover, ncRNAs associated with the anthocyanin biosynthesis pathway have also been reported in various regulatory modules, such as NEW41-CHI in litchi [60][67], miR396-FtsZs and miR_n10-BAG1 associated with blueberry [70][77] and miR172-AP2, miR7125-MYB16/MYB1 and MdLNC499-ERF109 involved in apple [71][72][73][78,79,80], all of which have been identified in anthocyanin accumulation (Table 1). In addition, miR828 triggers the biogenesis of phasiRNAs that, in trans or in cis, regulate multiple MYBs that are involved in anthocyanin accumulation [61][62][74][8,68,69]. These MYBs belong to the R2R3 class, which is integrated with multiple biological processes, particularly in plant anthocyanin biosynthesis [75][81]. In Arabidopsis, the overexpression of miR828 reduces anthocyanin accumulation by repressing genes encoding MYB transcription factors [61][68]. In tomato, miR858 plays a negative role in anthocyanin biosynthesis, and the blockage of miR858 leads to increased anthocyanin accumulation by modulating the expression of SlMYB7 and SlMYB48 [63][70]. Another report demonstrates that miR858a represses the translation of MYBL2 in Arabidopsis seedlings as a positive regulator of anthocyanin biosynthesis [65][72]. In grape, miR828/miR858 targets VvMYB114, which is reported as being essential for anthocyanin and flavonol accumulation [62][69]. The transient overexpression of miR858 reduces anthocyanin accumulation in kiwifruit (Actinidia arguta) by repressing the target gene MYBC1 [64][71]. Among them, miR828 and miR858 could directly or indirectly control anthocyanin biosynthesis in apple [16][26]; for example, a recent study found that the overexpression of mdm-miR828 inhibited anthocyanin synthesis through the cleavage of MdTAS4 in the late fruit coloration stage, and MdMYB1 was induced in a feedback regulatory mechanism through binding to the promoter of mdm-MIR828b to promote its expression [76][82]. Fruit ripening is a complex biological process and is associated with many aspects of fruit flavor and textural alterations. In persimmon (Diospyros kaki Thunb.), miR395p-3p and miR858b regulate bHLH and MYB, respectively, which synergistically regulate the structural genes responsible for tannin biosynthesis [77][83]. In addition, many miRNAs’ target genes have been identified through high-throughput sequencing associated with regulating persimmon fruit ripening, such as miR156-SPL, miR396-UFGT, miR858-MYB19/20 and miR2991-ADH [77][83]. Another study on strawberry shows that the overexpression of miR399 can improve fruit quality by targeting PHO2 [78][84]. A novel miRNA, Fan-miR73, negatively regulates its target gene, ABI5, to control strawberry fruit ripening [79][85]. Knocking down pre-slymiR157 or mature slymiR157 delays tomato fruit ripening by targeting LeSPL-CNR, in turn regulating the expression of LeMADS-RIN, LeHB1, SlAP2a and SlTAGL1 [80][81][86,87]. Additionally, miR164-NAC6/7 and miR393-AFB2 are associated with fruit ripening in kiwifruit and melon (Cucumis melo) [82][83][88,89] (Table 1). These sophisticated regulatory networks might provide the accurate regulation of fruit ripening in different plants. LncRNAs also play important roles in the fleshy fruit-ripening process. The genome-wide discovery and characterization of novel species-specific lncRNAs in fruits were conducted in various fleshy fruit species, including tomato [84][85][10,90], strawberry [46][53], apple [86][91], grape [87][92], kiwifruit [88][93], peach, mume (Prunus mume) [89][94], sea buckthorn [90][95] and melon [91][96]. These results present the global function of lncRNAs in different fruit species, which provides new insights into the regulation of fruit quality. In strawberry, color change in wild varieties of Fragaria pentaphylla (F. pentaphylla) may be largely regulated by lncRNAs [92][97]. In tomatoes, silencing two lncRNAs, lncRNA1459 and lncRNA1840, delayed the fruit-ripening processes, which indicated the positive regulatory roles of the two members in the fruit-ripening process [84][10]. Furthermore, knocking down lncRNA1459 by CRISPR/Cas9 genome editing technology affected lycopene, carotenoid and ethylene biosynthesis [93][17]. Moreover, in tomato, 187 lncRNAs were found to be direct targets of the MADS-box transcription factor (TF) RIPENING INHIBITOR (RIN), which is a critical TF of fruit ripening [94][95][98,99]. In the fleshy fruit species, lncRNAs were also reported to be the key regulators with miRNAs under sophisticated control to perform their proper function. Some research has shown that long non-coding RNA (lncRNA) could regulate miRNAs as endogenous target mimics (eTMs) and participate in anthocyanin accumulation, such as MLNC3.2 and MLNC4.6 in apple [68][75] and LNC1 in sea buckthorn [69][76]. In strawberry, the lncRNA FRILAIR serves as a miRNA sponge by functioning as a noncanonical target mimic of strawberry miR397, which can guide the mRNA cleavage of the fruit-ripening accelerating gene LAC11a, thereby regulating the fruit-ripening process [96][100]. Knocking out lncRNA2155 by CRISPR/Cas9 technology delayed tomato fruit ripening with downregulated ripening-related genes, including RIN, CNR, NOR, ACS4 and PSY1 [94][98]. Fruit softening and fruit texture are also crucial for optimizing fruit quality. In addition, several ncRNAs are involved in fruit softening. Knocking down pre-miR156a–c or their mature SlymiR156a sequences through the VIGS system accelerates tomato fruit softening after the red ripe stage [81][87]. Additionally, miR479-BGA, miR2950-CHS, miR22-PE, miR3627-PAL and miR399-ACO3 are associated with fruit softening in grapes [97][101] (Table 1). Furthermore, the overexpression of miR399a can promote the accumulation of fructose and glucose in wild strawberry fruit [78][84]. In apple, the overexpression of miR7125 reduces lignin biosynthesis by targeting MdCCR during light induction [72][79]. Taken together, it will be important to extensively explore the underlying mechanisms in fruit ripening. Plant hormones in fruit ripening are necessary, and the molecular mechanism and the signaling cascades of plant hormones during fruit ripening have been extensively studied in horticultural plants [98][102]. Non-coding RNAs are also involved in phytohormone regulation networks, such as ethylene (ETH), which is the major phytohormone in climacteric fruit ripening [99][100][103,104]. Tomato miR172 targets SlAP2 cleavage to accelerate fruit ripening and enhance ethylene biosynthesis [101][105]. Furthermore, slymiR1917 was reported as a negative regulator of two ET-related CTR4 splicing variants, but it is also regulated to ACS2 and ACS4, which are key genes for the establishment of the type of ET synthesis pathway [102][106]. In particular, Ethylene Insensitive 2 (EIN2) is targeted by miR828 [103][107], therefore for the onset of ethylene-dependent ripening events, a strong reduction of the expression of both miR394 and miR828 is required in tomato [104][108]. Moreover, some other miRNAs were found as regulators of some ET-related genes, such as the overexpression of the miR166-resistant version of SlREV downregulating EIN3, ERFs, AP2 and CTR3 in tomato [45][52]. The interplay may provide a mechanism to enable flexible fruit ripening. Several different types of non-coding RNAs are involved in regulating the expression of ripening genes, but further clarification of their diverse mechanisms of action is required. Further investigation might help to understand whether this behavior is relevant for development and if there are some other offset mechanisms in terms of time to ripen.4. Functions of miRNAs and lncRNAs in Fruit Responses to Biotic and Abiotic Stress
Stress tolerance is an important breeding objective and selection criteria in breeding that is critical for fruit quality, such as disease-resistant varieties, cold-resistant varieties and drought-resistant varieties. Besides the role of miRNAs and lncRNAs in growth, development and ripening, they also act as important signaling components in stress responses. They are key modulators of the transcriptional and post-transcriptional expression of genes during defense responses, and they are shown to be required for adaptation to the changes in ambient environments. Stress-induced changes occur in multiple species and correlate with a conserved mechanism involving non-coding RNA regulations. Salinity stress usually causes physiological disorders in fruit crops. During salinity conditions, numerous gene transcripts are variably regulated by miRNAs. The auxin signaling plays an important role in the biotic stress response, and the miR393-mediated regulation of the auxin receptor TIR1 is involved in the response to salt stress resistance and ABA-signaling pathways [105][106][109,110]. Furthermore, the miR396-GRF module was shown in pitaya (Hylocereus polyrhizus) and Arabidopsis [107][108][111,112]. Interestingly, a wide range of miRNAs was induced in date palms (Phoenix dactylifera L.) and mandarin (Citrus reticulata Blanco) under salt stress conditions [109][110][113,114], which provides insight into plants’ adaptation to salinity. High or low temperature stress at the fruit development stage is an important factor that determines fruit quality and fruit storage time, and hot or cold temperatures influence plant growth and yields. Several miRNAs induced by high-stress conditions have been identified through the bioinformatic prediction or RLM-5′ RACE-based validation in tomato, suggesting that a miRNA-mediated regulatory network is involved in high temperature [111][115]. In Arabidopsis, miR160 repressed ARF10, ARF16 and ARF17 to release the expression level of heat shock protein genes to allow the plants to survive heat stress [112][116], while miR160-ARF18 mediated salt tolerance in peanut [113][117]. In pear, a novel miRNA, Novel_188, is validated to target Pbr027651.1 to mediate fruit senescence under high- or low-temperature conditions [114][118]. Ptr-miR396b was determined to target 1-aminocyclopropane-1carboxylic acid oxidase (ACO) in response to cold stress in orange (Poncirus trifoliata) [115][119]. In mango, bioinformatic analysis reveals that MmiR78769 and MmiR101928 target phospholipase A and phospholipase D, respectively, both of which are associated with plant temperature stress-responsive process [116][120]. Moreover, degradome-wide analyses have revealed that miR393-TIR1/AFB displays a cold stress-specific response and miR156-SPL-mediated heat stress response in banana [117][121]. In particular, the lncRNAs’ temperature stress responses were found to be very specific. High temperature-induced LNC_000862 is likely to delay pear fruit senescence by competing with miR390a to derepress the expression of Pbr031098.1 [118][122]. LncRNAs involved in the response of chilling injury in tomato fruit have been systematically identified, providing a new perspective on lncRNA roles in chilling tolerance in fruits [119][123]. In mango, the cold-responsive lncRNA CRlnc26299 can interact with RC12B, which is the low-temperature and salt-responsive protein [116][120]. A novel lncRNA, COLD INDUCED lncRNA 1 (CIL1), is a positive regulator in plant response to cold stress by regulating the expression of endogenous reactive oxygen species (ROS) in Arabidopsis [120][124]. Drought stress adversely affects fruit crops’ productivity and quality. Drought stress response modulation via the miRNA pathway has also been found in several plant species. In tomato, miR159, miR169, miR160, miR167 and miR393 are associated with dehydration stress tolerance, by controlling hormonal signal transduction, stomatal closure and auxin-responsive genes [121][122][125,126]. The overexpression of miR396 showed lower densities of stomata and induced drought tolerance in Arabidopsis by suppressing the expression of GRF [123][127], which was consistent with the finding that the miR396-GRF module is involved in stress tolerance in tomato and pitaya [108][121][112,125]. Moreover, ABA-induced miR159 inhibits the transcripts of MYB101 and MYB33 during seedling stress responses in Arabidopsis [124][128]. A novel lncRNA, named DROUGHT INDUCED lncRNA (DRIR), has a positive role in the response of Arabidopsis to drought and salt stress [125][129]. Pathogen defense is associated with fruit quality and postharvest quality. Plants are constantly exposed to a range of microbial pathogens with different lifestyles and modes of attack, including fungal, bacterial and viral pathogens, whereas RNA-based mechanisms largely regulate plant–virus interactions. Many key miRNA regulators of the stress response in fruits during pathogen infection were identified, such as miRNAs engineer Botrytis cinerea (B. cinerea) in kiwifruit [126][130] and specific miRNAs’ response to stress in Amur grape (vitis amurensis Rupr.) [127][131]. In particular, Md-miRLn11 targeted an apple nucleotide-binding site (NBS)–leucine-rich repeat (LRR) class protein coding gene (Md-NBS) to trigger host immune responses during pathogen infection [128][132]. SlymiR482e-3p knocking-out lines showed enhanced resistance to tomato wilt disease and regulated ethylene signaling by suppressing the expression of ethylene response factors (SlERFs) [129][133]. The can-miRn37a further confirmed anthracnose resistance in chili (Capsicum annuum L.) by repressing ERFs and preventing fungal colonization [130][134]. In tomato, sly-MIR156d/e were found induced under biotic and abiotic stress [19][131][29,135]. In addition, miR159/319 and miR172 accumulation positively correlated with immune responses during Tomato leaf curl virus (ToLCV) infection, indicating that miR159/319 and miR172 might be associated with the response to viral infection in tomato [132][136]. In pear, pbr-miR156, pbr-miR164, pbr-miR399 and pbr-miR482 are induced during Apple stem pitting virus (ASPV) infection and then trigger its target genes to participate in viral defense pathways [133][137]. Overexpressed miR396 not only plays roles in drought response in A. thaliana [123][127] and cold tolerance in orange [115][119], but also has resulted in plant tolerance under the attack of necrotrophic fungal pathogens [134][138]. Previous reports have shown that lncRNA not only plays essential roles in diverse biological processes, but also in various stress responses. LncRNA4504 positively regulated methyl jasmonate (MeJA)-induced tomato fruit resistance to B. cinerea by promoting the accumulation of total phenols and flavonoids and upregulating the expression of JA signal pathway genes [135][139]. The most effective postharvest technology to maintain fruit quality is to delay the fruit senescence process, such as cold storage after the fruit is harvested. Thus, in incorporating the dynamic environments, important alterations in non-coding RNA transcriptomes are observed in many plant species, which has led to the general view that plants utilize ncRNAs as part of their arsenal to cope with the wide array of microbial pathogens they encounter (Table 1). Further investigation might help to find clues to a better understanding of the consequences of ncRNA attenuation under biotic and abiotic stress and its putative success under field conditions.| Fruit Biology | Classification | Species | Non-Coding RNA | Targets/Downstream | Functionally in Fruit Quality | Research Methods | References |
|---|---|---|---|---|---|---|---|
| Fruit development | Fruit size and number | arabidopsis | miR172C | APETALA2-like | silique fruit expansion | stable (MIR172C::GUS, MIR172CAuxRE::GUS) | [11][21] |
| miR159a/b | MYB33/MYB65 | altered growth habit, curled leaves, small siliques, and small seeds | T-DNA mutants (mir159ab double mutant) | [31][40] | |||
| apple | miR172p | AP2 | reduced fruit size, altered floral organ development | stable (MIR172p OE in tomato) | [12][13][22,23] | ||
| tomato | miR156 | SPL | fruit growth, ovary and fruit development | stable (AtMIR156b OE) | [20][15] | ||
| miR159 | SlGAMYB2 (GA biosynthesis gene) | larger fruits | STTM-miR159 | [32][41] | |||
| miR172d | AP2 | floral organ identity and number | CRISPR/Cas9 (slmir172c-dCR) | [14][24] | |||
| miR396a/b | GRF | a larger plant, with bigger flowers, leaves, and fruits | STTM-miR396 | [26][27][28][35,36,37] | |||
| miR1917 | CTR4 (altered ethylene response) | fruit size, bigger fruit | STTM-miR1917 | [30][39] | |||
| miR171a | SlGRAS24 and SlGRAS40 (altered gibberellin and auxin) | cell number and size, smaller tomato fruit | GRAS24 OE | [25][34] | |||
| miR164a | NAM2/3 | decreased fruit size | CRISPR/Cas9 (slmir164a, slmir164b, slmir164d, slmir164CR) | [29][38] | |||
| Fruit development | fruit set | tomato | miR159 | SlGAMYB2 (GA biosynthesis gene) | fruit morphology, precocious fruit initiationflattened, fruit with more locules inside | SlMIR159 OE | [42][11] |
| miR160 | ARF10, ARF16 and ARF17 | sugar accumulation, leaf and flower development, somatic embryo development, pear-shaped fruit | STTM-miR160 | [37][38][39][12,46,47] | |||
| miR166 | SlREV | fruit formation | Overexpression of a microRNA166-resistant version of SlREV (35S::REVRis) | [43][50] | |||
| miR168 | SlAGO1s | fruit initiation and growth | miR168 loss-of-function (four-point-mutated miR168-resistant 4m-SlAGO1A and 4m-SlAGO1B) | [49][56] | |||
| pear | PbrmiR397a | LACs | stone cell formation, reduced lignin content and stone cell number | transient (PbrmiR397a OE, pear), stable (PbrmiR397a OE, tobacco) | [44][51] | ||
| longan | miR160 | ARF10, -16, and -17 | somatic embryo development | target mimics down-regulate miR160 | [45][52] | ||
| seed development/parthenocarpy | tomato | miR159 | GAMyb-like1 and GAMyb-like2 | parthenocarpy | SlMIR159 OE | [42][11] | |
| miR166 | SlHB15A | parthenocarpic fruit set | used TILLING to screen for SlHB15A miR166-resistant alleles | [50][57] | |||
| miR167 | SlARF8 | parthenocarpy | downregulation of miR167 | [42][11] | |||
| miR168 | SlAGO1s | parthenocarpy | miR168-resistant 4m-SlAGO1A | [49][56] | |||
| miR172 | AP2 | small parthenocarpic fruit-like organ | CRISPR/Cas9 (slmir172c-dCR) | [14][24] | |||
| Fruit ripening | fruit color | litchi | miR156a * | LcSPL1/2 | anthocyanin biosynthesis | High-Throughput Sequencing and Degradome Analysis | [60][67] |
| NEW41 * | CHI | anthocyanin accumulation | |||||
| pear | miR156 * | SPL | Red Peel Coloration, anthocyanin biosynthesis | Degradome Library | [66][73] | ||
| blueberry | miR156a | VcSPL12 | anthocyanin accumulation | VcMIR156a OE in tomato | [59][66] | ||
| miR396 * | FtsZs | coloration | Small RNA and Degradome Sequencing | [70][77] | |||
| miR_n10 * | BAG1 | coloration | |||||
| apple | miR172 | AP2-MYB10 | flavonoidse, reduction in red coloration | miR172 OE | [73][80] | ||
| MLNC3.2 and MLNC4.6 (lncRNA) | miR156a-SPL2-like and SPL33 | anthocyanin biosynthesis | transient (35S::MLNC3.2, 35S::MLNC4.6, OE-miR156a) | [68][75] | |||
| miR7125 (light-induced) | MYB16/MYB1-CCRs | promoted anthocyanin synthesis, reduced lignin biosynthesis | transient (miR7125 OE) | [72][79] | |||
| MdLNC499 (lncRNA) | MdERF109 | fruit coloration | transient (TRV-MdLNC499, TRV-MdERF109, apple fruit), stable (MdLNC499 OE, MdLNC499 RNAi, MdERF109 OE, MdERF109 RNAi, apple calli) | [71][78] | |||
| mdm-miR828 | TAS4-MdMYB1 | inhibited anthocyanin synthesis | transient (mdm-miR828 OE, apple, stable (mdm-miR828 OE, Arabidopsis) | [76][82] | |||
| miR858 * | MYB | anthocyanin biosynthesis | small RNA-seq | [16][26] | |||
| sea buckthorn | LNC1 (lncRNA)-miR156a | SPL9 | anthocyanin accumulation | transient (TRV-LNC1) | [69][76] | ||
| Fruit ripening | fruit color | grape | miR858 | VvMYB114 | anthocyanin and flavonol accumulation | Degradom, transient/stable (VvMYB114 OE, tobacco) | [62][69] |
| miR156 | SPL9 | promoted fruit coloration | miR156b/c/d OE in tomato | [67][74] | |||
| miR3627 * | calcium-transporting ATPase10 | anthocyanin accumulation | sequencing small RNAs, bioinformatics analysis | [97][101] | |||
| miR828 | VvMYB113/VvMYB114 | anthocyanin and flavonol accumulation | vvi-miR828 OE, Arabidopsis | [62][69] | |||
| arabidopsis | miR828 | MYB75, MYB90, and MYB113 | anthocyanin accumulation | AtmiR828 OE | [61][68] | ||
| miR858a | MYB2 | anthocyanin accumulation, anthocyanin biosynthesis | STTM-miR858 | [65][72] | |||
| miR156 | SPL9 and SPL15 | anthocyanin biosynthesis | MIR156b OE | [58][65] | |||
| tomato | miR858 | SlMYB7 and SlMYB48 | anthocyanin accumulation | STTM-miR858 | [63][70] | ||
| kiwifruit | miR858 | AaMYBC1 | anthocyanin biosynthesis | transient (miR858 OE) | [64][71] | ||
| fruit ripening, fruit softening and fruit quality | persimmon | miR395 * | bHLH | tannin biosynthesis | high-throughput sequencing | [77][83] | |
| miR156 * | SPL | tannin biosynthesis | |||||
| miR396 * | Flavonoid 3-O-glucosyltransferase (UFGT) | tannin biosynthesis | |||||
| miR858 * | MYB19/20 | reduced the content of proanthocyanidin (PA) | |||||
| miR2991 * | ADH | tannin biosynthesis | |||||
| Fruit ripening | fruit ripening, fruit softening and fruit quality | strawberry | FRILAIR (lncRNA)-miR397 | LAC11a | delayed fruit ripening | transient (miR397 OE, Cas13b-miR397, ocotoploid strawberry) | [96][100] |
| fan-miR73 | ABI5 | fruit ripening | 5′ -RACE analysis | [79][85] | |||
| miR399 | PHO2 | flavor, sugar content | miR399a OE (woodland strawberry) | [78][84] | |||
| tomato | miR157 | SPL-CNR | delayed fruit ripening | miR157 OE | [80][81][86,87] | ||
| miR156 | SPL | accelerates tomato fruit softening | VIGS-miR156a | [81][87] | |||
| miR172 | AP2a | accelerates fruit ripening with enhanced ethylene biosynthesis | miR172 OE | [101][105] | |||
| miR166 | SlREV | fruit ripening | 35S::REVRis (EIN3, ERFs, AP2, and CTR3 downregulated) | [43][50] | |||
| miR828 * | EIN2 | ethylene-dependent ripening | high throughput sequencing | [104][108] | |||
| miR1917 | CTR4 | enhances ethylene response and accelerates fruit ripening | miR1917 OE | [103][107] | |||
| lncRNA2155 (lncRNA) | RIN, CNR, NOR, ACS4, PSY1 | delayed fruit ripening | CRISPR/Cas9 (lncRNA2155 KO) | [94][98] | |||
| lncRNA1459 (lncRNA) | PSY1, PDS, ZDS | ripening, ethylene biosynthesis | CRISPR/Cas9 (lncRNA1459 KO) | [84][93][10,17] | |||
| lncRNA1840 (lncRNA) | ripening-related genes | ripening, ethylene biosynthesis | TRV-lncRNA1840 | [84][10] | |||
| kiwifruit | miR164 | NAC6/7 | fruit ripening | miR164 OE (kiwifruit callus) | [82][88] | ||
| apple | miR7125 | MYB16/MYB1-CCRs | reduced lignin biosynthesis | transient (miR7125 OE, apple fruit) | [72][79] | ||
| melon | cme-miR393 | CmAFB2 | delayed fruit ripening | cme-miR393-OE | [83][89] | ||
| Fruit ripening | fruit ripening, fruit softening and fruit quality | grapes | miR479 * | BGA | fruit softing | deep sequencing, bioinformatics analysis | [97][101] |
| miR399 * | ACO3 | ||||||
| miR397 * | LOX | ||||||
| miR3627 * | Grip22/PAL | ||||||
| miR2950 * | CHS | ||||||
| miR22 * | PE | ||||||
| biotic and abiotic stress in fruit | cold response | arabidopsis | CIL1 (lncRNA) | ROS | enhances cold stress tolerance | T-DNA insertion mutants | [120][124] |
| orange | miR396b | GRF | cold tolerance | ptr-miR396b OE (transgenic lemon (Citrus limon)) | [115][119] | ||
| banana | miR393 * | TIR1/AFB | cold stress-specific response | bioinformatics analysis | [117][121] | ||
| mango | CRlnc26299 * (lncRNA) | RC12B | chilling tolerance | Computational Identification | [116][120] | ||
| salt tolerance | arabidopsis | miR396 | GRF | salt tolerance | target mimicry (eTM) transgene specific to miR396 | [107][111] | |
| miR393a/b | TIR1 | salt stress resistance and ABA signaling pathways | mir393ab double mutant | [105][106][109,110] | |||
| pitaya | miR396 * | GRF | stress response | bioinformatics analysis | [108][112] | ||
| heat tolerance | tomato | miR396 * | GRF | drought and heat stress | bioinformatics analysis | [121][125] | |
| arabidopsis | miR160 | ARF10, ARF16, and ARF17 | heat stress tolerance | eTM-miR160 | [112][116] | ||
| banana | miR156 * | SPL | heat stress response | bioinformatics analysis | [117][121] | ||
| mango | MmiR78769 and MmiR101928 (lncRNA) | phospholipase A and phospholipase D | biotic and abiotic stresses | Computational Identification | [116][120] | ||
| biotic and abiotic stress in fruit | heat tolerance | pear | Novel_188 | Pbr027651.1 | mediate fruit senescence | transient (Novel_188 OE) | [114][118] |
| LNC_000862 * (lncRNA) | miR390a-Pbr031098.1 | heat tolerance | bioinformatics analysis | [118][122] | |||
| drought response | arabidopsis | miR396a/b | GRF | drought tolerance | 35S::MIR396a and 35S::MIR396b | [123][127] | |
| miR159 | MYB101 and MYB33 | drought tolerance | miR159 OE | [124][128] | |||
| DRIR (lncRNA) | genes involved in ABA signaling | Enhances Drought and Salt Stress Tolerance | DRIR OE | [125][129] | |||
| tomato | miR169 | NFYA | drought and heat stress | STTM-miR169 | [122][126] | ||
| miR159 * | MYB | bioinformatics analysis | [121][125] | ||||
| miR160 * | ARF | ||||||
| miR167 * | ARF | ||||||
| miR393 * | auxin receptor homologous genes | ||||||
| pathogen defense | arabidopsis | miR396 | GRF | pathogen defense | miR396 target mimics lines | [134][138] | |
| apple | Md-miRLn11 | Md-NBS | pathogen defense | bioinformatics analysis | [128][132] | ||
| tomato | SlymiR482e-3p | NBS-LRR | enhanced resistance to tomato wilt disease | slymiR482e-3p KO lines | [129][133] | ||
| miR156 * | SPL | response to ToLCV infections | bioinformatics analysis | [19][131][29,135] | |||
| miR159/319 | AP2-like | viral response (tomato leaf curl new delhi virus (tolcndv)) | MicroRNA profiling | [132][136] | |||
| miR172 | TCP, bHLH | [132][136] | |||||
| LncRNA4504 (lncRNA) | JA signal pathway genes | pathogen defense (Botrytis cinerea) | TRV-lncRNA4504 | [135][139] | |||
| pear | pbr-miR156 * | pbRPS6 | viral defense | bioinformatics analysis | [133][137] | ||
| pbr-miR164 * | pbNAC | ||||||
| pbr-miR399 * | pbTLR | ||||||
| pbr-miR482 * | pbRX-CC |
