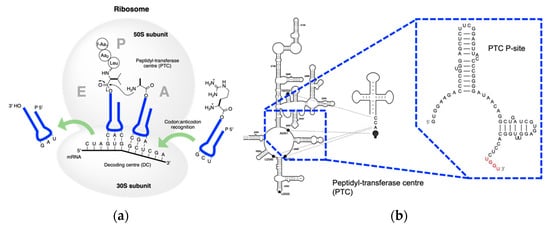
Extant biology uses RNA to record genetic information and proteins to execute biochemical functions. Nucleotides are translated into amino acids via transfer RNA in the central dogma. tRNA is essential in translation as it connects the codon and the cognate amino acid. Among the three steps in the central dogma, translation is the most important as it bridges the world of nucleic acids and the world of amino acids. In the “RNA world” hypothesis, RNA came first from the primordial environment, recorded the genetic information, and catalyzed fundamental biochemical reactions. Later, RNA alienated the catalytic function of peptides and proteins and released the information storage function to DNA. DNA self-copy, i.e., replication, and DNA-templated RNA polymerization, i.e., transcription, are more intuitive and practicable in prebiotic settings compared to RNA-coded peptide formation. Translation takes place in the endoplasmic reticulum membrane in the cytoplasm with messenger RNA as the template, transfer RNA as the adaptor, and ribosome RNA as the catalytic core.
- RNA world
- transfer RNA
- ribosome
- peptidyl transfer
1. The Evolution of tRNA
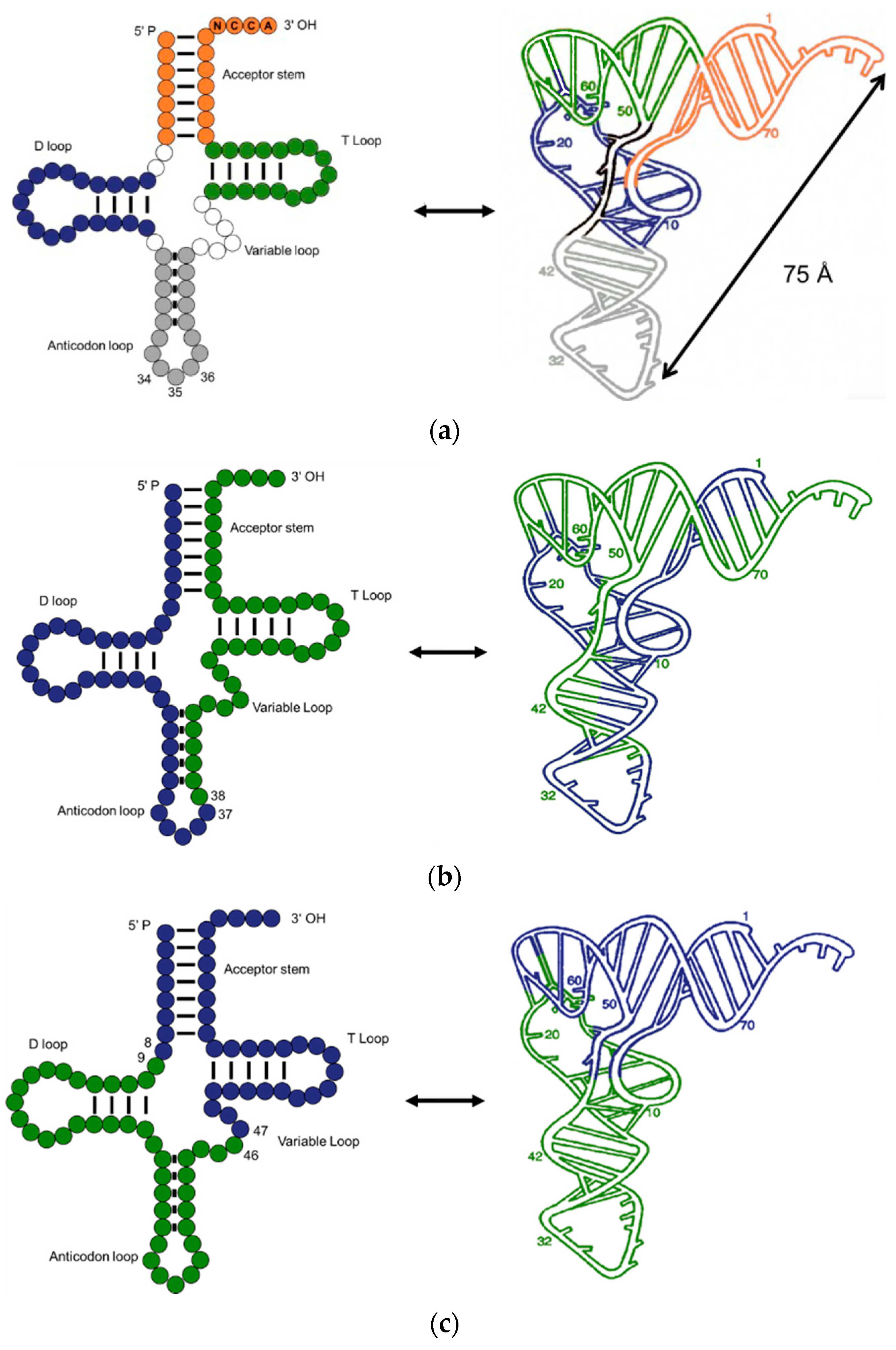
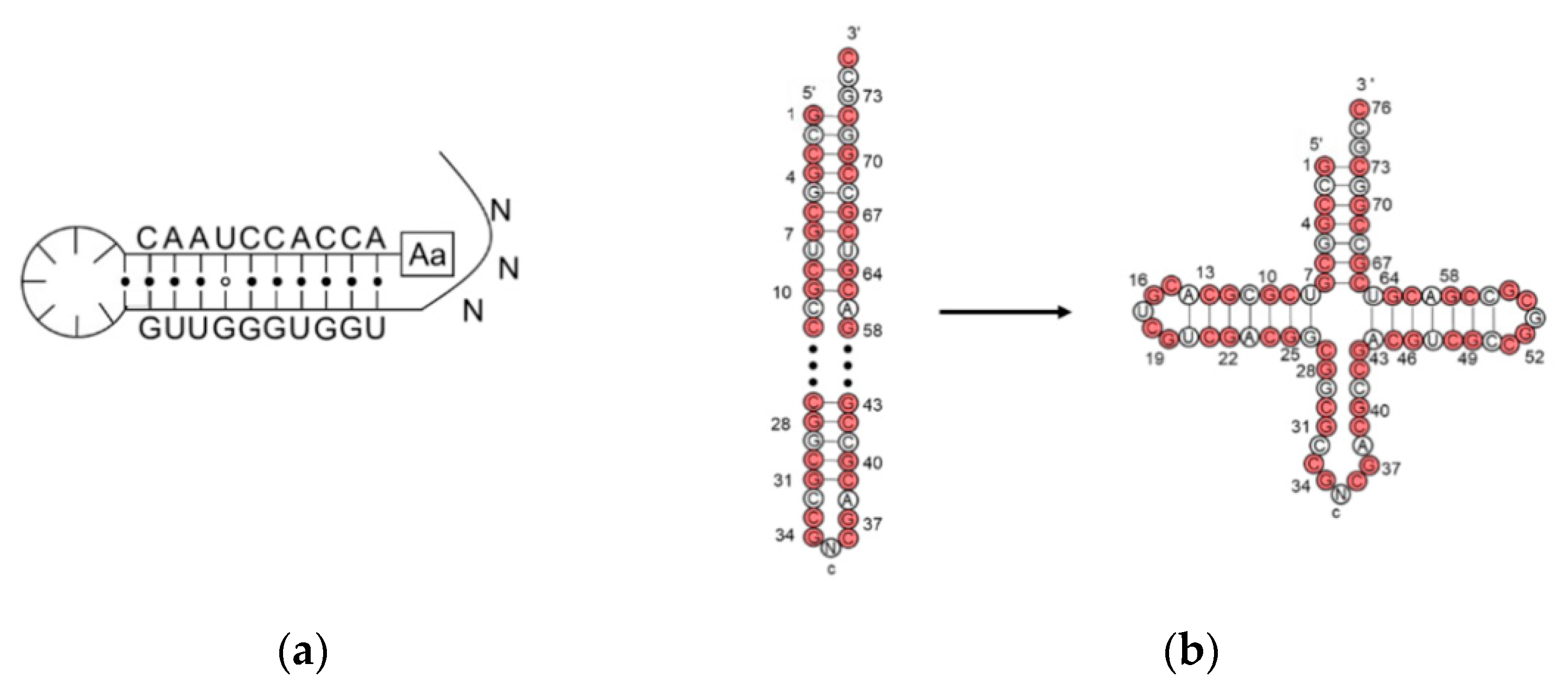
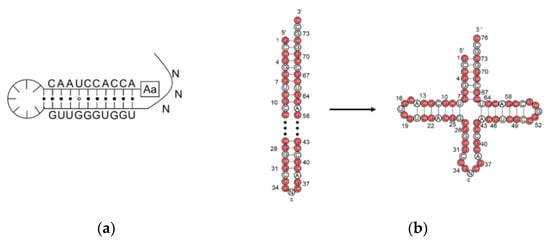
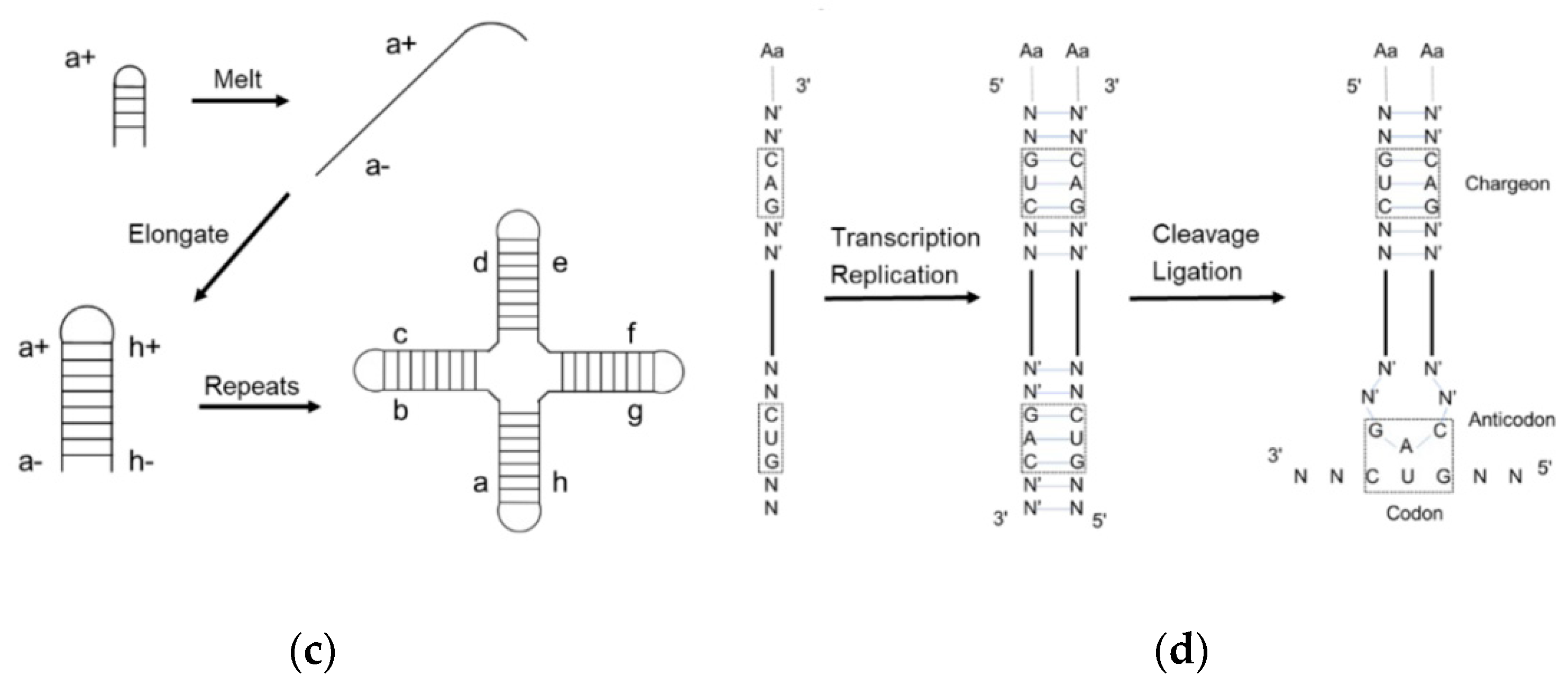
2. Assignment of the Amino Acids to Genetic Codes
3. Coded Peptide Formation
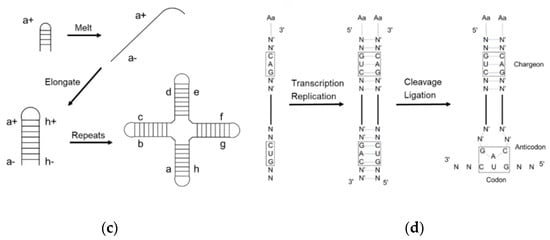
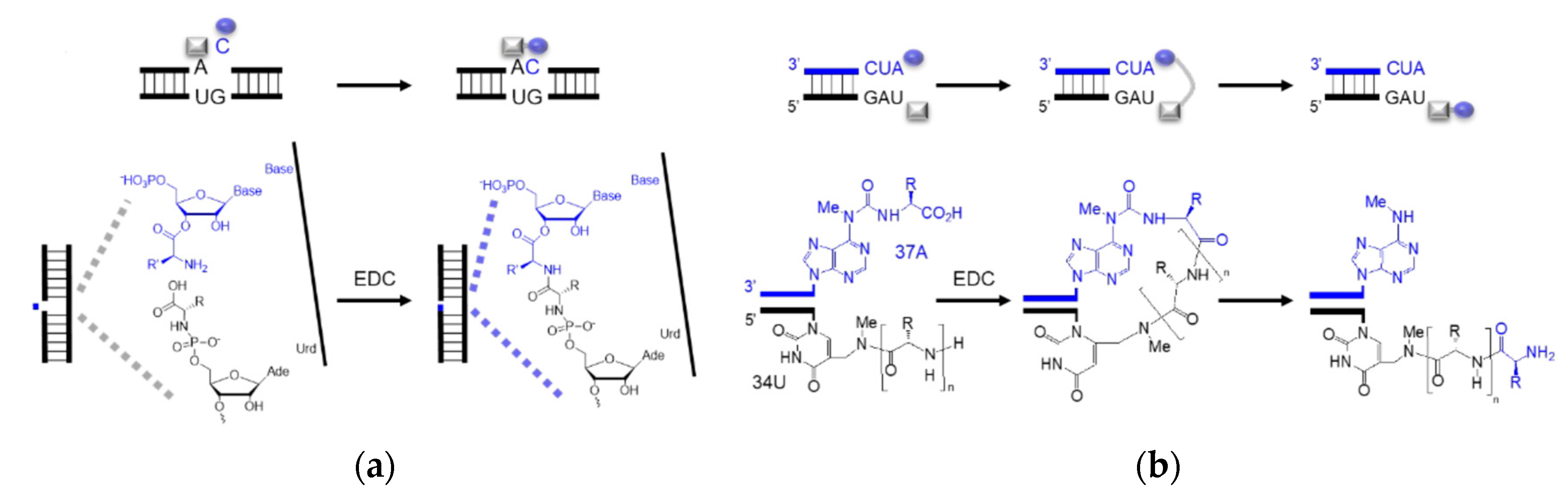
Dipeptides can be produced through the amidation of two amino acids without coupling reagents, but the yield is low [78][40]. Typical inorganic catalysts include layered double hydroxides [80][41] and titanium dioxide [81][42]. Cyanamide, diaminomaleonitrile, ferricyanide, trimetaphosphate, etc. are organic activators that likely catalyze the amide bond formation under prebiotic conditions. Prebiotically plausible N-carbamoyl amino acids (NCA) [82][43], 5(4H)-oxazolone [83[44][45],84], and cyclic acylphosphoramidate (CAPA) [85][46], activated from the respective amino acids, further yield mixed anhydrides and aminoacyl esters. Although prebiotic peptide formation is prevalent and short random peptides can be functional, the function cannot self-propagate until the amino acid sequence is recorded in RNA or prebiotic nucleotide analogues [86][47]. In the extant biology, peptide synthesis starts with the acylation of the 3′-terminus of the tRNA using aaRS (Figure 43a). In the prebiotic context, acylating RNA strands without the presence of pre-synthesized enzymes initiates RNA-coded peptide synthesis [87][48]. Tamura et al. [88][49] and Wu et al. [89][50] discovered that aminoacyl spontaneously transferred from aminoacyl phosphate mixed anhydride at the 5′-terminus of a donor strand to the 3′-terminus of an acceptor strand in a nicked duplex (Figure 43b) or nicked loop (Figure 43c). The transfer showed good stereoselectivity of L- over D-amino acids and chemical selectivity among amino acids, which indicates that the conformation of the D-ribose and β-nucleoside resulted in the single-chirality of amino acids. In the case of the nicked duplex transfer, tri-phenylalanine-RNA ester was detected [90][51]. Similar esterification was achieved via phosphoramidate (Figure 43d,e) [91,92][52][53]. While the prebiotic synthesis of mixed anhydride is not yet solved, amino acid phosphoramidate is prebiotic accessible [61][54]. To achieve the RNA-coded peptide synthesis, either the formation of amino acid donors or the aminoacyl transfer should be processed in an RNA sequence-dependent manner; however, no sequence-dependent acylation has been reported to date.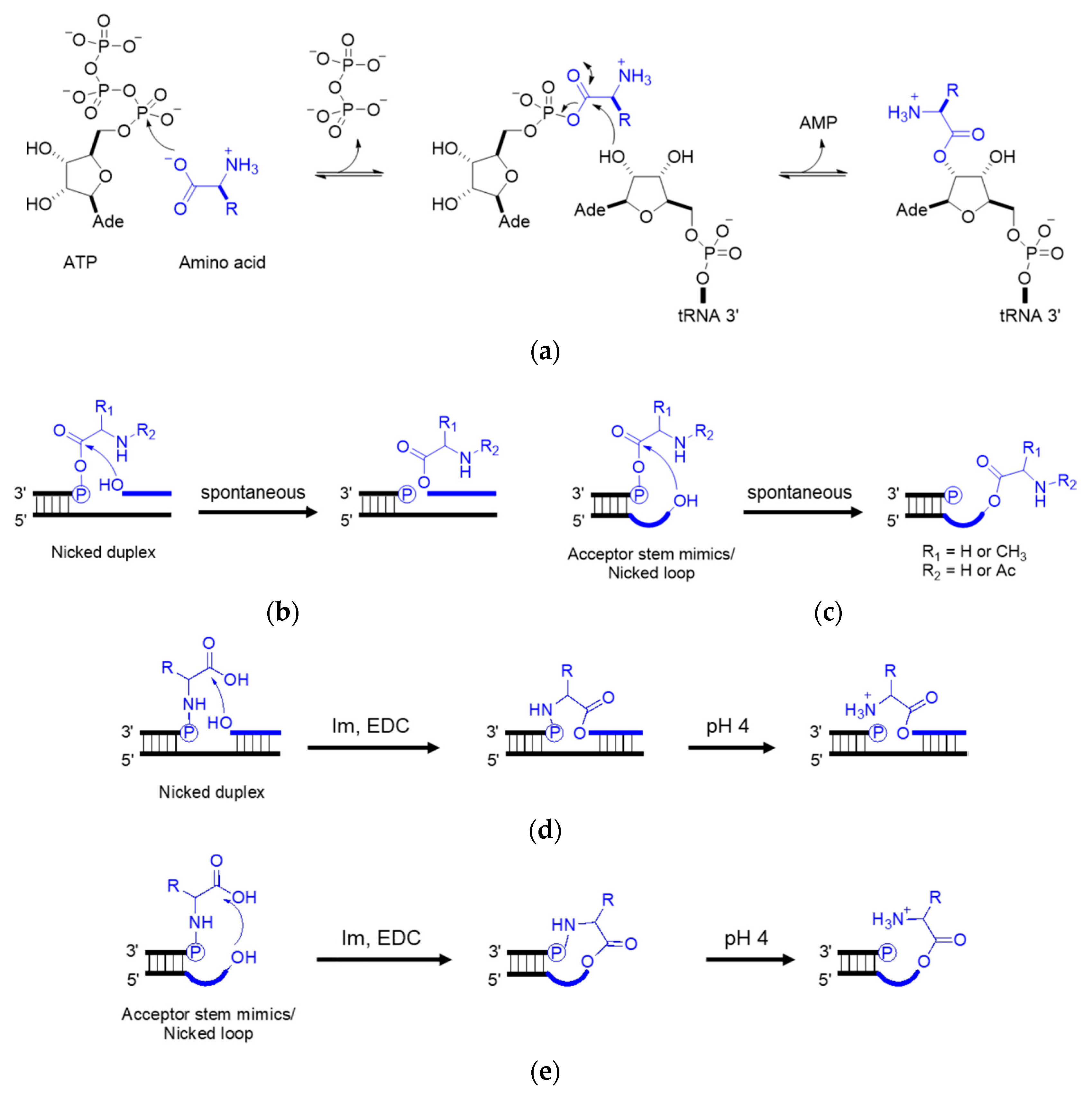
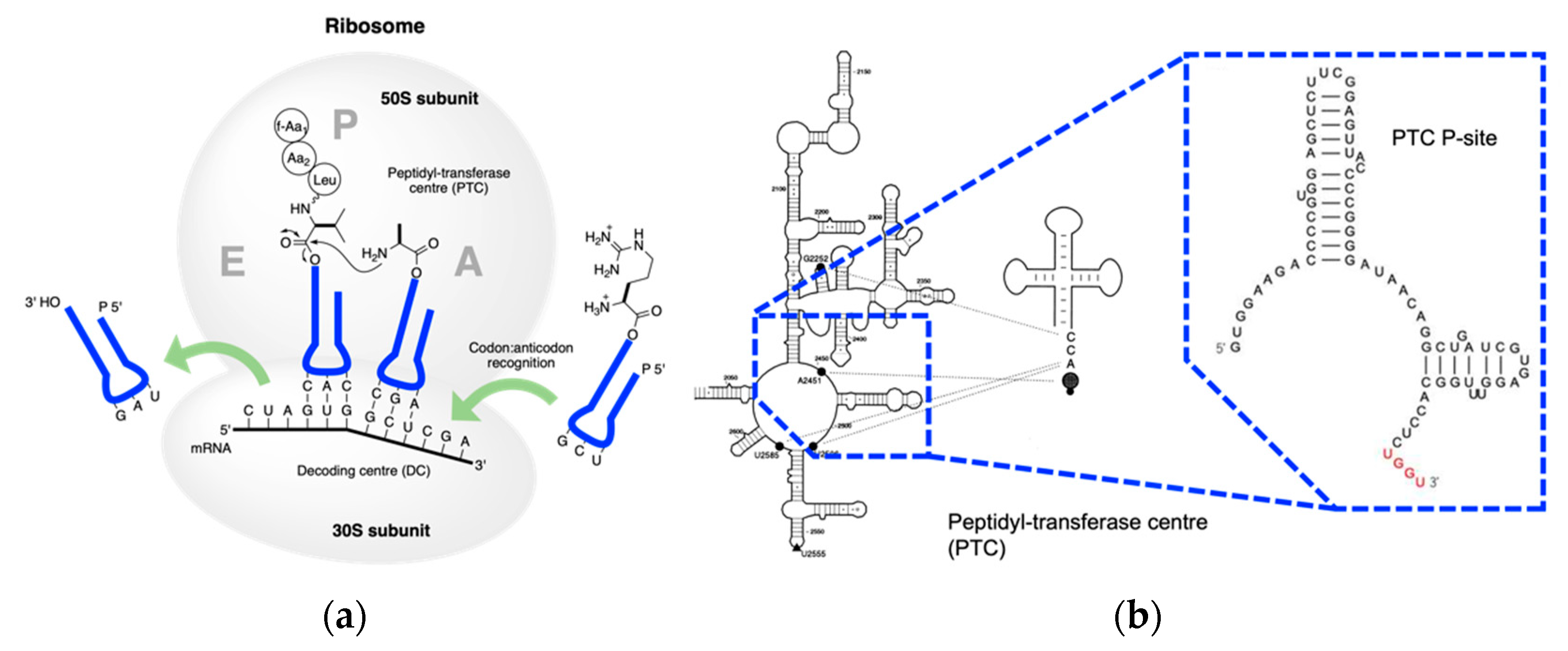
References
- Crick, F.H.C. The origin of the genetic code. J. Mol. Biol. 1968, 38, 367–379.
- Hoagland, M.B.; Stephenson, M.L.; Scott, J.F.; I Hecht, L.; Zamecnik, P.C. A soluble ribonucleic acid intermediate in protein synthesis. J. Biol. Chem. 1958, 231, 241–257.
- Ohtsuki, T.; Kawai, G.; Watanabe, K. The minimal tRNA: Unique structure of Ascaris suum mitochondrial tRNA(Ser)(UCU) having a short T arm and lacking the entire D arm. FEBS Lett. 2002, 514, 37–43.
- Masta, S.E.; Boore, J.L. Parallel evolution of truncated transfer RNA genes in arachnid mitochondrial genomes. Mol. Biol. Evol. 2008, 25, 949–959.
- Ohtsuki, T.; Watanabe, Y. T-armless tRNAs and elongated elongation factor Tu. IUBMB Life 2007, 59, 68–75.
- Jovine, L.; Djordjevic, S.; Rhodes, D. The crystal structure of yeast phenylalanine tRNA at 2.0 A resolution: Cleavage by Mg2+ in 15-year old crystals. J. Mol. Biol. 2000, 301, 401–414.
- Fujishima, K.; Kanai, A. tRNA gene diversity in the three domains of life. Front. Genet. 2014, 5, 142.
- Tocchini-Valentini, G.D.; Fruscoloni, P.; Tocchini-Valentini, G.P. Coevolution of tRNA intron motifs and tRNA endonuclease architecture in Archaea. Proc. Natl. Acad. Sci. USA 2005, 102, 15418–15422.
- Randau, L.; Söll, D. Transfer RNA genes in pieces. EMBO Rep. 2008, 9, 623–628.
- Di Giulio, M. The non-monophyletic origin of the tRNA molecule and the origin of genes only after the evolutionary stage of 1the last universal common ancestor (LUCA). J. Theor. Biol. 2006, 240, 343–352.
- Woese, C.R. The biological significance of the genetic code. In Progress in Molecular and Subcellular Biology; Hahn, F.E., Ed.; Springer: Berlin/Heidelberg, Germany, 1969; pp. 5–46.
- Hopfield, J.J. Origin of the genetic code: A testable hypothesis based on tRNA structure, sequence, and kinetic proofreading. Proc. Natl. Acad. Sci. USA 1978, 75, 4334–4338.
- Thulasi, P.; Pandya, L.K.; Znosko, B.M. Thermodynamic characterization of RNA triloops. Biochemistry 2010, 49, 9058–9062.
- Eigen, M.; Winkler-Oswatitsch, R. Transfer-RNA, an early gene? Naturwissenschaften 1981, 68, 282–292.
- Bloch, D.P.; McArthur, B.; Mirrop, S. tRNA-rRNA sequence homologies: Evidence for an ancient modular format shared by tRNAs and rRNAs. Biosystems 1985, 17, 209–225.
- Möller, W.; Janssen, G.M. Statistical evidence for remnants of the primordial code in the acceptor stem of prokaryotic transfer RNA. J. Mol. Evol. 1992, 34, 471–477.
- Maizels, N.; Weiner, A.M. Phylogeny from function: Evidence from the molecular fossil record that tRNA originated in replication, not translation. Proc. Natl. Acad. Sci. USA 1994, 91, 6729–6734.
- Harvey, S.C.; McCammon, J.A. Intramolecular flexibility in phenylalanine transfer RNA. Nature 1981, 294, 286–287.
- Sun, F.J.; Caetano-Anollés, G. The origin and evolution of tRNA inferred from phylogenetic analysis of structure. J. Mol. Evol. 2008, 66, 21–35.
- Johnson, A.P.; Cleaves, H.J.; Dworkin, J.P.; Glavin, D.P.; Lazcano, A.; Bada, J.L. The Miller volcanic spark discharge experiment. Science 2008, 322, 404.
- Miller, S.L. A production of amino acids under possible primitive earth conditions. Science 1953, 117, 528–529.
- Miller, S.L. Production of some organic compounds under possible primitive earth conditions. J. Am. Chem. Soc. 1955, 77, 2351–2361.
- Patel, B.H.; Percivalle, C.; Ritson, D.J.; Duffy, C.D.; Sutherland, J.D. Common origins of RNA, protein and lipid precursors in a cyanosulfidic protometabolism. Nat. Chem. 2015, 7, 301–307.
- Crick, F.H.C. An error in model building. Nature 1967, 213, 798.
- Lehmann, J.; Libchaber, A. Degeneracy of the genetic code and stability of the base pair at the second position of the anticodon. RNA 2008, 14, 1264–1269.
- Gamow, G. Possible relation between deoxyribonucleic acid and protein structures. Nature 1954, 173, 318.
- Crick, F.H.C. Origin of the genetic code. Nature 1967, 213, 119.
- Pelc, S.R. Correlation between coding-triplets and amino-acids. Nature 1965, 207, 597–599.
- Pelc, S.R.; Welton, M.G. Stereochemical relationship between coding triplets and amino-acids. Nature 1966, 209, 868–870.
- Woese, C.R. The Genetic Code; Harper & Row: New York, NY, USA, 1967.
- Dunnill, P. Triplet nucleotide-amino-acid pairing; a stereochemical basis for the division between protein and non-protein amino-acids. Nature 1966, 210, 1265–1267.
- Wong, J.T. A co-evolution theory of the genetic code. Proc. Natl. Acad. Sci. USA 1975, 72, 1909–1912.
- Di Giulio, M. Reflections on the origin of the genetic code: A hypothesis. J. Theor. Biol. 1998, 191, 191–196.
- Rogers, S.O. Evolution of the genetic code based on conservative changes of codons, amino acids, and aminoacyl tRNA synthetases. J. Theor. Biol. 2019, 466, 1–10.
- Woese, C.R. Nature of the biological code. Nature 1962, 194, 1114–1115.
- Błażej, P.; Pawlak, K.; Mackiewicz, D.; Mackiewicz, P. Model of genetic code structure evolution under various types of codon reading. Int. J. Mol. Sci. 2022, 23, 1690.
- Woese, C.R. Order in the genetic code. Proc. Natl. Acad. Sci. USA 1965, 54, 71–75.
- Rodin, S.; Ohno, S.; Rodin, A. Transfer RNAs with complementary anticodons: Could they reflect early evolution of discriminative genetic code adaptors? Proc. Natl. Acad. Sci. USA 1993, 90, 4723–4727.
- Rodin, S.; Rodin, A.; Ohno, S. The presence of codon-anticodon pairs in the acceptor stem of tRNAs. Proc. Natl. Acad. Sci. USA 1996, 93, 4537–4542.
- Martin, R.B. Free energies and equilibria of peptide bond hydrolysis and formation. Biopolymers 1998, 45, 351–353.
- Erastova, V.; Degiacomi, M.T.; Fraser, D.G.; Greenwell, H.C. Mineral surface chemistry control for origin of prebiotic peptides. Nat. Commun. 2017, 8, 2033.
- Pantaleone, S.; Ugliengo, P.; Sodupe, M.; Rimola, A. When the surface matters: Prebiotic peptide-bond formation on the TiO2 (101) anatase surface through periodic DFT-D2 simulations. Chem. A Eur. J. 2018, 24, 16292–16301.
- Abou Mrad, N.; Ajram, G.; Rossi, J.C.; Boiteau, L.; Duvernay, F.; Pascal, R.; Danger, G. The prebiotic C-terminal elongation of peptides can be bnitiated by N-carbamoyl amino acids. Chem. A Eur. J. 2017, 23, 7418–7421.
- Beaufils, D.; Danger, G.; Boiteau, L.; Rossi, J.-C.; Pascal, R. Diastereoselectivity in prebiotically relevant 5(4H)-oxazolone-mediated peptide couplings. Chem. Commun. 2014, 50, 3100–3102.
- Liu, Z.; Beaufils, D.; Rossi, J.-C.; Pascal, R. Evolutionary importance of the intramolecular pathways of hydrolysis of phosphate ester mixed anhydrides with amino acids and peptides. Sci. Rep. 2014, 4, 7440.
- Ni, F.; Gao, X.; Zhao, Z.-X.; Huang, C.; Zhao, Y.-F. On the electrophilicity of cyclic acylphosphoramidates (CAPAs) postulated as intermediates. Eur. J. Org. Chem. 2009, 2009, 3026–3035.
- Fialho, D.M.; Karunakaran, S.C.; Greeson, K.W.; Martínez, I.; Schuster, G.B.; Krishnamurthy, R.; Hud, N.V. Depsipeptide nucleic acids: Prebiotic formation, oligomerization, and self-assembly of a new proto-nucleic acid candidate. J. Am. Chem. Soc. 2021, 143, 13525–13537.
- Weber, A.L.; Orgel, L.E. Amino acid activation with adenosine 5′-phosphorimidazolide. J. Mol. Evol. 1978, 11, 9–16.
- Tamura, K.; Schimmel, P. Chiral-selective aminoacylation of an RNA minihelix. Science 2004, 305, 1253.
- Wu, L.-F.; Su, M.; Liu, Z.; Bjork, S.J.; Sutherland, J.D. Interstrand aminoacyl transfer in a tRNA acceptor stem-overhang mimic. J. Am. Chem. Soc. 2021, 143, 11836–11842.
- Tamura, K.; Schimmel, P. Peptide synthesis with a template-like RNA guide and aminoacyl phosphate adaptors. Proc. Natl. Acad. Sci. USA 2003, 100, 8666–8669.
- Jash, B.; Richert, C. Templates direct the sequence-specific anchoring of the C-terminus of peptido RNAs. Chem. Sci. 2020, 11, 3487–3494.
- Roberts, S.J.; Liu, Z.; Sutherland, J.D. Potentially prebiotic synthesis of aminoacyl-RNA via a bridging phosphoramidate-ester intermediate. J. Am. Chem. Soc. 2022, 144, 4254–4259.
- Weber, A.L.; Lacey, J.C., Jr. Genetic code correlations: Amino acids and their anticodon nucleotides. J. Mol. Evol. 1978, 11, 199–210.
- Jash, B.; Tremmel, P.; Jovanovic, D.; Richert, C. Single nucleotide translation without ribosomes. Nat. Chem. 2021, 13, 751–757.
- Müller, F.; Escobar, L.; Xu, F.; Węgrzyn, E.; Nainytė, M.; Amatov, T.; Chan, C.; Pichler, A.; Carell, T. A prebiotically plausible scenario of an RNA-peptide world. Nature 2022, 605, 279–284.
- Nainytė, M.; Müller, F.; Ganazzoli, G.; Chan, C.; Crisp, A.; Globisch, D.; Carell, T. Amino acid modified RNA bases as building blocks of an early earth RNA-peptide world. Chem. A Eur. J. 2020, 26, 14856–14860.
- Ban, N.; Nissen, P.; Hansen, J.; Moore, P.B.; Steitz, T.A. The complete atomic structure of the large ribosomal subunit at 2.4 A resolution. Science 2000, 289, 905–920.
- Polikanov, Y.S.; Steitz, T.A.; Innis, C.A. A proton wire to couple aminoacyl-tRNA accommodation and peptide-bond formation on the ribosome. Nat. Struct. Mol. Biol. 2014, 21, 787–793.
- Nissen, P.; Hansen, J.; Ban, N.; Moore, P.B.; Steitz, T.A. The structural basis of ribosome activity in peptide bond synthesis. Science 2000, 289, 920–930.
- Noller, H.F. RNA structure: Reading the ribosome. Science 2005, 309, 1508–1514.
- Cech, T.R. The ribosome is a ribozyme. Science 2000, 289, 878–879.
- Fahnestock, S.; Neumann, H.; Shashoua, V.; Rich, A. Ribosome-catalyzed ester formation. Biochemistry 1970, 9, 2477–2483.
- Fahnestock, S.; Rich, A. Ribosome-catalyzed polyester formation. Science 1971, 173, 340–343.
- Lohse, P.A.; Szostak, J.W. Ribozyme-catalysed amino-acid transfer reactions. Nature 1996, 381, 442–444.
- Frenkel-Pinter, M.; Haynes, J.W.; Mohyeldin, A.M.; Sargon, A.B.; Petrov, A.S.; Krishnamurthy, R.; Hud, N.V.; Williams, L.D.; Leman, L.J. Mutually stabilizing interactions between proto-peptides and RNA. Nat. Commun. 2020, 11, 3137.
- Bocchetta, M.; Xiong, L.; Mankin, A.S. 23S rRNA positions essential for tRNA binding in ribosomal functional sites. Proc. Natl. Acad. Sci. USA 1998, 95, 3525–3530.
