Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Conner Chen and Version 1 by Kamlesh Kumar.
People in the life sciences who work with Artificial Intelligence (AI) and Machine Learning (ML) are under increased pressure to develop algorithms faster than ever. The possibility of revealing innovative insights and speeding breakthroughs lies in using large datasets integrated on several levels. However, even if there is more data at our disposal than ever, only a meager portion is being filtered, interpreted, integrated, and analyzed. Both an increase in the learning capacity and the provision of a decision support system at a size that is redefining the future of healthcare are enabled by AI and ML.
- medical infrastructure
- healthcare infrastructure
- artificial intelligence
1. Introduction
Artificial Intelligence (shown in Figure 1) was initially introduced in the medical sector in 1976 when a computer algorithm was used to determine the reasons for intense abdominal pain [1]. From the first healthcare implementation of Artificial I ntelligence (AI) to today, numerous applications of AI have been introduced to enhance the strength and overcome the shortcomings of available medical infrastructure. These implementations include assistance in disease detection, like diabetes detection or cancer detection; enhancement of pathology classification, such as classification of radiology scans and outlining electrocardiogram qualities for cardiac study [2]; and forecasting illnesses with algorithms based on Machine Learning (ML) and Deep Learning (DL) developed to solve problems such as the pandemic of COVID-19 [3[3][4],4], serving as an epitome. However, despite the healthcare industry’s considerable investment in technological advancements, its deployment and integration in healthcare are still in their preliminary stages [5]. Workforce scarcity and exhaustion, and the transition to long-term illness care, are among the most significant concerns in healthcare. Thus, AI can significantly enhance the healthcare infrastructure through its extensive applicability.
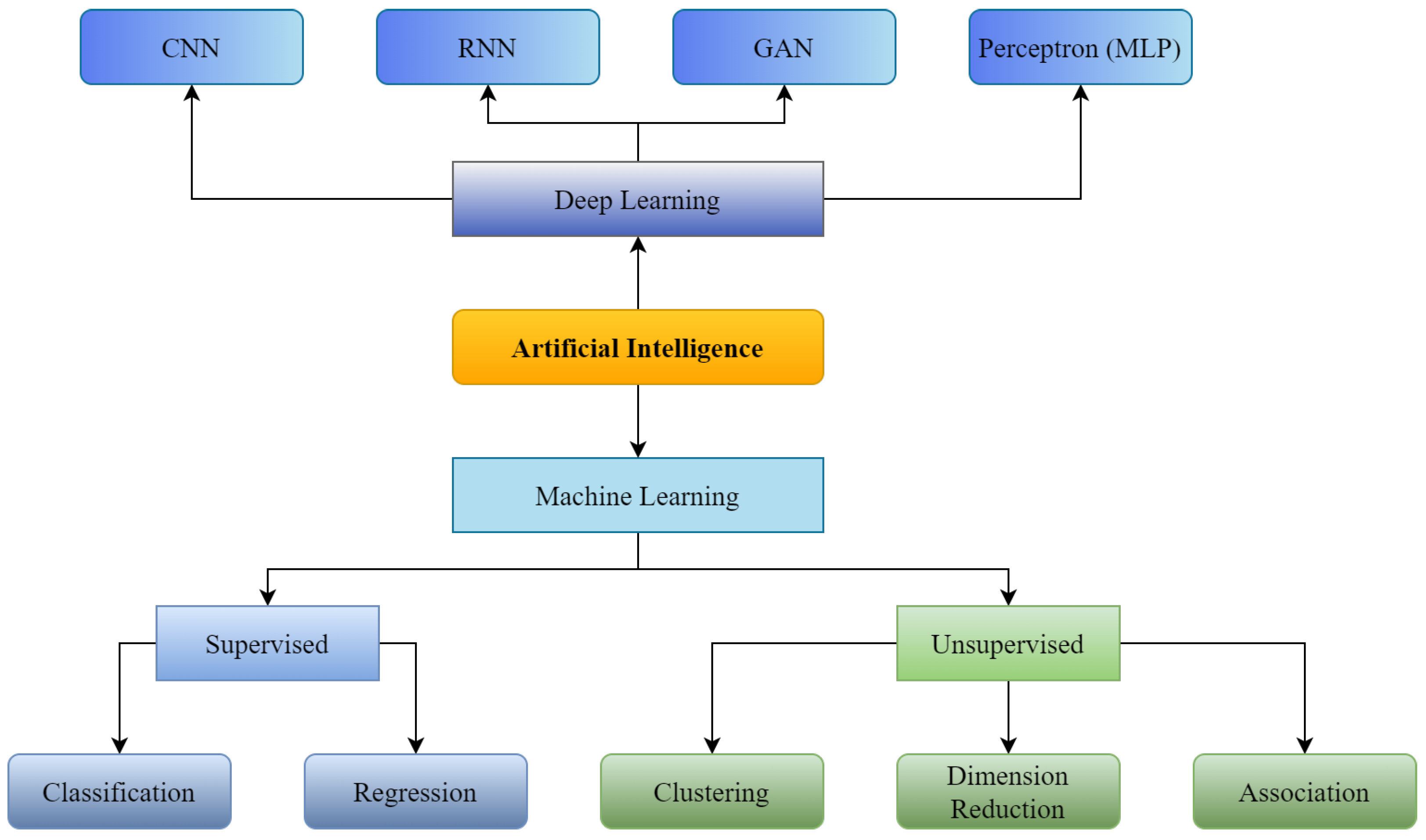
Figure 1. Supervised and Unsupervised machine learning with Convolutional Neural Network (CNN), Recurrent Neural Neural Network (RNN), Generative Adversarial Network (GAN) as branches of Deep Learning.
AI is revolutionizing medical infrastructure significantly in diagnosing various diseases, using medical imaging from various available medical imaging formats like- X-rays, MRI, CT, etc. AI can easily detect diseases related to the skin, lungs, organs, and viral issues. For instance, some skin diseases include skin cancer, acne, and rashes. Early identification of such skin illnesses can prevent critical future problems. Furthermore, in this direction, researchers like- Shoieb et al. [6] classified skin cancer using available data consisting of cancer images. Their results showed a considerable increase in skin diagnostic accuracy and precision compared to earlier studies. Zaher et al. [7] and Charan et al. [8] presented such a model to detect breast cancer using radiology scans. Moreover, like skin and breast cancers, lung cancer is amongst the deadliest ailments across the world [9,10][9][10] that causes 7.6 million yearly deaths worldwide [11]. Moreover, early detection of such a deadly disease is the only possible cure to reduce this number [12]. Many researchers [13,14,15,16,17][13][14][15][16][17] have proposed AI and ML-based approaches for predicting lung cancer using various sources. Apart from these applications, researchers have used AI for the detection of tumor [18], tuberculosis [19], and even COVID-19 diagnosis [20] as well, mainly using chest X-rays. Medical imaging for disease diagnosis and prognosis is widely accepted and increasing with boundless expectations and improvements in conventional medical infrastructure.
The imaging data is machine-readable, allowing the ML and DL algorithms to be run after adequate preprocessing or quality check steps. Moreover, a substantial chunk of healthcare data, including clinical laboratory reports, physical examinations, discharge summaries, and operation notes, usually remains narrative, which would be amorphous and inaccessible to computer algorithms. In this situation, Natural Language Processing (NLP) aims to gather relevant data from the available chunk to support clinical judgments [21]. Based on existing records, NLP uses text processing to define disease-related phrases in medical documentation [22]. Subsequently, keywords are selected after assessing their influence on categorizing normal and abnormal instances. For example, Miller et al. [23] employed NLP to track undesirable events in the laboratory environment. In addition, NLP pipelines can aid in illness detection. This technology has also been used for detecting various disease-related factors for cerebral aneurysms using clinical notes [24] to distinguish normal individuals from patients suffering from cerebral issues.
Moreover, Afzal et al. [22] used NLP to extract peripheral arterial disease-related keywords from clinical narratives. These were then utilized for differentiation between peripheral arterial disease and normal patients. Not only to collect documentation about disease-related information but NLP is being explored to learn various suicide factors [25] from suicide notes by developing a vocabulary or language-specific database. Moreover, this branch of Artificial Intelligence is utilized for evaluating mental illness [26], understanding the clinical workflow [27[27][28],28], classifying medical prescriptions [29], forecasting patient predilection [30,31][30][31], predicting risk and stratification of a patient [32], making decision support system [33], and question answering [34]. Juhn et al. [35] have also introduced an autonomous system that can significantly reduce the burden of medical triage by collecting patient data and understanding it with NLP to help the patient while choosing a consultant and completing other procedures, which usually take a long time in any hospital building.
Robotics focuses on designing and developing robots. When combined with AI, the result is an intelligent machine that can be taught to undertake complicated processes requiring much thought and continual learning. Consequently, a further branch of AI is interested in educating a robot to interpret the world in predicated but generic ways, control things in intractable surroundings, and communicate with humans. Robots that may undertake complex surgical treatments, such as minimally invasive and surgeon-less surgeries, are known as “Surgical Robots”. The systems represented [36,37][36][37] are the gold standard of care in many laparoscopic operations, with approximately a million operations performed each year. Robotic surgery enhances the effectiveness, precision, and reliability of surgical operations allowing quicker recovery and better patient outcomes. Apart from surgical tasks, in healthcare, there are several duties related to management. The application of AI in this domain has less adaptability than acute services, but it can deliver substantial productivity. It is necessary for hospitals because, for instance, a US nurse spends an average of 25% of her job tenure on administrative tasks [38]. This aim is most likely connected to robotic process automation technology. It is used in various medical systems, such as user registration, medical documentation, payment flow administration, and clinical record-keeping [39,40][39][40]. Besides patient interactions, mental well-being, telemedicine, and chatbots are often used in other medical contexts.
Research and development are some of the most critical areas, and boosting these areas can significantly strengthen healthcare infrastructure. For example, machine (and deep) learning algorithms have been used in a variety of drug discovery processes, including physio-chemical, poly-pharmacology, drug repositioning, quantitative structure-activity relationship, pharmacophore modeling, drug monitoring and revealing, toxicity prediction, ligand-based virtual screening, structure-based virtual screening, and peptide synthesis activities [41]. In addition, pharmacogenetics and molecular biomarker technologies may forecast drug efficacy and medication reactions within subjects, essential to precision medicine progress [42].
A significant number of studies [43,44][43][44] conducted in revolutionizing the conventional drug design include DeepMind at Google and AlphaFold, a tool based on AI, trained on protein binding domain spatial information to estimate the multi-dimensional shape of a protein from the sequence of amino acids. AI has become an effective tool in today’s technology because it saves time and money. Such rapid discovery and development of drugs can save millions of lives in critical conditions like a pandemic, which can be defined as an explicit strengthening of overall infrastructure by reducing overall development costs with increased drug efficacy [45,46,47][45][46][47].
Furthermore, supplying incorrect dosage is one of the hackneyed issues in this sector that not only causes the loss of millions of dollars but also weakens the whole infrastructure by increasing the mortality rate with undesired and deadly side effects [48]. With the rise of AI, numerous scientists are turning to ML and DL techniques to identify optimal medicine dosages. For example, Shen et al. [49] created an AI-based system called AI-PRS to discover the best medication doses and combinations for HIV treatment using antiretroviral therapy. Julkunen et al. [50] also created comboFM, a unique ML-based tool for determining optimal medication coalescing and dosing in pre-clinical investigations such as cancer cells. CombinationFM uses factorization machines—a machine learning framework to analyze multi-dimensional data and discover optimum medicine combinations and doses. Xue et al. [51] have also identified a suitable bioactive agent and inspected the drug delivery.
As discussed above, AI has become an expert in many stages of drug distribution and optimization. Studies also show how AI can further help in rapid discoveries and development of drugs by working on various stages like predicting interactions between proteins and their foldings [52], ligand and structure-base virtual screening [53[53][54],54], quantitative structure-activity relationship modeling and drug re-purposing [55,56][55][56], estimating physicochemical properties and bioactivity [57[57][58],58], toxicity and mode of action prediction of the compound [59[59][60],60], recognition of molecular pathways polypharmacology [61[61][62],62], de novo drug designing [63], pharmaceutical manufacturing and clinical trial design [64], and related ones [65[65][66],66], to various crucial, even may be incurable, diseases with its unbounded intelligence and memory power on 0.9-micron thick silicon bridges, also known as memory chips. All these studies show how AI enhances drug research and development for strengthening medical infrastructure economically and in terms of rapid processing.
2. Evolution of AI and ML in Medical Infrastructure
As shown in Figure 2, there are numerous possible applications of AI and ML algorithms to develop efficient tools to strengthen the healthcare infrastructure. Over the last five decades, AI has dramatically impacted the medical infrastructure. The scope of AI and ML applications has increased, opening the doors to individualized medicine rather than algorithm-based treatment. Predictive models that predict illness, treatment responses, and even preventive medicine in the future may be developed using such models [68][67]. AI may strengthen the healthcare infrastructure by improving diagnostic accuracy, clinical operations and workflow, procedure accuracy, treatment monitoring, and overall patient satisfaction. The evolution of AI and ML in medicine is detailed in the following timeline.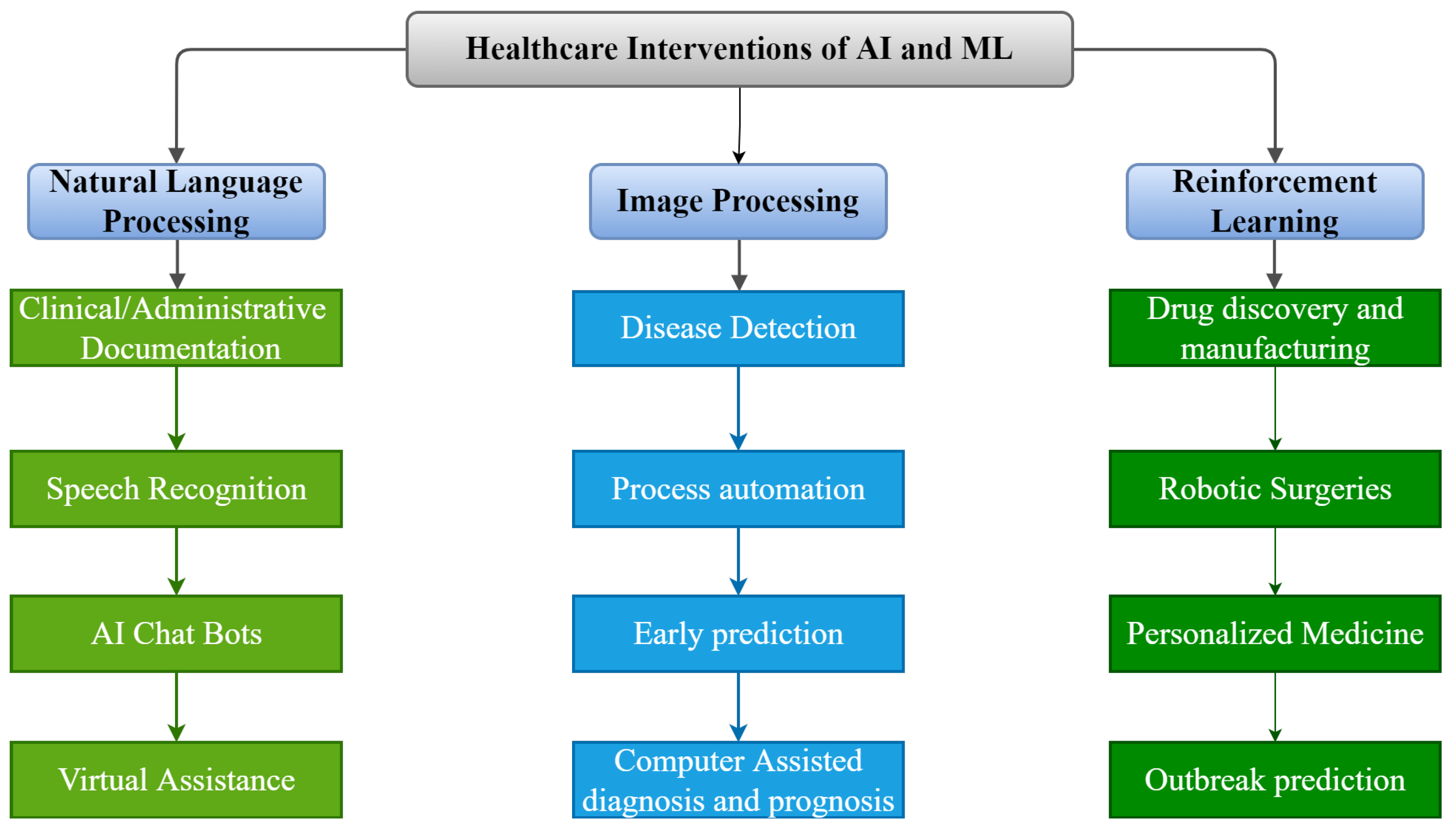
Figure 2.
Applications of AI and ML in Medical Infrastructure.
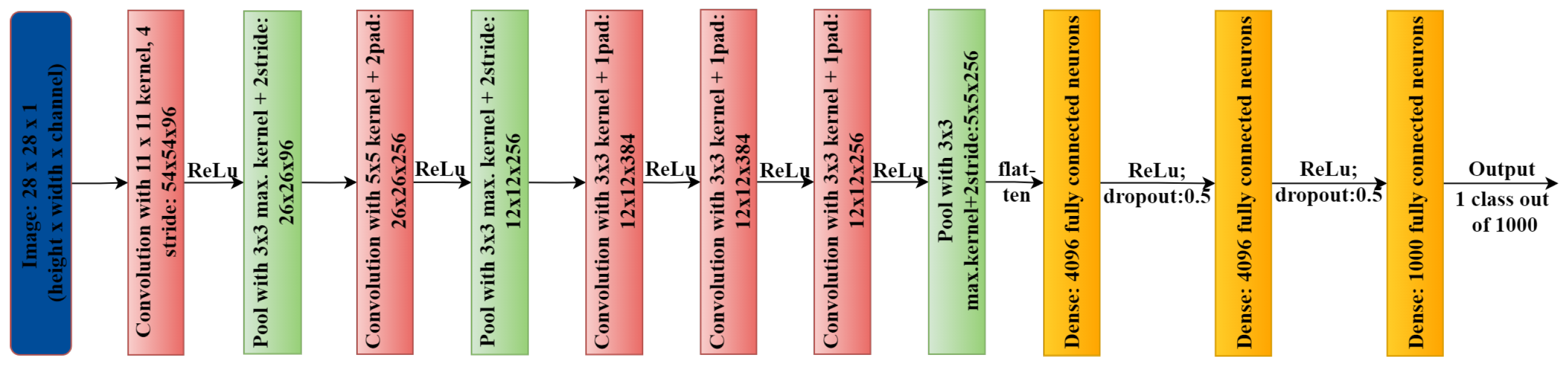
Figure 3.
AlexNet architecture.
References
- Fogel, A.L.; Kvedar, J.C. Artificial intelligence powers digital medicine. NPJ Digit. Med. 2018, 1, 5.
- Topol, E.J. High-performance medicine: The convergence of human and artificial intelligence. Nat. Med. 2019, 25, 44–56.
- Vaishya, R.; Javaid, M.; Khan, I.H.; Haleem, A. Artificial Intelligence (AI) applications for COVID-19 pandemic. Diabetes Metab. Syndr. Clin. Res. Rev. 2021, 14, 337–339.
- Gozes, O.; Frid-Adar, M.; Greenspan, H.; Browning, P.D.; Zhang, H.; Ji, W.B.; Bernheim, A.; Siegel, E. Rapid AI Development Cycle for the Coronavirus (COVID-19) Pandemic: Initial Results for Automated Detection & Patient Monitoring using Deep Learning CT Image Analysis. arXiv 2020, arXiv:2003.05037.
- Chen, J.; See, K.C. Artificial Intelligence for COVID-19: Rapid Review. J. Med. Internet Res. 2021, 22, e21476.
- Shoieb, D.; Youssef, S.; Aly, W. Computer-Aided Model for Skin Diagnosis Using Deep Learning. J. Image Graph. 2016, 4, 116–121.
- Abdel-Zaher, A.M.; Eldeib, A.M. Breast cancer classification using deep belief networks. Expert Syst. Appl. 2016, 46, 139–144.
- Charan, S.; Khan, M.J.; Khurshid, K. Breast cancer detection in mammograms using convolutional neural network. In Proceedings of the 2018 International Conference on Computing, Mathematics and Engineering Technologies (iCoMET), Sukkur, Pakistan, 3–4 March 2018.
- Cheng, J.Z.; Ni, D.; Chou, Y.H.; Qin, J.; Tiu, C.M.; Chang, Y.C.; Huang, C.S.; Shen, D.; Chen, C.M. Computer-Aided Diagnosis with Deep Learning Architecture: Applications to Breast Lesions in US Images and Pulmonary Nodules in CT Scans. Sci. Rep. 2016, 6, 24454.
- Suzuki, K.; Li, F.; Sone, S.; Doi, K. Computer-aided diagnostic scheme for distinction between benign and malignant nodules in thoracic low-dose CT by use of massive training artificial neural network. IEEE Trans. Med. Imaging 2005, 24, 1138–1150.
- Nie, L.; Wang, M.; Zhang, L.; Yan, S.; Zhang, B.; Chua, T.S. Disease Inference from Health-Related Questions via Sparse Deep Learning. IEEE Trans. Knowl. Data Eng. 2015, 27, 2107–2119.
- Nie, L.; Zhang, L.; Yang, Y.; Wang, M.; Hong, R.; Chua, T.S. Beyond Doctors: Future Health Prediction from Multimedia and Multimodal Observations. In Proceedings of the 23rd ACM international conference on Multimedia, Brisbane, Australia, 26–30 October 2015.
- Zhou, Y.; Lu, Y.; Pei, Z. Accurate diagnosis of early lung cancer based on the convolutional neural network model of the embedded medical system. Microprocess. Microsyst. 2021, 81, 103754.
- Orozco, H.M.; Villegas, O.O.V.; Maynez, L.O.; Sanchez, V.G.C.; de Jesus Ochoa Dominguez, H. Lung nodule classification in frequency domain using support vector machines. In Proceedings of the 2012 11th International Conference on Information Science, Signal Processing and their Applications (ISSPA), Montreal, QC, Canada, 2–5 July 2012.
- Shao, H.; Cao, L.; Liu, Y. A detection approach for solitary pulmonary nodules based on CT images. In Proceedings of the 2012 2nd International Conference on Computer Science and Network Technology, Changchun, China, 29–31 December 2012.
- da Silva, G.L.F.; da Silva Neto, O.P.; Silva, A.C.; de Paiva, A.C.; Gattass, M. Lung nodules diagnosis based on evolutionary convolutional neural network. Multimed. Tools Appl. 2017, 76, 19039–19055.
- de Sousa Costa, R.W.; da Silva, G.L.F.; de Carvalho Filho, A.O.; Silva, A.C.; de Paiva, A.C.; Gattass, M. Classification of malignant and benign lung nodules using taxonomic diversity index and phylogenetic distance. Med. Biol. Eng. Comput. 2018, 56, 2125–2136.
- Anitha, R.; Raja, D.S.S. Development of computer-aided approach for brain tumor detection using random forest classifier. Int. J. Imaging Syst. Technol. 2017, 28, 48–53.
- Rahman, T.; Khandakar, A.; Kadir, M.A.; Islam, K.R.; Islam, K.F.; Mazhar, R.; Hamid, T.; Islam, M.T.; Kashem, S.; Mahbub, Z.B.; et al. Reliable Tuberculosis Detection Using Chest X-Ray With Deep Learning, Segmentation and Visualization. IEEE Access 2021, 8, 191586–191601.
- Azeem, M.A.; Khan, M.I.; Khan, S.A. COVID-19 Detection via Image Classification using Deep Learning on Chest X-Ray. In Proceedings of the 2021 Ethics and Explainability for Responsible Data Science (EE-RDS), Johannesburg, South Africa, 27–28 October 2021.
- Kantor, P. Foundations of Statistical Natural Language Processing; MIT Press: Cambridge, MA, USA, 1999; Volume 2, p. 91.
- Afzal, N.; Sohn, S.; Abram, S.; Scott, C.G.; Chaudhry, R.; Liu, H.; Kullo, I.J.; Arruda-Olson, A.M. Mining peripheral arterial disease cases from narrative clinical notes using natural language processing. J. Vasc. Surg. 2017, 65, 1753–1761.
- Miller, T.P.; Li, Y.; Getz, K.D.; Dudley, J.; Burrows, E.; Pennington, J.; Ibrahimova, A.; Fisher, B.T.; Bagatell, R.; Seif, A.E.; et al. Using electronic medical record data to report laboratory adverse events. Br. J. Haematol. 2017, 177, 283–286.
- Castro, V.M.; Dligach, D.; Finan, S.; Yu, S.; Can, A.; Abd-El-Barr, M.; Gainer, V.; Shadick, N.A.; Murphy, S.; Cai, T.; et al. Large-scale identification of patients with cerebral aneurysms using natural language processing. Neurology 2016, 88, 164–168.
- Morrow, D.; Zamora-Resendiz, R.; Beckham, J.C.; Kimbrel, N.A.; Oslin, D.W.; Tamang, S.; Crivelli, S. A case for developing domain-specific vocabularies for extracting suicide factors from healthcare notes. J. Psychiatr. Res. 2022, 151, 328–338.
- Zhang, T.; Schoene, A.M.; Ji, S.; Ananiadou, S. Natural language processing applied to mental illness detection: A narrative review. NPJ Digit. Med. 2022, 5, 46.
- Friedman, C.; Alderson, P.O.; Austin, J.H.M.; Cimino, J.J.; Johnson, S.B. A General Natural-language Text Processor for Clinical Radiology. J. Am. Med. Inform. Assoc. 1994, 1, 161–174.
- Ou, Y.; Patrick, J. Automatic Structured Reporting from Narrative Cancer Pathology Reports. Electron. J. Health Inform. 2014, 8, 20.
- Carchiolo, V.; Longheu, A.; Reitano, G.; Zagarella, L. Medical prescription classification: A NLP-based approach. In Proceedings of the 2019 Federated Conference on Computer Science and Information Systems, Leipzig, Germany, 1–4 September 2019.
- Miotto, R.; Li, L.; Kidd, B.A.; Dudley, J.T. Deep Patient: An Unsupervised Representation to Predict the Future of Patients from the Electronic Health Records. Sci. Rep. 2016, 6, 26094.
- Jensen, K.; Soguero-Ruiz, C.; Mikalsen, K.O.; Lindsetmo, R.O.; Kouskoumvekaki, I.; Girolami, M.; Skrovseth, S.O.; Augestad, K.M. Analysis of free text in electronic health records for identification of cancer patient trajectories. Sci. Rep. 2017, 7, 46226.
- Ye, C.; Fu, T.; Hao, S.; Zhang, Y.; Wang, O.; Jin, B.; Xia, M.; Liu, M.; Zhou, X.; Wu, Q.; et al. Prediction of Incident Hypertension Within the Next Year: Prospective Study Using Statewide Electronic Health Records and Machine Learning. J. Med. Internet Res. 2018, 20, e22.
- Imler, T.D.; Morea, J.; Imperiale, T.F. Clinical Decision Support With Natural Language Processing Facilitates Determination of Colonoscopy Surveillance Intervals. Clin. Gastroenterol. Hepatol. 2014, 12, 1130–1136.
- Abacha, A.B.; Zweigenbaum, P. MEANS: A medical question-answering system combining NLP techniques and semantic Web technologies. Inf. Process. Manag. 2015, 51, 570–594.
- Juhn, Y.; Liu, H. Artificial intelligence approaches using natural language processing to advance EHR-based clinical research. J. Allergy Clin. Immunol. 2021, 145, 463–469.
- Kim, W.; Choi, Y.; Cho, J.Y.; Yoon, Y.S.; Han, H.S. Robot single incision left lateral sectionectomy via da Vinci® Xi™ Single Site™ & vaginal extraction of the specimen. Surg. Oncol. 2020, 33, 254–255.
- Morelli, L.; Guadagni, S.; Caprili, G.; Candio, G.D.; Boggi, U.; Mosca, F. Robotic right colectomy using the Da Vinci Single-Site® platform: Case report. Int. J. Med. Robot. Comput. Assist. Surg. 2013, 9, 258–261.
- Commins, J. Nurses Say Distractions Cut Bedside Time by 25%. Health Leaders. 2021. Available online: https://www.healthleadersmedia.com/nursing/nurses-say-distractions-cut-bedside-time-25 (accessed on 30 November 2022).
- Berg, S. Nudge Theory Explored to Boost Medication Adherence. 2021. Available online: https://www.ama-assn.org/delivering-care/patient-support-advocacy/nudge-theory-explored-boost-medication-adherence (accessed on 30 November 2022).
- Utermohlen, K. Four Robotic Process Automation (RPA) Applications in the Healthcare Industry. 2022. Available online: https://medium.com/@karl.utermohlen/4-robotic-process-automation-rpa-applications-in-the-healthcare-industry-4d449b24b613 (accessed on 30 November 2022).
- Gupta, R.; Srivastava, D.; Sahu, M.; Tiwari, S.; Ambasta, R.K.; Kumar, P. Artificial intelligence to deep learning: Machine intelligence approach for drug discovery. Mol. Divers. 2021, 25, 1315–1360.
- Zhang, D.H.; Wu, K.L.; Zhang, X.; Deng, S.Q.; Peng, B. In silico screening of Chinese herbal medicines with the potential to directly inhibit 2019 novel coronavirus. J. Integr. Med. 2020, 18, 152–158.
- Powles, J.; Hodson, H. Google DeepMind and healthcare in an age of algorithms. Health Technol. 2017, 7, 351–367.
- Bennett, W.F.D.; He, S.; Bilodeau, C.L.; Jones, D.; Sun, D.; Kim, H.; Allen, J.E.; Lightstone, F.C.; Ingólfsson, H.I. Predicting Small Molecule Transfer Free Energies by Combining Molecular Dynamics Simulations and Deep Learning. J. Chem. Inf. Model. 2020, 60, 5375–5381.
- Davenport, T.H.; Ronanki, R. Artificial Intelligence for the Real World. 2018. Available online: https://hbr.org/webinar/2018/02/artificial-intelligence-for-the-real-world (accessed on 15 November 2022).
- Zhavoronkov, A.; Vanhaelen, Q.; Oprea, T.I. Will Artificial Intelligence for Drug Discovery Impact Clinical Pharmacology? Clin. Pharmacol. Ther. 2020, 107, 780–785.
- Watson, O.P.; Cortés-Ciriano, I.; Taylor, A.R.; Watson, J.A. A decision theoretic approach to model evaluation in computational drug discovery. arXiv 2018, arXiv:1807.08926.
- Dimmitt, S.; Stampfer, H.; Martin, J.H. When less is more—Efficacy with less toxicity at the ED50. Br. J. Clin. Pharmacol. 2017, 83, 1365–1368.
- Shen, Y.; Liu, T.; Chen, J.; Li, X.; Liu, L.; Shen, J.; Wang, J.; Zhang, R.; Sun, M.; Wang, Z.; et al. Harnessing Artificial Intelligence to Optimize Long-Term Maintenance Dosing for Antiretroviral-Naive Adults with HIV-1 Infection. Adv. Ther. 2019, 3, 1900114.
- Julkunen, H.; Cichonska, A.; Gautam, P.; Szedmak, S.; Douat, J.; Pahikkala, T.; Aittokallio, T.; Rousu, J. Leveraging multi-way interactions for systematic prediction of pre-clinical drug combination effects. Nat. Commun. 2020, 11.
- Xue, R.; Liao, J.; Shao, X.; Han, K.; Long, J.; Shao, L.; Ai, N.; Fan, X. Prediction of Adverse Drug Reactions by Combining Biomedical Tripartite Network and Graph Representation Model. Chem. Res. Toxicol. 2019, 33, 202–210.
- Cunningham, J.M.; Koytiger, G.; Sorger, P.K.; AlQuraishi, M. Biophysical prediction of protein–peptide interactions and signaling networks using machine learning. Nat. Methods 2020, 17, 175–183.
- Gazgalis, D.; Zaka, M.; Abbasi, B.H.; Logothetis, D.E.; Mezei, M.; Cui, M. Protein Binding Pocket Optimization for Virtual High-Throughput Screening (vHTS) Drug Discovery. ACS Omega 2021, 5, 14297–14307.
- Amin, S.A.; Ghosh, K.; Gayen, S.; Jha, T. Chemical-informatics approach to COVID-19 drug discovery: Monte Carlo based QSAR, virtual screening and molecular docking study of some in-house molecules as papain-like protease (PLpro) inhibitors. J. Biomol. Struct. Dyn. 2020, 39, 4764–4773.
- Ha, E.J.; Lwin, C.T.; Durrant, J.D. LigGrep: A tool for filtering docked poses to improve virtual-screening hit rates. J. Cheminformatics 2021, 12, 69.
- Spiegel, J.O.; Durrant, J.D. AutoGrow4: An open-source genetic algorithm for de novo drug design and lead optimization. J. Cheminform. 2020, 12, 1–16.
- Schneider, P.; Walters, W.P.; Plowright, A.T.; Sieroka, N.; Listgarten, J.; Goodnow, R.A.; Fisher, J.; Jansen, J.M.; Duca, J.S.; Rush, T.S.; et al. Rethinking drug design in the artificial intelligence era. Nat. Rev. Drug Discov. 2020, 19, 353–364.
- Rashid, M. Design, synthesis and ADMET prediction of bis-benzimidazole as anticancer agent. Bioorganic Chem. 2020, 96, 103576.
- Uygun, M.T.; Amudi, K.; Turaçlı, İ.D.; Menges, N. A new synthetic approach for pyrazolopyrazine-4(5H)-one derivatives and their antiproliferative effects on lung adenocarcinoma cell line. Mol. Divers. 2021, 26, 113–124.
- Srivastava, A.; Siddiqui, S.; Ahmad, R.; Mehrotra, S.; Ahmad, B.; Srivastava, A.N. Exploring nature’s bounty: Identification of Withania somnifera as a promising source of therapeutic agents against COVID-19 by virtual screening and in silico evaluation. J. Biomol. Struct. Dyn. 2020, 40, 1858–1908.
- Gu, S.; Lai, L.-h. Associating 197 Chinese herbal medicine with drug targets and diseases using the similarity ensemble approach. Acta Pharmacol. Sin. 2019, 41, 432–438.
- Taha, K.F.; Khalil, M.; Abubakr, M.S.; Shawky, E. Identifying cancer-related molecular targets of Nandina domestica Thunb. by network pharmacology-based analysis in combination with chemical profiling and molecular docking studies. J. Ethnopharmacol. 2020, 249, 112413.
- Domenico, A.; Nicola, G.; Daniela, T.; Fulvio, C.; Nicola, A.; Orazio, N. De Novo Drug Design of Targeted Chemical Libraries Based on Artificial Intelligence and Pair-Based Multiobjective Optimization. J. Chem. Inf. Model. 2020, 60, 4582–4593.
- Wong, C.H.; Siah, K.W.; Lo, A.W. Estimation of clinical trial success rates and related parameters. Biostatistics 2018, 20, 273–286.
- Yousefian-Jazi, A.; Sung, M.K.; Lee, T.; Hong, Y.H.; Choi, J.K.; Choi, J. Functional fine-mapping of noncoding risk variants in amyotrophic lateral sclerosis utilizing convolutional neural network. Sci. Rep. 2020, 10, 12872.
- Gupta, R.; Ambasta, R.K.; Kumar, P. Identification of novel class I and class IIb histone deacetylase inhibitor for Alzheimer’s disease therapeutics. Life Sci. 2021, 256, 117912.
- Ruffle, J.K.; Farmer, A.D.; Aziz, Q. Artificial Intelligence-Assisted Gastroenterology— Promises and Pitfalls. Am. J. Gastroenterol. 2019, 114, 422–428.
- Kulikowski, C.A. An Opening Chapter of the First Generation of Artificial Intelligence in Medicine: The First Rutgers AIM Workshop, June 1975. Yearb. Med. Inform. 2015, 24, 227–233.
- Kulikowski, C.A. Beginnings of Artificial Intelligence in Medicine (AIM): Computational Artifice Assisting Scientific Inquiry and Clinical Art – with Reflections on Present AIM Challenges. Yearb. Med. Inform. 2019, 28, 249–256.
- Weiss, S.; Kulikowski, C.A.; Safir, A. Glaucoma consultation by computer. Comput. Biol. Med. 1978, 8, 25–40.
- Shortliffe, E.H.; Davis, R.; Axline, S.G.; Buchanan, B.G.; Green, C.; Cohen, S.N. Computer-based consultations in clinical therapeutics: Explanation and rule acquisition capabilities of the MYCIN system. Comput. Biomed. Res. 1975, 8, 303–320.
- Using Decision Support to Help Explain Clinical Manifestations of Disease. 2017. Available online: http://www.mghlcs.org/projects/dxplain (accessed on 30 October 2022).
- Ferrucci, D.; Levas, A.; Bagchi, S.; Gondek, D.; Mueller, E.T. Watson: Beyond Jeopardy! Artif. Intell. 2015, 199–200, 93–105.
- Mintz, Y.; Brodie, R. Introduction to artificial intelligence in medicine. Minim. Invasive Ther. Allied Technol. 2019, 28, 73–81.
- Bakkar, N.; Kovalik, T.; Lorenzini, I.; Spangler, S.; Lacoste, A.; Sponaugle, K.; Ferrante, P.; Argentinis, E.; Sattler, R.; Bowser, R. Artificial intelligence in neurodegenerative disease research: Use of IBM Watson to identify additional RNA-binding proteins altered in amyotrophic lateral sclerosis. Acta Neuropathol. 2017, 135, 227–247.
- Comendador, B.E.V.; Francisco, B.M.B.; Medenilla, J.S.; Nacion, S.M.T.; Serac, T.B.E. Pharmabot: A Pediatric Generic Medicine Consultant Chatbot. J. Autom. Control. Eng. 2015, 3, 137–140.
- Ni, L.; Lu, C.; Liu, N.; Liu, J. MANDY: Towards a Smart Primary Care Chatbot Application. In Communications in Computer and Information Science; Springer: Singapore, 2017; pp. 38–52.
- LeCun, Y.; Boser, B.; Denker, J.S.; Henderson, D.; Howard, R.E.; Hubbard, W.; Jackel, L.D. Backpropagation Applied to Handwritten Zip Code Recognition. Neural Comput. 1989, 1, 541–551.
- Krizhevsky, A.; Sutskever, I.; Hinton, G.E. ImageNet Classification with Deep Convolutional Neural Networks. Commun. ACM 2017, 60, 84–90.
- Liu, S.; Deng, W. Very deep convolutional neural network based image classification using small training sample size. In Proceedings of the 2015 3rd IAPR Asian Conference on Pattern Recognition (ACPR), Kuala Lumpur, Malaysia, 3–6 November 2015.
- Szegedy, C.; Liu, W.; Jia, Y.; Sermanet, P.; Reed, S.; Anguelov, D.; Erhan, D.; Vanhoucke, V.; Rabinovich, A. Going deeper with convolutions. In Proceedings of the 2015 IEEE Conference on Computer Vision and Pattern Recognition (CVPR), Boston, MA, USA, 7–12 June 2015.
- He, K.; Zhang, X.; Ren, S.; Sun, J. Deep Residual Learning for Image Recognition. In Proceedings of the 2016 IEEE Conference on Computer Vision and Pattern Recognition (CVPR), Las Vegas, NV, USA, 27–30 June 2016; pp. 770–778.
- Babenko, B.; Mitani, A.; Traynis, I.; Kitade, N.; Singh, P.; Maa, A.Y.; Cuadros, J.; Corrado, G.S.; Peng, L.; Webster, D.R.; et al. Detection of signs of disease in external photographs of the eyes via deep learning. Nat. Biomed. Eng. 2022, 6, 1370–1383.
- Domain-Specific Language Model Pretraining for Biomedical Natural Language Processing. Available online: https://www.microsoft.com/en-us/research/blog/domain-specific-language-model-pretraining-for-biomedical-natural-language-processing/ (accessed on 15 November 2022).
More
