Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 3 by Jessie Wu and Version 2 by Jessie Wu.
The success of a prosthetic treatment is closely related to the periodontal health of the individual. The periodontium constitutes the tissues that support the teeth. It is made up of two soft tissues (which are the gingiva and periodontal ligament) and two hard tissues (which are the root cementum and alveolar bone).
- prosthetic biomaterials
- health
- fixed dental prosthesis
1. Introduction
It is now widely accepted that periodontal disease (PD) is a multifactorial pathological entity induced by polymicrobial dysbiosis and host-mediated inflammation [1][2]. Key components in the pathophysiology of PD and its associated clinical features include gingival inflammation, periodontal ligament destruction, bone loss, bacterial colonization and invasion, increased numbers of polymorphonuclear (PMN) and epithelial cells, increased volume and decreased pH of the gingival crevicular fluid (GCF), as well as increased periodontal and gingival indices [3][4][5]. PD has a high prevalence worldwide, which is estimated to be 30–50% [6][7]. Currently, the most recent classification of PD is based on the severity (stages I–IV) and progression (grade A–C) of the disease [8]; however, for practical purposes, we can divide it into gingivitis, which refers to inflammation of the gums, and periodontitis, where in addition to inflammation there is also the destruction of periodontal tissues [9].
One of the factors that precisely leads to the development of periodontal disease is the use of poorly fabricated prosthetic restorations, with a poor marginal and internal fit greater than 120 μm [3]. In this way, and due to a greater marginal discrepancy, the cement forms a thicker layer and comes into contact with the oral cavity environment, which causes the dissolution of the cement and leads to increased accumulation and retention of bacteria in the area causing irreversible damage to the periodontal and pulpal tissues if not detected in time [10][11].
In addition, prosthetic restorative biomaterials can affect the formation of biofilms mainly because of their rough and irregular surfaces creating a series of niches in which microorganisms are protected from tooth brushing, muscular action and salivary flow favoring bacterial colonization and thus in turn the generation of an immunological response by the patient [12]. For this reason, the choice of a restorative material is an important part for the success of a prosthetic treatment in patients who need it, with the main objective of restoring function and esthetics, without leaving aside the biocompatibility and periodontal health that firmly accompany this process, ensuring greater durability of the restoration [13].
Nowadays, ceramics have become increasingly popular as prosthetic restorative materials due to the previously mentioned characteristics [14]; in fact, the most frequently used ceramic restorations in the dental area are porcelain-fused-to-metal crowns [15]; however, the use of metal-free ceramic restorations such as two-layer and single-layer (monolithic) zirconia prostheses has also increased in recent years, becoming a very promising alternative [16]. One of the main disadvantages in the use of metal-ceramic restorations is that the metal alloy can produce allergic reactions in some patients [17][18] and changes in the subgingival microbiota [19]. Finally, it has been observed through studies that this type of restoration fabricated by the conventional method has a lower marginal fit compared to zirconia restorations fabricated by the computer-aided design/computer-aided manufacturing (CAD/CAM) method, a system qualified as an effective and safe fabrication technology for producing fixed prosthetic restorations [20][21], which favors the accumulation of dentobacterial plaque, the formation of dental caries and the development of periodontal and pulp disease [3].
The diagnosis of periodontal disease is mainly performed by evaluating certain clinical and radiographic parameters that allow the dentist to determine the periodontal condition of the patient [22]. In addition, during the last three decades, research has advanced significantly in the field of oral biomarkers, and a wide variety of them have been detected in different fluids such as saliva and GCF, with the main purpose of improving early detection rates of periodontal disease and, in the future, replacing the form of diagnosis to a less invasive and more practical tool [23][24].
2. Biomaterials Used in Fixed Dental Prosthesis
Several prosthetic materials are used for the fabrication of fixed dental prostheses (as shown in Figure 1), including metal-ceramic and metal-free ceramics such as zirconium oxide and lithium disilicate [25][26][27][28][29][30][31][32][33][34][35][36][37][38][39][40], as well as polymeric materials such as polymethylmethacrylate (PMMA), with the latter mainly used for provisional purposes [27][41].
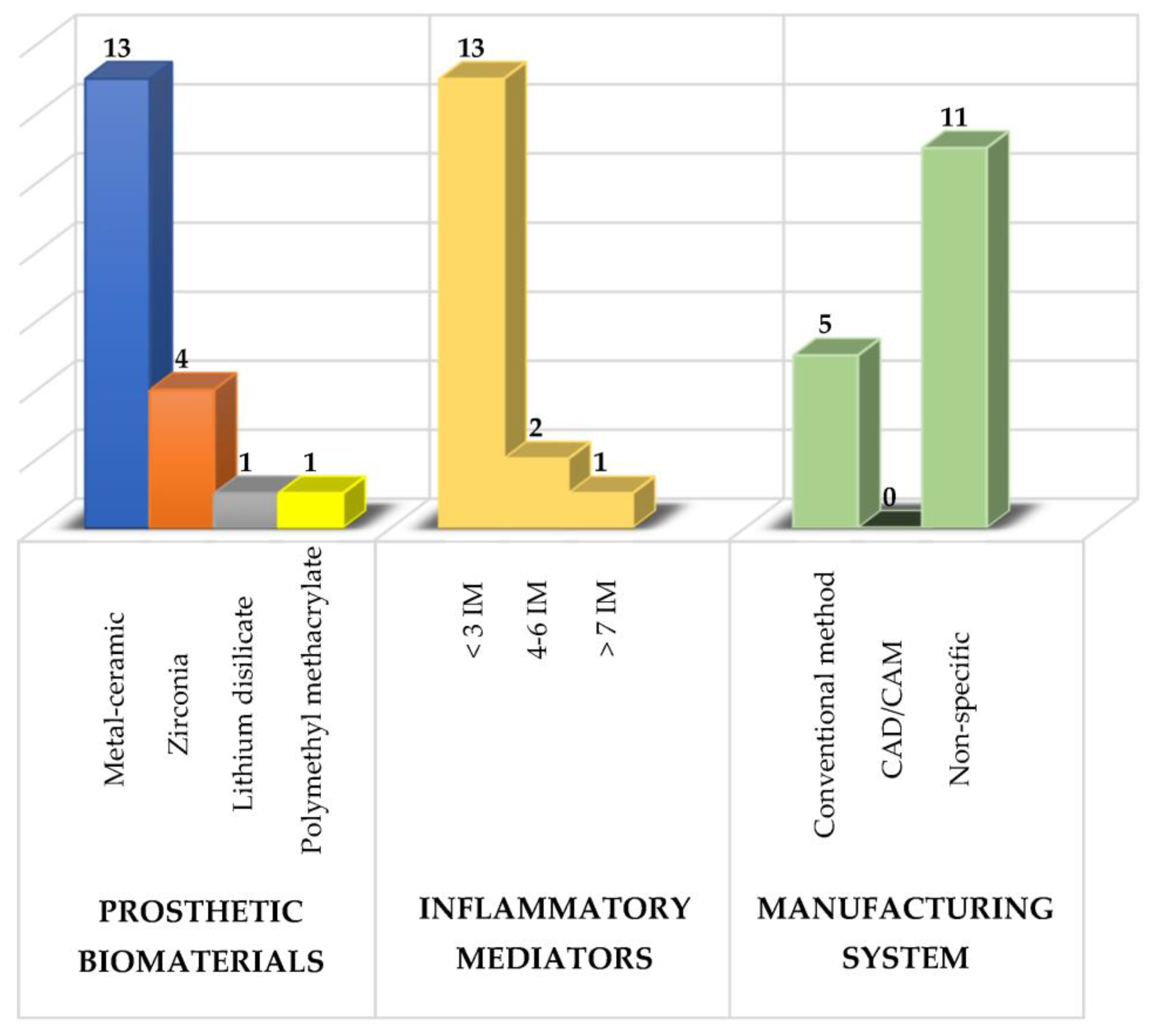
Figure 1. Summary of the study characteristics used in the revisewarch. IM: inflammatory mediators.
Of these prosthetic biomaterials, researchers have most frequently analyzed the use of metal-ceramic prostheses followed by zirconium prostheses with the main purpose of knowing their effects on the composition of the subgingival microbiota, the levels of various inflammatory mediators and the periodontal condition to determine which type of prosthetic biomaterial induces a lower inflammatory response and thus to have a therapeutic alternative that maintains the patient’s periodontal health [3][5].
Metal-ceramic prostheses are composed of a metal coping that supports the overlying ceramic. They are characterized because they are ideal where there is little tooth structure and are more economical compared to metal-free ceramics [42]. In relation to the use of this type of prosthesis, twenty-one different inflammatory mediators have been analyzed [25][28][29][31][32][33][34][35][36][37][38][39][40], and currently, it is very well documented that metal ceramic prostheses increase bacterial levels; therefore, there is a greater production of proinflammatory cytokines which leads to the destruction of the supporting tissues of the teeth [19][25]. This is partly due to the fact that the bacteria in the biofilm lower the pH by producing acidic substances that dissolve the surface oxides of the dental alloys, reducing the resistance to corrosion and, therefore, generating rough and irregular surfaces that favor a greater accumulation and retention of bacteria in the area [43].
On the other hand, the use of metal-free ceramic prostheses, mainly zirconia restorations [44], has increased in recent years, becoming a very promising alternative [16]. This type of restoration is very durable, require a minimally invasive preparation, which allows a greater preservation of the dental tissue, a high resistance to bending and fracture, as well as their translucency property to be retained; however, they are more expensive than metal-ceramic prostheses [45]. As a consequence of the use of this type of biomaterials, less biofilm formation has been found and therefore a more accentuated decrease in the levels of proinflammatory cytokines [25][27][28][30][46].
3. Periodontal Health in Patients with Fixed Dental Prosthesis
An ideal prosthetic treatment should not only be limited in restoring function and esthetics in the patient, but also achieve a healthy relationship with the periodontal tissues [47][48][49][50]. Thus, there are some factors that influence periodontal health such as cervical emergence profile, periodontal phenotype, biological thickness, materials and fabrication method of the restoration, prosthetic margin location, prepared tooth finish line, cementing materials and marginal and internal fit [9][11][51].
The cervical emergence profile or apical third design of a restoration is defined as the contour of the tooth and crown as they cross the soft tissues and rise toward the interproximal contact zone and the height of the facial and lingual contour [52]. On the other hand, the periodontal phenotype corresponds to the gingival thickness and width of the keratinized tissue (gingival phenotype) and bone morphotype [53]. This can be divided into thick and thin phenotypes. The thin periodontal phenotype represents a small proportion of cases [54]; however, it is considered a risk factor for additional bone loss [55], is associated with gingival recession [56] and also more prone to increasing the severity of peri-implantitis [57]. Having knowledge of the different periodontal phenotypes helps to minimize tissue damage and provides better results both in preparing the tooth for prosthetic placement and in gum recession. The biological thickness is defined as the dentogingival junction constituted by the junctional epithelium and the insertion of the supraalveolar connective tissue (2.04 mm). This space must be respected in order to maintain and protect periodontal health. Prostheses made and placed in an iatrogenic manner, i.e., violating the biological thickness, predispose an individual to the development of subgingival caries and result in an inflammatory process, which ultimately leads to the destruction of periodontal tissue [58][59].
In relation to biomaterials and the method of fabrication of prosthetic restorations, it has been observed that patients with zirconia prostheses obtain better results in terms of periodontal health, reduction of inflammation and maintenance of oral hygiene compared to metal-ceramic prostheses [56]. On the other hand, the cytomorphometric analysis of the periodontium before and after the insertion of fixed Cr-Co metal-ceramic prostheses fabricated by the conventional method and the CAD/CAM system, as well as the use of zirconia prostheses fabricated by the latter technique has shown an increase in the oral epithelial cell count and a decrease in the PMN count. Moreover, the cytological method is an informative test that allows us to identify etiological risk factors of the periodontitis, since it reveals the dynamics of the disease during its progression in prosthetic treatment [60].
The location of the prosthetic margin in relation to the completion line can be subgingival (below the gingival margin), juxtagingival (at the level of the gingival margin) or supragingival (above the gingival margin) [61]. The finishing line of a dental preparation is defined as the junction of the prepared and unprepared tooth structure with the margin of the restoration [48], so there are three types: horizontal, including straight shoulder, beveled shoulder, curved and sloped chamfer; vertical, including knife-edge preparation; and the preparation without a finishing line (BOPT). In fact, it has been shown that anterior teeth treated with the biologically oriented preparation technique (BOPT) present better plaque indices, stable probing depth, greater gingival thickness and stable gingival margins. In addition, prosthetic treatment using this technique has a positive impact on patient satisfaction, and based on these results, the authors highly recommend this technique, especially in cases of retreatment with prosthetic crowns [13]. The marginal and internal fit corresponds to the space between the margin of the restoration and the finishing line of the prepared tooth [10]. It is accepted that this space should not be >120 μm. In this way, with a thorough evaluation of the periodontium, an individualized and precise periodontal treatment can be proposed, since each case is different. It is also very important to motivate the patient to place greater emphasis on brushing and plaque control. In addition, maintenance appointments should be taken into account to avoid possible negative effects on the periodontium associated with the use of prosthetic restorations [62].
4. Changes in the Composition of the Subgingival Microbiota Related to the Use of Fixed Dental Prosthesis
The subgingival microbiota corresponds to diverse microbial communities (bacteria, archaea, fungi and viruses) that live attached to the root surface of teeth or dental implants with their outer surface in contact with the gingival tissue [63][64]. Bacteria are the most abundant component, and it is estimated that there are approximately 500 species that live in a state of eubiosis with the host [65][66][67]. However, the microbiota can undergo substantial changes as a result of various factors (unbalanced diet, smoking and poor oral hygiene) [68][69] that disrupt bacterial homeostasis and lead to a state of dysbiosis, where one or more types of periodontopathogenic bacteria proliferate, at least temporarily taking over the immune system, as happens in gingivitis and periodontitis [70][71]. A representation showing the associations between bacterial species colonizing the gingival sulcus is that of bacterial complexes in ecological equilibrium [66], where Porphyromonas gingivalis, Tannerella forsythia and Treponema denticola, which are the most periodontopathogenic bacteria constituting the red complex, have been detected in higher proportions in periodontitis conditions compared to healthy subjects [72][73][74][75] and even in patients with periodontitis and other systemic diseases [76].
On the other hand, in addition to natural teeth, dental implants and dentures are substrates for biofilm formation [77][78]. In relation to prosthetic restorations, it has been observed that the formation of biofilms on different types of dental ceramics is highly dependent on the genus and species of the microorganism [79]. In fact, the ability of microorganisms to adhere to prosthetic restorative materials has been mainly associated with the chemical composition of the biomaterial, the surface roughness, the surface free energy, its irregular topography and the release of metal ions, which could contribute to biofilm growth and generate a pathological process [43]. The most recent studies regarding the influence of metal-ceramic prosthetic restorations on the composition of the subgingival microbiota indicate a higher proportion of orange and red complex bacteria associated with a higher accumulation of dentobacterial plaque and bleeding on probing which is why they could be more vulnerable to future periodontal deterioration in case of a sudden change in the host immune response [19][35][80][81]. Additionally, the subgingival microbiota around single tooth implants has been evaluated and compared with natural teeth, finding a higher proportion of Klebsiella pneumonie, Pseudomonas aeruginosa and Streptococcus species compared with their controls, taking into account that the first two species are infrequently found in the oral cavity and are related to cases of periodontitis and peri-implantitis [82]. On the other hand, it has also been demonstrated that, in CAD/CAM fabricated zirconia prosthetic restorations, bacterial levels are more compatible with periodontal health, producing a more pronounced change towards clinical recovery in these patients, since zirconia is a highly biocompatible material with periodontal tissues, has less negative effects on gingival margins and also greatly inhibits biofilm formation producing a more subdued inflammatory response compared to metal-ceramic prostheses fabricated mainly by the conventional method [46].
With this, what is expected in the future is to be able to implement therapies that help to control and reduce the formation of biofilm around prosthetic restorations. In fact, in addition to conventional treatment (scaling and root planning), the use of photoactivation antimicrobial therapy, which combines the activation of a photosensitizer with a light source in the presence of oxygen, producing free radicals that generate damage to bacteria, has had very important microbiological and clinical results, especially in cases of patients with severe periodontitis and fixed dental prosthesis. For this reason, it is expected that this therapy will help to solve the problems and difficulties faced by conventional antimicrobial therapy and can function as a complement to conventional mechanical treatments [83][84].
References
- Dahlen, G.; Fejerskov, O.; Manji, F. Current concepts and alternative perspective on periodontal disease. BMC Oral Health 2020, 20, 235.
- Kwon, T.; Lamster, I.B.; Levin, L. Current concepts in the management of periodontitis. Int. Dent. J. 2021, 6, 462–476.
- Srimaneepong, V.; Heboyan, A.; Zafar, M.S.; Khurshid, Z.; Marya, A.; Fernandes, G.V.O.; Rokaya, D. Fixed prosthetic restorations and periodontal health: A narrative review. J. Funct. Biomater. 2022, 13, 15.
- Lim, G.; Janu, U.; Chiou, L.L.; Gandhi, K.K.; Palomo, L.; John, V. Periodontal health and systemic conditions. Dent. J. 2020, 8, 130.
- Heboyan, A.; Manrikyan, M.; Markaryan, M.; Vardanyan, I. Changes in the parameters of gingival crevicular fluid in masticatory function restoration by various prosthodontic construct ions. Int. J. Pharm. Sci. Res. 2020, 12, 2088–2093.
- Kassebaum, N.J.; Smith, A.G.C.; Bernabé, E.; Fleming, T.D.; Reynolds, A.E.; Vos, T.; Murray, C.J.L.; Marcenes, W.; GBD 2015 Oral Health Collaborators. Global, regional, and National prevalence, incidence, and Disability-Adjusted life year for oral conditions for 195 countries, 1990–2015: A systematic analysis for the global burden of diseases, injuries, and risk factors. J. Dent. Res. 2017, 4, 380–387.
- Martínez-García, M.; Castrejón-Pérez, R.C.; Rodríguez-Hernández, A.P.; Sandoval-Motta, S.; Vallejo, M.; Borges-Yáñez, S.A.; Hernández-Lemus, E. Incidence of arterial hypertension in people with periodontitis and characterization of the oral and subgingival microbiome: A study protocol. Front. Cardiovasc. Med. 2022, 8, 763293.
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and grading of periodontitis: Framework and proposal of new classification and case definition. J. Periodontol. 2018, 45, 149–161.
- Tsuchida, S.; Satoh, M.; Takiwaki, M.; Nomura, F. Current status of proteomic technologies for discovering and identifying gingival crevicular fluid biomarkers for periodontal disease. Int. J. Mol. Sci. 2018, 26, 86.
- Heboyan, A. Marginal and internal fit of fixed prosthodontic constructions: A literature review. IJDRR 2019, 2, 19.
- Heboyan, A.; Vardanyan, A.; Avetisyan, A. Cement selection in dental practice. World Sci. 2019, 2, 4–9.
- Monteiro, D.R.; De Souza Batista, V.E.; Caldeirã, A.C.; Jacinto, R.; Pessan, J.P. Oral prosthetic microbiology: Aspects related to the oral microbiome, Surface Properties, and strategies for controlling biofilms. Biofouling 2021, 37, 353–371.
- Serra-Pastor, B.; Bustamante-Hernández, N.; Fons-Font, A.; Solá-Ruíz, M.F.; Ravilla-León, M.; Agustín-Panadero, R. Periodontal behavior and patient satisfaction of anterior teeth restored with single zirconia crowns using a biological oriented preparation technique: A 6-year prospective clinical study. J. Clin. Med. 2021, 6, 3482.
- Kontonasaki, E.; Rigos, A.E.; Ilia, C.; Istantsos, T. Monolithic zirconia: An Update to Current knowledge. Optical properties, wear, and clinical performance. Dent. J. 2019, 2, 90.
- Warreth, A.; Elkareimi, Y. All-ceramic restorations: A review of the literature. Saudi Dent. J. 2020, 32, 365–372.
- Da Silva, L.H.; Lima, E.; Miranda, R.B.; Favero, S.S.; Lohbauer, U.; Cesar, P.F. Dental ceramics: A review of new materials and processing methods. Braz. Oral Res. 2017, 31, 133–146.
- Takaoka, Y.; Akiba, Y.; Nagasawa, M.; Ito, A.; Masui, Y.; Akiba, N.; Eguchi, K.; Miyazawa, H.; Tabeta, K.; Uoshima, K. The relationship between dental metal allergy, periodontitis, and palmoplantar pustulosis: An observational study. J. Prosthodont. Res. 2022, 30, 438–444.
- Rokaya, D.; Bohara, S.; Srimaneepong, V.; Kongkiatkamon, S.; Khurshid, Z.; Heboyan, A.; Zafar, M.S. Metallic Biomaterials for Medical and Dental Prosthetic Applications. In Functional Biomaterials; Jana, S., Ed.; Springer: Singapore, 2022.
- Rademacher, S.W.; Zaura, E.; Kleverlaaan, C.J.; Buijs, M.J.; Crielaard, W.; Loos, B.G.; Laine, M.L. Qualitative and quantitative differences in the subgingival microbiome of the restored and unrestored teeth. J. Periodontal Res. 2019, 54, 405–412.
- Dimitriadis, K.; Sfikas, A.K.; Kamnis, S.; Tsolka, P.; Agathopoulos, S. Influence of heat treatment on the microstructure and the physical and mechanical properties of dental highly translucent zirconia. J. Adv. Prosthodont. 2022, 14, 96–107.
- Heboyan, A.; Marya, A.; Syed, A.U.Y.; Khurshid, Z.; Zafar, M.S.; Rokaya, D.; Anastasyan, M. In vitro microscopic evaluation of metal- and zirconium-oxide-based crowns’ marginal fit. Pesqui Bras. Odontopediatria Clín. Integr. 2022, 22, e210144.
- Ko, T.J.; Byrd, K.M.; Kim, S.A. The chairside periodontal diagnostic toolkit: Past, present, and future. Diagnostics 2021, 22, 932.
- Steigmann, L.; Maekawa, S.; Sima, C.; Travan, S.; Wang, C.W.; Giannobile, W.V. Biosensor and Lab-on-a-chip Biomarker-identifying Technologies for Oral and Periodontal Diseases. Front. Pharmacol. 2020, 9, 1–18.
- Marya, A.; Rokaya, D.; Heboyan, A.; Fernandes, G.V.d.O. Biomolecular and Biochemical Aspects of the Oral Cavity. Molecules 2022, 24, 8676.
- Zhang, L.; Tao, Z.; Wang, X. Comparison of short-term restorative effects and periodontal health status of restorations made of different materials in full-crown restoration of mandibular premolar tooth defects. Dis. Markers 2022, 1, 3682741.
- Alrahlah, A.; Altwaim, M.; Alshuwaier, A.; Eldesouky, M.; Alzahrani, K.M.; Attar, E.A.; Alshahrani, A.; Abrar, E.; Vohra, F.; Abduljabbar, T. Influence of ceramic lumineers on inflammatory periodontal parameters and gingival crevicular fluid IL-6 and TNF- α levels-A clinical trial. Appl. Sci. 2021, 11, 2829.
- Elmagd, A.A.A.; Sabry, D.; Mohammed, E. Interleukin-1β activity in gingival crevicular fluid of abutment teeth with temporary fixed restorations versus final fixed restorations: Prospective observational study. Saudi Dent. J. 2021, 33, 322–327.
- Saravanakumar, P.; Veeravalli, P.; Kumar, A.; Mohamed, K.; Mani, U.; Grover, M.; Thangarajan, T.S. Effect of different crown materials on the interleukin-one beta content of gingival crevicular fluid endodontically treated molars: An original research. Cureus 2017, 9, 1–15.
- Kumar, N.K.; Kasa, V.K.; Padakandla, P.; Togau, H.; Kalagatla, S.; Chandra, S.N. Evaluation of chemokines in gingival crevicular fluid in children with dental caries and stainless steel crowns: A clinic-biochemical study. J. Indian Soc. Pedod. Prev. Dent. 2017, 34, 237–239.
- Ariaans, K.; Heussen, N.; Schiffer, H.; Wienert, A.L.; Plümäkers, B.; Lothar, R.; Wolfart, S. Use of molecular indicators of inflammation to assess the biocompatibility of all-ceramic restorations. J. Clin. Periodontol. 2016, 43, 173–179.
- Sakallioğlu, E.E.; Lütfioğlu, M.; Sakallioğlu, U.; Ceylan, G.K.; Pamuk, F.; Dede, F.Ö.; Dede, D. Gingival crevicular fluid levels of neuropeptides following dental restorations. J. Appl. Biomater. Funct. Mater. 2015, 13, 186–193.
- Chang, K.-C.J.; Wheater, M.A.; Jacobs, L.C.; Litonjua, L.A. Interleukins in gingival crevicular fluid in patients with definitive full-coverage restorations. Compend. Contin. Educ. Dent. 2014, 35, 18–24.
- Yu, L.; Su, J.; Zou, D.; Mariano, Z. The concentrations of IL-8 and IL-6 in gingival crevicular fluid during nickel-chromium alloy porcelain crown restoration. J. Mater. Sci. Mater. Med. 2013, 24, 1717–1722.
- Kushlinskii, N.E.; Solovykh, E.A.; Karaoglanova, T.B.; Boyar, U.; Gershtein, E.S.; Troshin, A.A.; Maksimovskaya, L.N.; Yanushevich, O.O. Matrix metalloproteinases and inflammatory cytokines in oral fluid of patients with chronic generalized periodontitis and various construction materials. Bull. Exp. Biol. Med. 2012, 153, 72–76.
- Passariello, C.; Puttini, M.; Virga, A.; Gigola, P. Microbiological and host factors are involved in promoting the periodontal failure of metaloceramic crowns. Clin. Oral Investig. 2012, 16, 987–995.
- Moretti, L.A.; Barros, R.R.; Costa, P.P.; Oliveira, F.S.; Ribeiro, F.J.; Novaes, A.B., Jr.; Palioto, D.B. The influence of restorations and prosthetic crowns finishing lines on inflammatory levels after non-surgical periodontal therapy. J. Int. Acad. Periodontol. 2011, 13, 65–72.
- Erdemir, E.O.; Baran, I.; Nalcaci, R.; Apan, T. IL-6 and IL-8 levels in GCF of the teeth supporting fixed partial denture. Oral Dis. 2010, 16, 83–88.
- Weishaupt, P.; Bernimoulin, J.P.; Lange, K.P.; Rothe, S.; Naumann, M.; Hägewald, S. Clinical and inflammatory effects of galvano-ceramic and metal-ceramic crowns on periodontal tissues. J. Oral Rehabil. 2007, 34, 941–947.
- Kurtiş, B.; Tüter, G.; Korkmaz, T.; Yücel, A.; Serdar, M.; Özcan, G. Clinical examination and interleukin-1β levels in gingival crevicular fluid in patients treated with removable partial dentures. Int. J. Prosthodont. 2003, 3, 59–63.
- Özen, J.; Beydemir, B.; Serdar, M.A.; Dalkiz, M.; Saygun, I.; Özdemir, A. The effect of fixed restoration materials on the IL-1β content of gingival crevicular fluid. Turk. J. Med. Sci. 2001, 3, 365–369.
- Mirchandani, B.; Zhou, T.; Heboyan, A.; Yodmongkol, S.; Buranawat, B. Biomechanical Aspects of Various Attachments for Implant Overdentures: A Review. Polymers 2021, 19, 3248.
- McLaren, E.A.; Figueira, J. Updating Classifications of Ceramic Dental Materials: A Guide to Material Selection. Compend. Contin. Educ. Dent. 2015, 6, 406–416.
- Hao, Y.; Huang, X.; Zhou, X.; Ren, B.; Peng, X.; Cheng, L. Influence of dental prosthesis and restorative materials interface on oral biofilms. Int. J. Mol. Sci. 2018, 14, 3157.
- Chen, Y.W.; Moussi, J.; Drury, J.L.; Wataha, J.C. Zirconia in biomedical applications. Exp. Rev. Med. Devices 2016, 10, 954–963.
- Alqutaibi, A.Y.; Ghulam, O.; Krsoum, M.; Binmahmoud, S.; Taher, H.; Elmalky, W.; Zafar, M.S. Revolution of Current Dental Zirconia: A Comprehensive Review. Molecules 2022, 5, 1699.
- Heboyan, A.; Manrikyan, M.; Zafar, M.S.; Rokaya, D.; Nushikyan, R.; Vardanyan, I.; Vardanyan, A.; Khurshid, Z. Bacteriological evaluation of gingival crevicular fluid in teeth restored using fixed dental prostheses: An in vivo study. Int. J. Mol. Sci. 2021, 22, 5463.
- Abduo, J.; Lyons, K.M. Interdisciplinary interface between fixed prosthodontics and periodontics. Periodontol. 2000 2017, 74, 40–62.
- Ercoli, C.; Tarnow, D.; Poggio, C.E.; Tsigarida, A.; Ferrari, M.; Caton, J.G.; Chochlidakis, K. The relationships between tooth-supported fixed dental prostheses and restorations and the periodontium. J. Prosthodont. 2021, 30, 305–317.
- Heboyan, A.; Zafar, M.S.; Rokaya, D.; Khurshid, Z. Insights and Advancements in Biomaterials for Prosthodontics and Implant Dentistry. Molecules 2022, 27, 5116.
- Heboyan, A.; Zafar, M.S.; Karobari, M.I.; Tribst, J.P.M. Insights into Polymeric Materials for Prosthodontics and Dental Implantology. Materials 2022, 15, 5383.
- Karobari, M.I.; Siddhartan, S.; Adil, A.H.; Khan, M.M.; Venugopal, A.; Rocaya, D.; Heboyan, A.; Marya, C.M.; Marya, A. Modifiable and Non-modifiable Risk Factors Affecting Oral and Periodontal Health and Quality of Life in South Asia. Open Dent. J. 2022, 16, 1874–2106.
- Chu, S.J.; Kan, J.Y.; Lee, E.A.; Lin, G.H.; Jahangiri, L.; Nevins, M.; Wang, H.L. Restorative emergence profile for single-tooth implants in healthy periodontal patients: Clinical guidelines and decision-Making strategies. Int. J. Periodontics Restor. Dent. 2019, 40, 19–20.
- Malpartida-Carrillo, V.; Tinedo-Lopez, P.L.; Guerrero, M.E.; Amaya-Pajares, S.P.; Özcan, M.; Rösing, C.K. Periodontal phenotype: A review of historical and Current classifications evaluating different methods and characteristics. J. Esthet. Restor. Dent. 2020, 33, 432–445.
- Yin, X.J.; Wei, B.Y.; Ke, X.P.; Zhang, T.; Jiang, M.Y.; Luo, X.Y.; Sun, H.Q. Correlation between clinical parameters of crown and gingival morphology of anterior teeth and periodontal biotypes. BMC Oral Health 2020, 19, 59.
- Aguirre-Zorzano, L.A.; Vallejo-Aisa, F.J.; Estefanía-Fresco, R. Supportive periodontal therapy and periodontal biotype as prognostic factors in implants placed in patients with a history of periodontitis. Med. Oral Patol. Oral Cir. Bucal 2013, 1, 786–792.
- Avetisyan, A.; Markaryan, M.; Rokaya, D.; Tovani-Palone, M.R.; Zafar, M.S.; Khurshid, Z.; Vardanyan, A.; Heboyan, A. Characteristics of periodontal tissues in prosthetic treatment with fixed dental prostheses. Molecules 2021, 2, 1331.
- Isler, S.C.; Uraz, A.; Kaymaz, O.; Cetiner, D. An evaluation of the relationship between periimplant soft tissue biotype and the severity of periimplantitis: A cross-sectional study. Int. J. Oral Maxillofac. Implants 2019, 34, 187–196.
- Zheng, Z.; Ao, X.; Xie, P.; Jiang, F.; Chen, W. The biological width around implant. J. Prosthodont. Res. 2021, 24, 11–18.
- Vlachodimou, E.; Fragkioudakis, I.; Vouros, I. Is there an association between the gingival phenotype and the width of keratinized gingiva? A systematic review. Dent. J. 2021, 23, 34.
- Heboyan, A.; Syed, A.U.I.; Rokaya, D.; Cooper, P.R.; Manrikyan, M.; Markaryan, M. Cytomorphometric analysis of inflammation dynamics in the periodontium following the use of fixed dental prostheses. Molecules 2020, 12, 4650.
- León- Martínez, R.; Montiel-Company, J.M.; Bellot-Arcís, C.; Solá-Ruíz, M.F.; Selva-Otaolaurruchi, E.; Agustín-Panadero, R. Periodontal behavior around teeth prepared with finishing line for restoration with fixed prostheses. A systematic review. J. Clin. Med. 2020, 17, 249.
- Ercoli, C.; Caton, J.G. Dental prostheses and tooth-related factors. J. Clin. Periodontol. 2018, 89, 223–236.
- Curtis, M.A.; Diaz, P.I.; Van Dyke, T.E. The role of the microbiota in periodontal disease. Periodontol. 2000 2020, 83, 14–25.
- Gopalakrishnan, U.; Murthy, R.T.; Felicita, A.S.; Alshehri, A.; Awadh, W.; Almaki, A.; Vinothkumar, T.S.; Baeshen, H.A.; Bhandi, S.; Kathir, A.; et al. Sulfate-reducing bacteria in patients undergoing fixed orthodontic treatment. Int. Dent. J. 2022.
- Ramzan, M.; Karobari, M.I.; Heboyan, A.; Mohamed, R.N.; Mustafa, M.; Basheer, S.N.; Desai, V.; Batool, S.; Ahmed, N.; Zeshan, B. Synthesis of Silver Nanoparticles from Extracts of Wild Ginger (Zingiber zerumbet) with Antibacterial Activity against Selective Multidrug Resistant Oral Bacteria. Molecules 2022, 27, 2007.
- Socransky, S.S.; Haffajee, A.D. Periodontal microbial ecology. Periodontol. 2000 2005, 38, 135–187.
- Ptasiewicz, M.; Grywalska, E.; Mertowska, P.; Glowniak, I.K.; Baran, A.P.; Rystwej, P.N.; Chalas, R. Armed to the teeth- The oral mucosa immunity system and microbiota. Int. J. Mol. Sci. 2022, 14, 882.
- Di Stefano, M.; Polizzi, A.; Santonocito, S.; Romano, A.; Lombardi, T.; Isola, G. Impact of oral microbiome in periodontal health and periodontitis: A critical review on prevention and treatment. Int. J. Mol. Sci. 2022, 5, 5142.
- Tuominen, H.; Rautava, J. Oral microbiota and cancer development. Pathobiology 2021, 88, 116–126.
- Hou, K.; Wu, Z.X.; Chen, X.Y.; Wang, J.Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Li, J.; Chen, Z.S. Microbiota in health and diseases. Signal Transduct. Target Ther. 2022, 23, 135.
- Teles, R.P.; Gursky, L.C.; Faveri, M.; Rosa, E.A.; Teles, F.R.F.; Feres, M.; Socransky, S.S.; Haffajee, A.D. Relationships between subgingival microbiota and GCF biomarkers in generalized aggressive periodontitis. J. Clin. Periodontol. 2010, 37, 313–323.
- Romero-Castro, N.S.; Vázquez-Villamar, M.; Muñoz-Valle, J.F.; Reyes-Fernández, S.; Serna-Radilla, V.O.; García-Arellano, S.; Castro-Alarcón, N. Relationship between TNF-a, MMP-8 and MMP-9 levels in gingival crevicular fluid and the subgingival microbiota in periodontal disease. Odontology 2019, 108, 25–33.
- Abusleme, L.; Hoare, A.; Hong, B.Y.; Diaz, P.I. Microbial signatures of health, gingivitis and periodontitis. Periodontol. 2000 2021, 86, 57–78.
- Esparbès, P.; Legrand, A.; Bandiaky, O.N.; Carpentier, M.C.; Martin, H.; Montassier, E.; Soueidan, A. Subgingival microbiota and cytokines profile changes in patients with periodontitis: A pilot study comparing healthy and diseased sites in the same oral cavities. Microorganisms 2021, 9, 2364.
- Mahendra, J.; Palathingal, P.; Mahendra, L.; Alzahrani, K.J.; Banjer, H.J.; Alsharif, K.F.; Halawani, I.F.; Muralidharan, J.; Annamalai, P.T.; Verma, S.S.; et al. Impact of red complex bacteria and TNF- α levels on the diabetic and renal status of chronic kidney disease patients the presence and absence of periodontitis. Biology 2022, 16, 451.
- Berger, D.; Rakhamimova, A.; Pollack, A.; Loewy, Z. Oral biofilms: Development, Control, and Analysis. High Throughput 2018, 7, 1–8.
- Mubaraki, M.Q.; Moaleem, M.M.A.; Alzahrani, A.H.; Shariff, M.; Alqahtani, S.M.; Porwal, A.; Al-Sanabani, F.A.; Bhandi, S.; Tribst, J.P.M.; Heboyan, A.; et al. Assessment of Conventionally and Digitally Fabricated Complete Dentures: A Comprehensive Review. Materials 2022, 15, 3868.
- Dobrzynski, M.; Pajaczkowska, M.; Nowicka, J.; Jaworski, A.; Kosior, P.; Szymonowicz, M.; Kuropka, P.; Rybak, Z.; Bogucki, Z.A.; Filipiak, J.; et al. Study of surface structure changes for selected ceramics used in the CAD/CAM system on the degree of microbial colonization, In Vitro test. Biomed. Res. Int. 2019, 12, 130806.
- Dantas, T.; Padrão, J.; Da Silva, M.R.; Pinto, P.; Madeira, S.; Vaz, P.; Zille, A.; Silva, F. Bacteria co-culture adhesion on different texturized zirconia surfaces. J. Mech. Behav. Biomed. Mater. 2021, 123, 104786.
- Wang, J.C.; Lai, C.H.; Listgasten, M.A. Porphyromonas gingivalis, Prevotella intermedia and Bacteroides forsythus in plaque subjacent to bridge pontics. J. Clin. Periodontol. 1998, 25, 330–333.
- Menini, M.; Delucchi, F.; Bagnasco, F.; Pera, F.; Di Tullio, N.; Pesce, P. Analysis of the subgingival microbiota in implant-supported full-arch rehabilitations. Dent. J. 2020, 5, 104.
- Tamrakar, A.K.; Murali, G.; Singh, S.; Shakila, R. Evaluation of subgingival microbiota around single tooth implants. J. Oral Biol. Craniofac. Res. 2020, 10, 180–183.
- Mocanu, R.C.; Martu, M.A.; Luchian, I.; Sufaru, I.G.; Maftei, G.A.; Ioanid, N.; Martu, S.; Tatarciuc, M. Microbiologic profiles of patients with dental prosthetic treatment and periodontitis before and after photoactivation therapy—Randomized clinical trial. Microorganisms 2021, 9, 713.
- Vardhan, P.K.; Paramashivaiah, R.; Prabhuji, M.L.V.; Bhavikatti, S.K.; Basha, S.; Arora, S.; Basheer, S.N.; Peeran, S.W.; Aldowah, O.; Heboyan, A. The Effect of Photodynamic Therapy on the Early Outcome of Implants Placed on Patients with Periodontitis. Photonics 2022, 9, 480.
More
