You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 1 by Mostafa Hassan Baky and Version 3 by Jessie Wu.
Bee products, viz., pollen (BP) and bread, are normally harvested from the flowering plants with the aid of bees. BP is further subjected to a fermentation process in bee hives to produce the more valuable and bioavailable BB. Owing to their nutritional and medicinal properties, bee products are considered as an important food supplements rich in macro-, micro-, and phytonutrients. Bee products are rich in carbohydrates, amino acids, vitamins, fatty acids, and minerals in addition to a myriad of phytonutrients such as phenolic compounds, anthocyanins, volatiles, and carotenoids.
- bee pollen
- bee bread
- phytonutrients
1. Introduction
Bee pollen (BP) is the natural pollen of flowering plants collected mainly by honeybees and is considered as the major source of feeding for bee growth [1]. Owing to its nutritional and medicinal value, BP plays pivotal role in human health [2]. Regular BP supplementation can induce several health benefits such as reducing capillary fragility, improving the cardiovascular system, maintaining gut functions, and promoting skin health [3]. Additionally, because of its anti-inflammatory activity along with its anti-androgen effect, BP is used in the treatment of chronic prostatitis [1]. The nutritional and medicinal value of BP is attributed to its richness in several macro- and micronutrients such as carbohydrates [3], proteins, vitamins, amino acids, minerals, lipids, flavonoids, phenolic compounds, glucosinlolates (GLSs), and essential oils [4]. BP is considered as a rich source of certain essential amino acids including arginine, histidine, lysine, tryptophan, phenylalanine, methionine, threonine, leucine, isoleucine, and valine [5]. Additionally, BP contains vitamins, macroelements such as phosphorus, sodium, calcium, magnesium, and potassium, and microelements including copper, manganese, iron, zinc, and selenium [6].
In contrast, bee bread [7] is produced via the fermentation of pollen, honey, and bee digestive enzymes in the presence of lactic acid bacteria in wax-sealed honeycombs [8]. Such fermented BB is considered to be more bioavailable than crude BP which is collected directly from the legs of the bees at the hive entrance with the aid of traps [9]. Owing to their richness in polyphenols and flavonoids, both BP and BB are considered as potent antioxidants and possess several human health benefits [10] including antitumor, anti-inflammatory and antimicrobial effects [11]. The total phenolic content (TPC) of both BP and BB collected from different locations varies between 10.5 and 24.6 mgGAE/g [12] and 2.5 and 37.1 mgGAE/g, respectively, and total flavonoid content (TFC) is reported to be from 1.9 to 4.5 mgQE/g [8]. Compared to BP, fermented BB is different in chemical composition as it contains higher levels of reducing sugars, vitamin K, and is considered as a rich source of polyunsaturated fatty acid (PUFA) that is essential for human health and improving lipid profile [13]. Moreover, both BP and BB have high nutritional value as they are considered as a rich source of fatty acids. BP is rich in long chain fatty acids such as linoleic, α-linolenic, and α-palmitic acid [14], whereas BB is richer in linoleic, oleic, and 11,14,17-eicosatrienoic acids [14]. Although the protein level in BP is higher than that of BB, BB protein is considered more bioavailable and digestible [15]. Recently, BB has been favored as a dietary supplement over BP, which has led to the enlargement of BB production and collection from honeycombs [8]. Standardization of nutraceuticals becomes essential for use to confirm its consistency and safety using different analytical tools [13]. Due to the change in several conditions such as climate, plant origin, collection tools, and collection time, the nutritional and chemical characteristics of bee products are subjected to differences [13].
2. Phytonutrients
Several phytochemical groups were reported in BP and BB samples such as volatiles, coenzyme Q10, carotenoids, anthocyanins, phenolics, and glucosinolates (Figure 12).
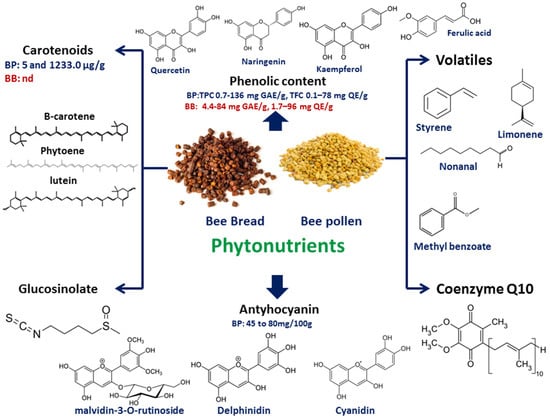
Figure 12. Major phytonutrients identified in both BP and BB, including phenolics, anthocyanins, carotenoids, glucosinolates, coenzyme Q10, and volatiles.
2.1. Volatile Compounds
4.1. Volatile Compounds
Volatile compounds of different aroma types are detected in BP as a result of the collection of nectar from flowers by the honey bee [16][57], and whether they can be used to trace BP flower origin is not reported extensively in the literature. The volatile content in 14 BP samples from Lithuania was analyzed using solid-phase micro-extraction (SPME)-GC-MS technique with a total of 42 volatiles including nonanal 1.5–20.1%, dodecane 1.2–34.6%, and tridecane 1.4–24.7% [17][35]. Likewise, the volatile content of three different polyfloral Lithuanian BP samples was evaluated using SPME-GC-MS. Results revealed that styrene was the most abundant, accounting for 19.6–27.0%. Other volatiles that appear more flower-specific included limonene, hexanal, nonanal, and 1-tridecene (43.3%) [18][36]. The volatile oil content of three stingless BP samples (genus Scaptotrigona sp.) collected from mid-north region of Brazil was determined using GC-MS. Results revealed the identification of 41 volatile compounds belonging to different classes with an abundance of hydrocarbons and esters; the most frequent volatiles among the analyzed samples were kaur-16-ene, methyl cinnamate, benzyl acetate, methyl benzoate, methyl hydrocinnamate, and ethyl phenylacetate [19][38]. Compared to other studies, hydrocarbons and esters were reported to be abundant in stingless BP samples. The volatile constituents in ground BP and its aqueous solution (1:1 w/v) were investigated using HS-SPME/GC–MS analysis [20][37]. A total of 25 compounds were identified in ground bee pollen mostly belonging to aldehydes, such as 2-methylbutanal, 3-methylbutanal, pentanal, hexanal, (E)-2-hexenal, heptanal, octanal, and nonanal. Meanwhile, ground BP aqueous solution showed the presence of 22 compounds, of which four new volatiles were identified as 2-methyl-propanal, hexanoic acid methyl ester, 2-heptanone, and 2-octene [20][37]. The volatile compounds detected in BP and BB are related to the floral source from which the bee products are collected [19][38]. The abundance of volatile constituents in bee products can be used as an indication of their freshness and further adds to the sensory characteristics of commercial products.
2.2. Coenzyme Q10
4.2. Coenzyme Q10
Coenzyme Q10 (CO-Q10) plays a fundamental role in the mitochondrial electron transport chain and as an antioxidant in plasma membranes and lipoproteins [3]. The COQ-10 concentration in BP was determined using accelerated solvent extraction (ASE) techniques such as HPLC-DAD, and 11 commercial samples, including rape bee pollen, tea bee pollen, apricot bee pollen, and mixed bee pollen, were analyzed for their CO-Q10 content [1]. The results revealed that extraction temperature had a considerable effect on extraction yield, where adjusting the temperature by more than 80 °C provided satisfactory extraction yields. The assay was well validated and found linear over a concentration range of 0.25–200 mg/L, and LOD and LOQ and were detected at 0.16 and 0.35 mg/kg, respectively. The percentage of recoveries were above 90% with below 6.3% inter- and intra-day precision [1]. In another study, the CO-Q10 amount in BB samples from Poland was determined using LC/MS-MS and detected at 11.5 μg/g [21][39].
2.3. Carotenoids
4.3. Carotenoids
The carotenoid composition of bee-pollen samples were analyzed by Salazar-González et al. using rapid resolution liquid chromatography (RRLC) coupled to UV–Vis spectrophotometry and Digital Image Analysis (DIA) [22][40]. Results led to the identification of α-tocopherol (4.7–95.9 μg/g) along with 10 other carotenoids, including phytoene (0.12–17.82 μg/g), lutein isomer 1 (1.62–131.03 μg/g), lutein isomer 2 (1.3–137.5 μg/g), anteraxanthin isomers 1 (1.6–131.0 μg/g), zeaxanthin (from 12.8 to 256.4 μg/g), zeinoxanthin (10.5–996.1 μg/g), lutein (0.99–4.6 μg/g), β-cryptoxanthin (2.1–16.3 μg/g), and β-carotene (0.68–3.6 μg/g). The correlation of colorimetric coordinates with carotenoid level was further estimated using multiple linear regression (MLR) [22][40]. In another study, 16 chestnut-derived BP samples collected from two regions in Turkey were analyzed for carotenoid level using HPLC-DAD [23][41]. Results showed the presence of five carotenoids, identified as lutein (3.8 to 33.4 mg/kg), zeaxanthin (4.8 to 36.3 mg/kg), β–cryptoxanthin (7.5 to 44.6 mg/kg), β-carotene (7.9 to 152.4 mg/kg), and β-carotene (from 2.4 to 395.0 mg/kg) [23][41]. By comparing carotenoid levels in BP to that of carrot (a major source carotenoids), the abundance of carotenoid in BP at higher levels was revealed, posing it as an essential carotenoids source [23][41].
2.4. Glucosinolates (GLS)
4.4. Glucosinolates (GLS)
Glucosinolates are secondary plant metabolites with potential health benefits and their quality and number differ among different bee pollens depending on plant source. Sulforaphane (SFN) is an isothiocyanate derivative produced upon the reaction of enzyme myrosinase with glucosinolate, typically upon cell crushing [24][69]. SFN was identified for the first time in five bee pollen samples using the LC-MS/MS method [25][42]. SFNs were detected at trace levels in some bee-pollen samples (<23 g/kg). The average analyte recovery rate ranged between 92 and 106% in all cases [25][42], with LC-MS/MS found selective, linear from 8 to 1000 g/kg, with both relative standard deviation (percent RSD) and relative error (percent RE) values less than 10%, and both LOD and LOQ limits at 3 g/kg and 8 g/kg, respectively [25][42]. GLS content in 49 BP samples from four different apiaries in Spain was determined using UPLC-Q-TOF/MS, revealing variation in GLS levels ranging from 34 to 9806 μg/kg [26][43].
2.5. Phenolics
4.5. Phenolics
Phenolics represent a group of phytochemicals found in almost all plants and are of potential value to human health. Phenolic acids and phenolics showed higher ability to activate enzymes of antioxidant protection in cells, which can prevent oxidative stress damage in cells [27][70]. Several phenolics belonging to flavonoids (listed in Table S1) and phenolic acids (Table S2) were reported in BP and BB. Total phenolic–flavonoid content (TPC-TFC) in BP and BB samples from the same bee hive from five different locations in Turkey [28][26] was assessed with TPC ranging from 8.2 to 43.4 mgGAE/g, whereas TFC ranged from 1.8 to 4.4 mgQE/g [28][26]. Moreover, BP showed much higher TPC levels than BB samples mostly affected by the extraction method, extraction solvents, and botanical origins of the samples, which was in accordance with several studies [12][29][12,71]. The polyphenolic content of 35 BP samples collected from different locations in India was investigated using UHPLC-DAD-MS/MS, with 60 identified compounds, including 38 flavonoids mostly comprising the flavonol subclass, 21 phenolic acids, and one glucosinolate [30][47]. On the other hand, 13 hydroxycinnamic acids derivatives, 4 hydroxybenzoic acids, and 4 phenolic glycerides were quantified as follows: catechin, 0.94–19.1 mg/100 g; rutin, 4.81–24.8 mg/100 g; quercetin, 3.14–15.9 mg/100 g; luteolin, 1.06–5.8 mg/100 g; kaempferol, 0.12–9.3 mg/100 g; and apigenin, 0.46–3.02 mg/100 g [30][47]. Likewise, nine polyphenols were identified from BP samples collected from Greece (Viannos, Crete), quantified as follows: ferulic acid, 149.1 µg/g; o-, p-coumaric acid, 36.6 µg/g; quercetin, 29.6 µg/g; cinnamic acid, 23.4 µg/g; naringenin, 21.9 µg/g; hesperitin, 3.0 µg/g; and kaempferol, 7.83 µg/g, using nano-liquid chromatography system [31][44]. The optimized analytical method was validated and the intra-day and inter-day RSD % for retention duration, retention factor, and peak area repeatability were below 4.68 and 5.57%, respectively [31][44]. Compared to traditional HPLC methods, the new method runs in nanoflow conditions, resulting in higher sensitivity and shorter analysis times [31][44]. The phenolic content from a BP methanol extract sample using HPLC analysis was investigated [32][45]. 3, 4-Dimethoxycinnamic acid (45.8 mg/mL) was identified as the most abundant phenolic, alongside gallic acid, catechin, and quercitin as major phenolics [32][45]. Flavonoid and phenolic acid contents in a commercial BP sample collected from local markets in Valladolid, Spain, were investigated using supercritical fluid chromatography (SFC) [33][46]. Nine phenolic compounds were identified and quantified as follows: catechin, 22.15 mg/kg; quercetin, 22.02 mg/kg; p-coumaric acid, 11.62; and cinnamic acid, 3.70 mg/kg [33][46]. Furthermore, the best results were obtained at LODs and LOQs less than 5 microg/mL; however, the RSD percentage values for method repeatability and inter-day reproducibility were less than 3% and 10%, respectively [33][46].
In another study, phenolic compounds in 16 chestnut BP samples from two different regions in Turkey were analyzed using HPLC-DAD [23][41]. Major phenolics included luteolin (from 0.3 to 0.9 mg/g), hyperoside (0.18 to 1.1 mg/g), vitexin (0.354 to 1.720 mg/g), chalcone (0.01 to 0.05 mg/g), rosmarinic acid (2.0 to 11.5 mg/g), pinocembrin (0.06 to 0.1 mg/g), and chrysin (0.01 to 0.04 mg/g). It could be deduced that BP richness in phenolics presents a good source of antioxidants for human health [23][41]. Five fresh samples of BP and BB were analyzed using LC-MS/MS [34][29] with phenolics detected at much higher levels in BP over BB, exemplified by caffeic acid (12.4–56.1 mg/100 g), ethyl gallate (0.04–3.1 mg/100 g), trans-ferulic acid (23.2–107.6 mg/100 g), and myricetin (20.4–244.7 mg/100 g) [34][29]. In contrast, other phenolic acids were found at higher levels in BB versus BP, such as protocatechuic acid (166.61 mg/100 g), p-coumaric acid (28.7–142.4 mg/100 g), quercetin (381–3918 mg/100 g), isorhamnetin (1227 mg/100 g), luteolin (21.9–3490 mg/100 g), salicylic acid (19.2–65.2 mg/100 g), chlorogenic acid (3.89–36.09 mg/100 g), 2,5-dihydroxybenzoic acid (2.69–35.09 mg/100 g), kaempferol (112.94–2681.20 mg/100 g), and gallic acid (34.65–347.37 mg/100 g) [34][29].
A mixture of pollen samples from different plant species collected from Bayburt, Turkey, were investigated for its phenolic and fatty acid content [35][48]. A total of 23 phenolics were quantified, represented by rutin (115.4 mg/kg) followed by kaempferol (9.8 mg/kg), quercetin (7.8 mg/kg), myricetin (2.2 mg/kg), and p-coumaric acid (0.5 mg/kg) [35][48].
Phenolic acid and flavonoid content from Lithuanian BP samples were detected using an HPLC-electrochemical detector (ECD) system [17][35], with major forms detected in all samples, including 2-hydroxycinnamic acid (43.4–179.9 μg/g), rutin (156.2–955.7 μg/g), and quercetin (24.0–529.8 μg/g) [17][35]. The polar extract of BP from C. nucifera was analyzed using HPLC-ESI-MS/MS [36][23]. Results identified flavonoid glycosides, such as isorhamnetin-3-O-(2″-O-rhamnosyl) glucoside, isorhamnetin-3-O-(2″,3″-O-dirhamnosyl) glucoside, isorhamnetin-di-3,7-O-glucoside, quercetin-3-O-rhamnosylglucoside, quercetrin, and isorhamnetin-3-O-(2″-O-rhamnosylacetyl) glucoside, along with hydroxycinnamic acid amide derivatives [36][23]. In another study, 33 phenolics were detected in Rhododendron ponticum L. BP using LC-MS/MS [37][30]. Among the detected compounds, myricetin was detected at the highest level (3744.3 μg/100 g), followed by epicatechin (1350.4 μg/100 g), catechin (1207.1 μg/100 g), and tyrosol (1137.6 μg/100 g) [37][30]. Differences in flavonoid compositions among studies are likely attributed to the BP floral source among other factors. The abundance of phenolics and flavonoids with anti-oxidant potential in bee products indicates its potential role in protecting human cells from oxidative stress adding to its health benefits.
2.6. Anthocyanins
4.6. Anthocyanins
Anthocyanins are colored water-soluble pigments belonging to flavonoids that impart color to fruits and flowers, aside from their several biological activities such as the treatment of neurodegenerative and vascular diseases due to its antioxidant activity [2]. Anthocyanin composition in five Spanish dark blue BP samples was studied using HPLC/PDA/MS. Results revealed that the anthocyanin content ranged from 45 to 80 mg/100 g of blue pollen, which was represented by delphinidin, cyanidin and petunidin-3-O-glucoside, delphinidin, cyanidin, peonidin and malvidin-3-O-rutinoside, and cyanidin-3-(6-malonylglucoside), with the major one being petunidin-3-O-rutinoside [2]. The pigments derived from the blue-black BP were compared to pigments collected from Fuchsia extorticata pollen using HPLC [7], identified as delphindin, petunidin, and malvidin-3-O-glucosides, as well as delphinidin-3-O-glucosides [7]. The flavonol glycosides kaempferol-3-sophoroside, quercetin-3-sophoroside, and kaempferol 3-neohesperidoside were detected alongside anthocyanins and confirmed using 1HNMR spectroscopy [7].
