You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Jessie Wu and Version 1 by Humaira Yasmin.
Nanofluids (NFs) synthesized via the suspension of diverse nanoparticles into conventional thermal fluids are known to exhibit better thermal, optical, tribological, and convective properties, photothermal conversion, and heat transfer performance in comparison with traditional thermal fluids. Stability is pivotal to NF preparation, properties, performance, and application.
- NF
- formulation
- stability
- Mono and Hybrid Nanofluids
1. Introduction
The advent of nanotechnology has brought about significant technological advancement in many fields of study. The birth of NFs as advanced thermal fluids in the area of thermal management is a laudable and notable feat. NFs (mono and hybrid) have been extensively researched and proven to be better than conventional thermal fluids, and this is due to their enhanced thermophysical and convective properties [1,2,3,4,5,6,7,8,9,10,11,12,13][1][2][3][4][5][6][7][8][9][10][11][12][13]. The application of diverse mono and hybrid NFs in various thermal systems has been studied experimentally [5,6,12][5][6][12] and numerically [14,15[14][15][16][17],16,17], and found to improve heat transfer characteristics better than traditional thermal fluids. NFs have been investigated in the various types of convective heat transfer studies, such as natural [18[18][19][20][21][22],19,20,21,22], mixed [23[23][24],24], and forced convection [25,26,27,28,29,30][25][26][27][28][29][30] at laminar, turbulent, and transition regimes. These studies showed the enhancement of heat transfer performance with the HNFs found to be better thermal fluids than MNFs. In addition, the use of mono and hybrid NFs in heat transfer systems such as solar collectors [12[12][31][32][33],31,32,33], radiators [34,35,36][34][35][36], refrigerators [37], mini-channel [38], microtubes [39[39][40],40], heat pipes [20[20][41],41], air-conditioning [42], heat exchangers [43], etc, have been studied. The deployment of NFs in these thermal transporting devices showed improvements in the heat transfer and flow characteristics than when conventional thermal fluids were used. Furthermore, mono and hybrid NFs/NPs have been employed as coolants (metal rolling process and metal machining operation) [44[44][45][46][47],45,46,47], lubricants (automobile) [48[48][49][50][51],49,50,51], thermal storage materials [52,53[52][53][54],54], sensors [55[55][56][57],56,57], drilling muds [58[58][59],59], chemically enhanced oil recovery material [60[60][61][62],61,62], etc, and are better thermal fluids/materials than the conventional thermal fluids/materials.
The suspension of diverse NPs into various base fluids to synthesize NFs has been proven to possess superior thermal properties compared with the traditional thermal fluids. Current research progress has revealed that the suspension of HNPs (mixing of two or more NPs) in different base fluids possessed better convective and thermal properties than MNFs [2,4,12][2][4][12]. MNF and HNF preparation appears to be a simple practice but complex in the true sense of it. Stability, which is the even distribution of mono and hybrid NPs in the base fluid, is key to the results associated with the thermal [63,64][63][64] and convective properties [65,66,67,68][65][66][67][68] and performance [68,69,70,71][68][69][70][71] of mono and hybrid NFs in various areas of their application. The stability of mono and hybrid NFs has been proven to significantly affect their thermal properties [72,73,74,75,76,77,78,79,80,81][72][73][74][75][76][77][78][79][80][81] and convective heat transfer performances [65,82,83][65][82][83]. The instability is marked by sedimentation and agglomeration of the mono and hybrid NPs suspended in the base fluid. This consequently leads to inaccurate results when the resultant mono and hybrid NFs are deployed in different applications [65,67,69,70,71,84,85][65][67][69][70][71][84][85]. This goes to show that obtaining good and desirable stability of mono and hybrid NFs is crucial. However, the stability of mono and hybrid NFs are strongly connected to preparation variables, such as stirring time, rate, temperature, sonication time, power, frequency, amplitude, and dispersion fraction (where a surfactant is used) [7,75,77,81,86,87,88,89][7][75][77][81][86][87][88][89]. The sonication variables (time, power, mode, frequency, and amplitude) are related to the sonication energy required to achieve homogenized and stable mono and hybrid NFs [74,78,90,91][74][78][90][91].
2. Formulation Techniques
MNFs and HNFs are formulated through the suspension of MNPs and HNPs, respectively, into conventional thermal fluids, namely, base fluids, of which their stability is very important to the measurement of the thermophysical properties and convective studies. Fundamentally, MNFs and HNFs are formulated using a one- and two-step process (Figure 1). By this, the latter entails two processes, namely, (i) synthesis of MNPs or HNPs in the powdery form and (ii) suspension of MNPs or HNPs into the base fluids. The most reported process in the literature is the two-step process for the formulation of MNFs and HNFs, which can encourage their large-scale formulation at a low cost and an industrial utilization. The shortcoming of the two-step process relates to the sedimentation and agglomeration of MNPs and HNPs due to the Van der Waals forces of attraction among the particles [92]. The one-step process consists of the simultaneous production of MNFs and HNFs by way of synthesis and suspension of MNPs and HNPs in the base fluids. This technique is advantageous as it improves the homogeneity and stability of MNFs and HNFs and eliminates arduous procedures, such as drying and storing in comparison with the single-step process by reducing the agglomeration tendency of MNPs and HNPs [92,93][92][93]. However, the industrial application of this technique is impracticable except for low vapor-pressure fluids. This technique is also not cost-effective [94]. In addition, various one-step process techniques have been reported in the literature [93,95,96][93][95][96].
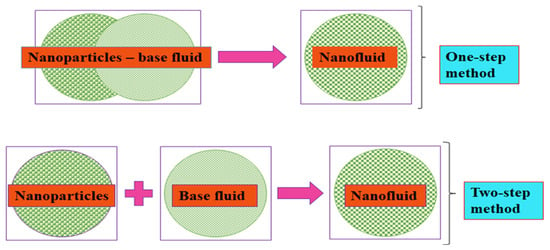
Figure 1.
Mono and hybrid NF formulation strategies.
2.2. Characterization Techniques
Numerous techniques have been reported in the literature for the characterization of MNFs and HNFs for their MNP and HNP shapes, sizes, distribution, functional groups, crystalline structure, surface morphology, dispersion, elemental composition, saturation, magnetization, etc. These techniques include Raman spectroscopy, X-ray diffractometer, high-resolution transmission electron microscopy, Fourier transform infrared spectroscopy, scanning electron microscopy, vibrating sample magnetometer, energy-dispersive X-ray spectroscopy, light scattering, and transmission electron microscopy [1,17,78,97,98,99,100,101][1][17][78][97][98][99][100][101]. The most used technique for characterizing MNFs and HNFs is transmission electron microscopy (TEM), followed by scanning electron microscopy (SEM) and the X-ray diffractometer (XRD). These most used techniques are often engaged as a stand-alone technique or with other techniques for MNF and HNF characterization. TEM is used to determine the size, shape, and dispersion of MNPs and HNPs in MNFs and HNFs, respectively, while the SEM detects surface morphology and elemental mapping. XRD is used to show the crystalline structure and grain size of MNPs and HNPs contained in MNFs and HNFs, respectively.
2.3. Stability Improvement and Tests
2.3.1. Stability of NFanofluids
The suspension of MNPs and HNPs in various base fluids introduces charges into the base fluids, which leads to the formation of an electrical double layer (EDL) around the particle surface [102]. Therefore, MNFs and HNFs are referred to as electrically conducting fluids. By applying a potential across these fluids, oppositely charged electrodes tend to attract the MNPs or HNPs and EDL. The formation of EDL is strongly connected to the volume fraction, size, surface charge of the particles, and concentration of ions in the base fluids. The stability and even distribution of MNPs or HNPs in the base fluids are vital in the application of MNFs and HNFs because the thermophysical (mostly κ and μ) and optical properties, and the efficiency of the same are significantly related to the concentration of MNPs or HNPs in the suspension [103,104][103][104]. Improving the stability of MNFs and HNFs to reduce agglomeration and sedimentation with the two-step process has led to the utilization of four techniques, namely, ultrasonication, surfactant addition, surface modification, and pH control.
2.3.2. Stability Improvement Techniques
Sonication
Sonication is one of the techniques deployed to obtain homogeneous mixtures of NPs suspended in selected base fluids. Several studies demonstrated that sonication affected κ, absorbance wavelength, μ, cluster size, surfactants, the diameter of CNTs, and particle size [74,78,79,85,105,106,107][74][78][79][85][105][106][107]. For NFs, a sonication time spanning a few minutes to several hours has been documented. It can be deduced that an optimum sonication time (mainly due to the Brownian motion of MNPs or HNPs) occurred where the variable investigated either reduced (for μ and κ) or increased (for CNT diameter, particle, and cluster size). An optimum sonication time ranging from 12 min [108] to 60 h [105] has been reported in the literature for MNFs and HNFs. This reflects the need to optimize sonication time as it relates to other variables to achieve improved stability. However, this is mostly not the case for most of the studies on the formulation of MNFs and HNFs, except for very limited studies that have optimized the sonication parameters [74,85,109][74][85][109]. Sonication of NFs has been reported to be carried out using the following three different types of ultrasonicators: probe-type, sonication-bath type, and shaker-type [68,80,110][68][80][110]. Figure 2 shows the sonication of HNF.
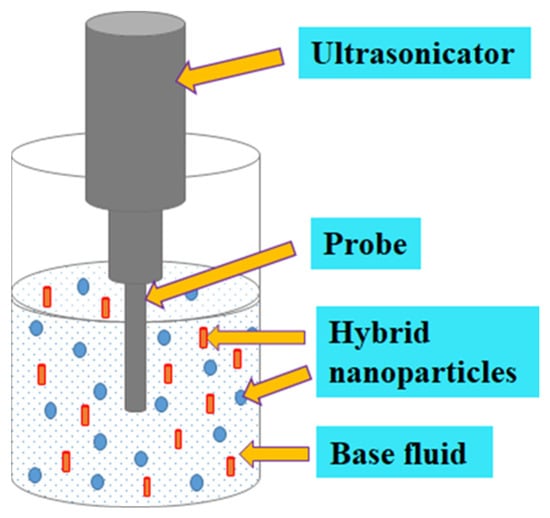
Figure 2.
Ultrasonication of HNF.
Addition of Surfactants
Surfactants are complex chemical compounds that create an electrostatic repulsion to overcome magnetic attraction (for magnetic NPs) and Van der Waals interaction between NPs to avoid their sedimentation in the suspended base fluids [111]. The primary reason for surfactant use in NF formulation is to aid the stability of NPs in the base fluid [111]. Surfactants lower the interfacial tension between NPs and base fluid to enhance the stability of NFs. The use of surfactants promotes the stability of NFs by increasing the EDL between NPs. Surfactants such as cetyl trimethyl ammonium bromide (CTAB), sodium dodecyl sulphate (SDS), gum Arabic (GA), oleic acid (OA), polyvinyl pyrrolidone (PVP), nanosperse AQ, dodecyl trimethyl ammonium bromide, sodium dodecylbenzene sulfonate (SDBS), and hexa decetyl trimethyl ammonium bromide have been used in the literature to stabilize MNFs and HNFs [73,95,104,112,113,114,115][73][95][104][112][113][114][115]. A list of some surfactants used in NF studies is given in Table 1. An increase in κ, zeta potential (ZP), surface tension, and the μ of MNFs and HNFs due to the use of surfactants has been reported [95,103,106,109][95][103][106][109]. However, the effectiveness of surfactants at >60 °C was reported to reduce due to weak bonds between surfactants and NPs, which, when finally broken, may lead to sedimentation and thus the instability of MNFs and HNFs [112]. Different surfactants have been used by various researchers to stabilize MNFs and HNFs formulated from diverse NPs and HNPs and suspended in different base fluids [13,18,73,78,109,116,117][13][18][73][78][109][116][117]. Therefore, it can be concluded that the stability of MNFs and HNFs based on the use of surfactants is dependent on the type and nature (magnetic or not) of NPs or HNPs, the base fluid type (ionic or non-ionic), and the type of surfactants used.
Table 1.
List of surfactants used in nanofluid studies.
| Nanofluids | pH | References |
|---|---|---|
| Al2O3-TiO2 (8:2, 6:4, 4:6, 2:8)/DW | 5.68–5.75 | Kumar and Sarkar [122] |
| Al2O3 + MgO/DW | 8.23 | Kumar and Sarkar [128] |
| Al2O3 + SiC/DW | 6.82 | Kumar and Sarkar [128] |
| Al2O3 + AIN/DW | 7.56 | Kumar and Sarkar [128] |
| Al2O3 + Cu/DW | 8.12 | Kumar and Sarkar [128] |
| Al2O3 + MWCNT/DW | 7.73 | Kumar and Sarkar [128] |
| Name |
|---|
23.
Table 12. Isoelectric points of mono and hybrid nanofluids.
| Al | |||
| 2 | |||
| O | |||
| 3 | |||
| /DW | |||
| 7.62 | Kumar and Sarkar | [128] | |
| Al2O3/DW | 8.0 | Wang and Li [129] | |
| Cu/DW | 9.5 | Wang et al. [106] | |
| Cu-Al2O3/DW | 5.5 | Momin et al. [130] | |
| SiO2-CuO/C/GL-EG (60:40) | 8–9 | Akilu et al. [131] | |
| SiO2-GNP/naphthenic mineral oil | 9–11 | Qing et al. [132] | |
| Fe3O4-GNP/DIW | 3, 5, 7, 8, and 10 | Askari et al. [101] | |
| Al2O3 and Al2O3-Fe (50:50)/DIW | 12 | Okonkwo et al. [133] | |
| Al2O3/W | 7–8.2 | Menbari et al. [85] | |
| CuO/W | 8–9 | Menbari et al. [85] | |
| Al2O3-CuO/W | 7.6–8.5 | Menbari et al. [85] | |
| Al2O3/EG | 6.5–7.5 | Menbari et al. [134] | |
| CuO/EG-W (50:50) | 8–9 | Menbari et al. [134] | |
| CuO/EG | 8.5–10 | Menbari et al. [134] | |
| Al2O3/EG-W (50:50) | 7–8 | Menbari et al. [134] | |
| Al2O3-CuO/EG | 7–8.2 | Menbari et al. [134] | |
| Al2O3-CuO/EG-W (50:50) | 7.2–8.5 | Menbari et al. [134] | |
| Al2O3-CuO/W | 7.5–8.5 | Menbari et al. [135] | |
| Al2O3-CuO/EG-W | 7–8.2 | Menbari et al. [135] | |
| Cu/DIW | 7.43–10.2 | Kamalgharibi et al. [86] | |
| Cu/EG-DIW (50:50 vol) | 7.8–9.6 | Kamalgharibi et al. [86] | |
| Cu/EG | 9.85–10.2 | Kamalgharibi et al. [86] | |
| Al2O3/W | 5.1–5.2 (sonicated) and 5.4 (without sonication) | Mahbubul et al. [123] | |
| MgO/EG | 9.66–10.84 | Adio et al. [120] | |
| Al2O3 (100 nm)/GL | 4.09 | Adio et al. [74] | |
| Al2O3 (80 nm)/GL | 6.26 | Adio et al. [74] | |
| Al2O3 (20–30 nm)/GL | 6.44 | Adio et al. [74] | |
| Cu-TiO2 | 7 | Sajid and Ali [92] | |
| Al2O3-Cu | 5.5 | Sajid and Ali [92] | |
| Ag-MgO | 5.74 | Sajid and Ali [92] | |
| SiO2-GNP | 11 | Sajid and Ali [92] | |
| ZnO/W | 4 | Sajid and Ali [92] | |
| Al2O3/BDW | 10 | Zawrah et al. [73] | |
| TiO2 | 7 | Chakraborty et al. [127] | |
| Ag-TiO2 | 7.3–8.35 (without sonication) and 7.70–8.69 (sonication) | Chakraborty et al. [127] | |
| Nanofluids | IEP | References | |
|---|---|---|---|
| Sodium dodecyl sulphate | |||
| Al2O3 | 9.1 | Kumar and Sarkar [122] | |
| Sodium dodecylbenzene sulfonate | |||
| Al2O3 | 6.2–6.8 | Zawrah et al. [73] | Gum Arabic |
| MgO | 6.82 | Kumar and Sarkar [122] | Oleic acid |
| SiC | 7.56 | Kumar and Sarkar [122] | Cetyl trimethyl ammonium bromide |
| AIN | 8.12 | Kumar and Sarkar [122] | Polyvinyl pyrolidone |
| Cu | 7.73 | Kumar and Sarkar [122] | Nanosperse AQ |
| MWCNT | 7.62 | Kumar and Sarkar [122] | Dodecyl trimethyl ammonium bromide |
| Al2O3 | 8.0 | Mahbubul et al. [123] | Hexa decetyl trimethyl ammonium bromide |
| Sodium hexa meta phosphate | |||
| Span 80 | |||
| eriochrome blackT | |||
| Triton X100 | |||
| Tween 80 | |||
| Citrus acid | |||
| 3-Aminopropyl) trimethoxysilane | |||
| Polyisobutene succinimide | |||
| Sodium deoxycholate | |||
| Poly(vinyl alcohol) | |||
| Polyisobutene succinimide | |||
| Tetramethylammonium hydroxide |
Control of pH
The stability of MNFs and HNFs can be improved by adjusting the pH. By suspending NPs into a base fluid, surface electric charges are produced on the resultant NF, which can be manipulated by altering the pH value. The surface electrostatic charges affect the stability of NF. An alteration of the pH value farther from the isoelectric point (IEP) enhances the NF stability. The pH of NF determines the IEP of the suspension, and this can be altered to improve the stability to avoid sedimentation and agglomeration. The IEP of some NFs is provided in Table 12. Additionally, the surface electric charge can be determined using ZP. The ZP measures the repulsion between NPs and increases with a rise in the particles suspended in the base fluid [73,118][73][118]. A high ZP (absolute value) indicates the stability of NFs due to a strong electrostatic repulsion between NPs, while a low ZP shows instability due to the weak electrostatic repulsion of particles. With a ZP value of >60 mV, a very stable NF is formulated; a value of >30 mV implies a stable NF, whereas <20 mV indicates weakness in NF stability [73,96,103][73][96][103]. Zawrah et al. [73] reported the modification of the pH of Al2O3/water NF (with a surfactant of SDBS) from 5 to 10 using NaOH because the IEP of the NF was around 6.3. Similar pH alterations to improve NF stability were carried out in other studies [85,106,119,120,121][85][106][119][120][121]. The pH of MNFs and HNFs is given in Table
Table 23.
pH of mono and hybrid nanofluids.
Functionalization of Nanoparticles
The surface modification or functionalization of NPs is another technique employed to improve the stability of NFs. This stability-enhancing method is surfactant-free but needs materials for functionalization. Although this technique is not widely studied, it is a promising method for the formulation of more stable MNFs and HNFs [104,105,136][104][105][136]. Owing to the importance of the stability of MNFs and HNFs, the measurement of this parameter is key to the further use of MNFs and HNFs in terms of thermophysical properties and convective heat transfer studies.
2.3.3. Stability Test Methods
Visual Inspection
The simplest method to check the stability of MNFs and HNFs is by visual inspection. In other words, it is a visual observation of the MNF and HNF samples at daily or weekly, or monthly intervals to see how the NPs or HNPs sediment with time. This is not a scientific method for checking the stability of MNFs and HNFs, as reported in the literature [74,104,137,138][74][104][137][138]. However, this method is always used in addition to other stability monitoring techniques that are scientific [74,104,109,137,138][74][104][109][137][138].
Zeta Potential
ZP is a method used to determine the stability of MNFs and HNFs. As earlier stated, the ZP of MNFs and HNFs is strongly connected to the repulsive force between the NPs or HNPs. This technique is mostly used to measure the stability of MNFs and HNFs, as reported in the literature by several authors [1,73,98,121,131][1][73][98][121][131]. The degree of stability of MNFs and HNFs can be determined using this method based on the obtained ZP values. It is worth mentioning that this stability-checking technique is often used along with other techniques.
Ultraviolet-Visible Spectrophotometer
This method seems to be the most employed of all the methods for monitoring the stability of MNFs and HNFs. The absorbance or transmittance of the MNFs and HNFs at the peak wavelength can be deployed to monitor the stability of MNFs and HNFs [20,79,85,109,139,140][20][79][85][109][139][140]. One distinguished merit of this method is the capability to check stability at regular intervals for a long time (days to months) [141], which other methods cannot offer. Thus, it provides an instantaneous measurement of the stability of MNFs and HNFs. Similar to other techniques, it is always used along with other methods such as visual inspection and ZP.
Checking of Thermophysical Properties
The stability of mono and hybrid NFs is also monitored by measuring their thermophysical properties over time. Garbadeen et al. [18] and Joubert et al. [20] monitored the stability of MWCNT/DIW and Fe2O3/DIW NFs for 250 min and 20 h, respectively, by measuring the μ. Likewise, in other studies, Yu et al. [142] and Ijam et al. [139] monitored the stability of Fe3O4/kerosene and GO/DIW-EG (60:40) NFs by measuring their κ for 360 min and 7 days, respectively. The use of κ to monitor the stability of NFs was corroborated by the work of Wang et al. [106], which reported a strong relationship between κ and the stability of NFs (Al2O3/W and Cu/W). Both Mahrood et al. [143] and Arani and Pourmoghadam [144] reported the use of density to monitor the stability of carboxymethyl cellulose-based Al2O3 and TiO2 NFs (before and after) and EG-based Al2O3-MWCNT NF (five times in 14 days), respectively. Additionally, Babu and Rao [145] used turbidity to check the stability of water-based Al2O3 NF. The literature showed that two or more of these reported NF stability monitoring techniques were used to check the stability of mono and hybrid NFs.
References
- Sharifpur, M.; Adio, S.A.; Meyer, J.P. Experimental investigation and model development for effective μ of Al2O3-glycerol NFs by using dimensional analysis and GMDH-NN methods. Int. Commun. Heat Mass Transf. 2015, 68, 208–219.
- Rostami, S.; Toghraie, D.; Shabani, B.; Sina, N.; Barnoon, P. Measurement of the κ of MWCNT-CuO/water hybrid NF using artificial neural networks (ANNs). J. Therm. Anal. Calorim. 2021, 143, 1097–1105.
- Osman, S.; Sharifpur, M.; Meyer, J.P. Experimental investigation of convection heat transfer in the transition flow regime of aluminium oxide-water NFs in a rectangular channel. Int. J. Heat Mass Transf. 2019, 133, 895–902.
- Giwa, S.O.; Sharifpur, M.; Ahmadi, M.H.; Sohel Murshed, S.M.; Meyer, J.P. Experimental investigation on stability, μ, and σ of water-based hybrid NF of mwcnt-Fe2O3. Nanomaterials 2021, 11, 136.
- Nwaokocha, C.; Momin, M.; Giwa, S.; Sharifpur, M.; Murshed, S.M.S.; Meyer, J.P. Experimental investigation of thermo-convection behaviour of aqueous binary NFs of MgO-ZnO in a square cavity. Therm. Sci. Eng. Prog. 2022, 28, 101057.
- Rostami, S.; Aghaei, A.; Hassani Joshaghani, A.; Mahdavi Hezaveh, H.; Sharifpur, M.; Meyer, J.P. Thermal–hydraulic efficiency management of spiral heat exchanger filled with Cu–ZnO/water hybrid NF. J. Therm. Anal. Calorim. 2021, 143, 1569–1582.
- Giwa, S.O.; Sharifpur, M.; Meyer, J.P. Effects of uniform magnetic induction on heat transfer performance of aqueous hybrid ferrofluid in a rectangular cavity. Appl. Therm. Eng. 2020, 170, 115004.
- Giwa, S.O.; Sharifpur, M.; Meyer, J.P. Experimental study of thermo-convection performance of hybrid NFs of Al2O3-MWCNT/water in a differentially heated square cavity. Int. J. Heat Mass Transf. 2020, 148, 119072.
- Sharifpur, M.; Yousefi, S.; Meyer, J.P. A new model for density of NFs including nanolayer. Int. Commun. Heat Mass Transf. 2016, 78, 168–174.
- Ghodsinezhad, H.; Sharifpur, M.; Meyer, J.P. Experimental investigation on cavity flow natural convection of Al2O3–water NFs. Int. Commun. Heat Mass Transf. 2016, 76, 316–324.
- Sharifpur, M.; Solomon, A.B.; Ottermann, T.L.; Meyer, J.P. Optimum concentration of NFs for heat transfer enhancement under cavity flow natural convection with TiO2– Water. Int. Commun. Heat Mass Transf. 2018, 98, 297–303.
- Sundar, L.S.; Manoj, A.H.M.; António, K.S.; Hafiz, C.M.S.; Ali, M. Efficiency analysis of thermosyphon solar flat plate collector with low mass concentrations of ND–Co3O4 hybrid NFs: An experimental study. J. Therm. Anal. Calorim. 2021, 143, 959–972.
- Sundar, L.S.; Singh, M.K.; Sousa, A.C.M. Turbulent heat transfer and friction factor of nanodiamond-nickel hybrid NFs flow in a tube: An experimental study. Int. J. Heat Mass Transf. 2018, 117, 223–234.
- Al-Hossainy, A.F.; Eid, M.R. Combined theoretical and experimental DFT-TDDFT and thermal characteristics of 3-D flow in rotating tube of C hybrid NF to enhancing oil extraction. Waves Random Complex Media 2021.
- Al-Hossainy, A.F.; Eid, M.R. Combined experimental thin films, TDDFT-DFT theoretical method, and spin effect on h hybrid NF flow with higher chemical rate. Surf. Interfaces 2021, 23, 100971.
- Eid, M.R.; Al-Hossainy, A.F. High-performance NF synthesis and DFT-TDDFT study of grapheme nanosheets along bent surface for enhanced. Case Stud. Therm. Eng. 2021, 25, 100983.
- Jamshed, W.; Eid, M.R.; Al-Hossainy, A.F.; Raizah, Z.; Tag El Din, E.S.M.; Sajid, T. Experimental and TDDFT materials simulation of thermal characteristics and entropy optimized of Williamson Cu-methanol and Al2O3-methanol NF flowing through solar collector. Sci. Rep. 2022, 12, 18130.
- Garbadeen, I.D.; Sharifpur, M.; Slabber, J.M.; Meyer, J.P. Experimental study on natural convection of MWCNT-water NFs in a square enclosure. Int. Commun. Heat Mass Transf. 2017, 88, 1–8.
- Solomon, A.B.; van Rooyen, J.; Rencken, M.; Sharifpur, M.; Meyer, J.P. Experimental study on the influence of the aspect ratio of square cavity on natural convection heat transfer with Al2O3/Water NFs. Int. Commun. Heat Mass Transf. 2017, 88, 254–261.
- Joubert, J.C.; Sharifpur, M.; Solomon, A.B.; Meyer, J.P. Enhancement in heat transfer of a ferrofluid in a differentially heated square cavity through the use of permanent magnets. J. Magn. Magn. Mater. 2017, 443, 149–158.
- Solomon, A.B.; Sharifpur, M.; Ottermann, T.; Grobler, C.; Joubert, M.; Meyer, J.P. Natural convection enhancement in a porous cavity with Al2O3-Ethylene glycol/water NFs. Int. J. Heat Mass Transf. 2017, 108, 1324–1334.
- Giwa, S.O.; Sharifpur, M.; Meyer, J.P. Heat transfer enhancement of dilute Al2O3-MWCNT water based hybrid NFs in a square cavity. In Proceedings of the International Heat Transfer Conference, Beijing, China, 10–15 August 2018; Volume 2018, pp. 5365–5372.
- Waini, I.; Ishak, A.; Groşan, T.; Pop, I. Mixed convection of a hybrid NF flow along a vertical surface embedded in a porous medium. Int. Commun. Heat Mass Transf. 2020, 114, 104565.
- Alsabery, A.I.; Ismael, M.A.; Chamkha, A.J.; Hashim, I. Mixed convection of Al2O3 -water NF in a double lid-driven square cavity with a solid inner insert using Buongiorno’s two-phase model. Int. J. Heat Mass Transf. 2018, 119, 939–961.
- Dalkılıç, A.S.; Türk, O.A.; Mercan, H.; Nakkaew, S.; Wongwises, S. An experimental investigation on heat transfer characteristics of graphite-SiO2/water hybrid NF flow in horizontal tube with various quad-channel twisted tape inserts. Int. Commun. Heat Mass Transf. 2019, 107, 1–13.
- Syam Sundar, L.; Otero-Irurueta, G.; Singh, M.K.; Sousa, A.C.M. Heat transfer and friction factor of multi-walled carbon nanotubes-Fe3O4 nanocomposite NFs flow in a tube with/without longitudinal strip inserts. Int. J. Heat Mass Transf. 2016, 100, 691–703.
- Kanti, P.K.; Sharma, K.V.; Minea, A.A.; Kesti, V. Experimental and computational determination of heat transfer, entropy generation and pressure drop under turbulent flow in a tube with fly ash-Cu hybrid NF. Int. J. Therm. Sci. 2021, 167, 107016.
- Gul, T.; Firdous, K. The experimental study to examine the stable dispersion of the graphene nanoparticles and to look at the GO–H2O NF flow between two rotating disks. Appl. Nanosci. 2018, 8, 1711–1727.
- Gul, T.; Khan, M.A.; Noman, W.; Khan, I. SS symmetry Fractional Order Forced Convection Carbon Nanotube NF Flow Passing over a Thin Needle. Symmetry 2019, 11, 312.
- Sundar, L.S.; Mesfin, S.; Venkata Ramana, E.; Said, Z.; Sousa, A.C.M. Experimental investigation of thermo-physical properties, heat transfer, pumping power, entropy generation, and exergy efficiency of nanodiamond + Fe3O4/60, 40% water-ethylene glycol hybrid NF flow in a tube. Therm. Sci. Eng. Prog. 2021, 21, 100799.
- Mubeen, I.; Shengyong, L.; Jianhua, Y.; Khan, M.S.; Yan, M.; Ali, H.M. Effect of milling material on characteristics and reactivity of mechanically treated fly ash to produce PCDD/F. J. Therm. Anal. Calorim. 2021, 143, 2707–2716.
- Choudhary, S.; Sachdeva, A.; Kumar, P. Investigation of the stability of MgO NF and its effect on the thermal performance of flat plate solar collector. Renew. Energy 2020, 147, 1801–1814.
- Okonkwo, E.C.; Wole-Osho, I.; Kavaz, D.; Abid, M.; Al-Ansari, T. Thermodynamic evaluation and optimization of a flat plate collector operating with alumina and iron mono and hybrid NFs. Sustain. Energy Technol. Assess. 2020, 37, 100636.
- Gopalsamy, V.; Senthil, R.; Varatharajulu, M.; Karunakaran, R. Application of response surface methodology to predict the optimized input quantities of parabolic trough concentrator. Int. J. Renew. Energy Dev. 2020, 9, 393–400.
- Efemwenkiekie, K.U.; Oyedepo, S.O.; Giwa, S.O.; Sharifpur, M.; Owoeye, T.F.; Akinlabu, K.D.; Meyer, J.P. Experimental investigation of heat transfer performance of novel bio-extract doped mono and hybrid NFs in a radiator. Case Stud. Therm. Eng. 2021, 28, 101494.
- Khan, A.; Ali, H.M.; Nazir, R.; Ali, R.; Munir, A.; Ahmad, B.; Ahmad, Z. Experimental investigation of enhanced heat transfer of a car radiator using ZnO nanoparticles in H2O–ethylene glycol mixture. J. Therm. Anal. Calorim. 2019, 138, 3007–3021.
- Lou, J.F.; Zhang, H.; Wang, R. Experimental investigation of graphite nanolubricant used in a domestic refrigerator. Adv. Mech. Eng. 2015, 7, 1–9.
- Ho, C.J.; Chen, W.C.; Yan, W.M.; Amani, P. Contribution of hybrid Al2O3-water NF and PCM suspension to augment thermal performance of coolant in a minichannel heat sink. Int. J. Heat Mass Transf. 2018, 122, 651–659.
- Jamil, M.; Khan, A.M.; Hegab, H.; Gong, L.; Mia, M.; Gupta, M.K. Effects of hybrid Al2O3-CNT NFs and cryogenic cooling on machining of Ti–6Al–4V. Int. J. Adv. Manuf. Technol. 2019, 102, 3895–3909.
- Hussien, A.A.; Abdullah, M.Z.; Yusop, N.M.; Al-Nimr, M.A.; Atieh, M.A.; Mehrali, M. Experiment on forced convective heat transfer enhancement using MWCNTs/GNPs hybrid NF and mini-tube. Int. J. Heat Mass Transf. 2017, 115, 1121–1131.
- Xu, Q.; Liu, L.; Feng, J.; Qiao, L.; Yu, C.; Shi, W.; Ding, C.; Zang, Y.; Chang, C.; Xiong, Y.; et al. A comparative investigation on the effect of different NFs on the thermal performance of two-phase closed thermosyphon. Int. J. Heat Mass Transf. 2020, 149, 119189.
- Ahmed, F.; Iqbal, M. Heat Transfer Analysis of MHD Power Law Nano Fluid Flow through Annular Sector Duct. J. Therm. Sci. 2020, 29, 169–181.
- Singh, S.K.; Sarkar, J. Experimental hydrothermal characteristics of concentric tube heat exchanger with V-cut twisted tape turbulator using PCM dispersed mono/hybrid NFs. Exp. Heat Transf. 2021, 34, 421–442.
- Guo, J.; Barber, G.C.; Schall, D.J.; Zou, Q.; Jacob, S.B. Tribological properties of ZnO and WS2 NFs using different surfactants. Wear 2017, 382–383, 8–14.
- Gao, T.; Li, C.; Zhang, Y.; Yang, M.; Jia, D.; Jin, T.; Hou, Y.; Li, R. Dispersing mechanism and tribological performance of vegetable oil-based CNT NFs with different surfactants. Tribol. Int. 2019, 131, 51–63.
- He, J.; Sun, J.; Meng, Y.; Pei, Y. Superior lubrication performance of MoS2-Al2O3 composite NF in strips hot rolling. J. Manuf. Process. 2020, 57, 312–323.
- Gugulothu, S.; Pasam, V.K. Experimental investigation to study the performance of CNT/MoS2 hybrid NF in turning of AISI 1040 steel. Aust. J. Mech. Eng. 2020, 20, 814–824.
- Ali, M.K.A.; Xianjun, H.; Mai, L.; Qingping, C.; Turkson, R.F.; Bicheng, C. Improving the tribological characteristics of piston ring assembly in automotive engines using Al2O3 and TiO2 nanomaterials as nano-lubricant additives. Tribol. Int. 2016, 103, 540–554.
- Nam, J.S.; Lee, P.H.; Lee, S.W. Experimental characterization of micro-drilling process using NF minimum quantity lubrication. Int. J. Mach. Tools Manuf. 2011, 51, 649–652.
- Ali, M.K.A.; Hou, X.; Abdelkareem, M.A.A. Anti-wear properties evaluation of frictional sliding interfaces in automobile engines lubricated by copper/graphene nanolubricants. Friction 2020, 8, 905–916.
- Ali, M.K.A.; Xianjun, H. Improving the heat transfer capability and thermal stability of vehicle engine oils using Al2O3/TiO2 nanomaterials. Powder Technol. 2020, 363, 48–58.
- Kiani, M.; Ansari, M.; Arshadi, A.A.; Houshfar, E.; Ashjaee, M. Hybrid thermal management of lithium-ion batteries using NF, metal foam, and phase change material: An integrated numerical–experimental approach. J. Therm. Anal. Calorim. 2020, 141, 1703–1715.
- Navarrete, N.; Mondragón, R.; Wen, D.; Navarro, M.E.; Ding, Y.; Juliá, J.E. Thermal energy storage of molten salt –based NF containing nano-encapsulated metal alloy phase change materials. Energy 2019, 167, 912–920.
- Sharma, H.K.; Verma, S.K.; Singh, P.K.; Kumar, S.; Paswan, M.K.; Singhal, P. Performance analysis of paraffin wax as PCM by using hybrid zinc-cobalt-iron oxide nano-fluid on latent heat energy storage system. Mater. Today Proc. 2019, 26, 1461–1464.
- Zaibudeen, A.W.; Philip, J. Temperature and pH sensor based on functionalized magnetic NF. Sens. Actuators B Chem. 2018, 268, 338–349.
- Soares, M.C.P.; Rodrigues, M.S.; Schenkel, E.A.; Perli, G.; Silva, W.H.A.; Gomes, M.K.; Fujiwara, E.; Suzuki, C.K. Evaluation of silica NFs in static and dynamic conditions by an optical fiber sensor. Sensors 2020, 20, 707.
- Abdullah, S.M.; Rafique, S.; Hamdan, K.S.; Roslan, N.A.; Li, L.; Sulaiman, K. Highly sensitive capacitive cell based on a novel CuTsPc-TiO2 nanocomposite electrolytic solution for low-temperature sensing applications. Sens. Actuators A Phys. 2019, 289, 94–99.
- Esmaeilzadeh, F.; Teja, A.S.; Bakhtyari, A. The κ, μ, and cloud points of bentonite NFs with n-pentadecane as the base fluid. J. Mol. Liq. 2020, 300, 112307.
- Salehnezhad, L.; Heydari, A.; Fattahi, M. Experimental investigation and rheological behaviors of water-based drilling mud contained starch-ZnO NFs through response surface methodology. J. Mol. Liq. 2019, 276, 417–430.
- Alnarabiji, M.S.; Yahya, N.; Nadeem, S.; Adil, M.; Baig, M.K.; Ben Ghanem, O.; Azizi, K.; Ahmed, S.; Maulianda, B.; Klemeš, J.J.; et al. NF enhanced oil recovery using induced ZnO nanocrystals by electromagnetic energy: Μ increment. Fuel 2018, 233, 632–643.
- Li, R.; Jiang, P.; Gao, C.; Huang, F.; Xu, R.; Chen, X. Experimental investigation of silica-based NF enhanced oil recovery: The effect of wettability alteration. Energy Fuels 2017, 31, 188–197.
- AfzaliTabar, M.; Rashidi, A.; Alaei, M.; Koolivand, H.; Pourhashem, S.; Askari, S. Hybrid of quantum dots for interfacial tension reduction and reservoir alteration wettability for enhanced oil recovery (EOR). J. Mol. Liq. 2020, 307, 112984.
- Shao, X.-F.; Mo, S.-P.; Chen, Y.; Yin, T.; Yang, Z.; Jia, L.-S.; Cheng, Z.-D. Solidification behavior of hybrid TiO2 NFs containing nanotubes and nanoplatelets for cold thermal energy storage. Appl. Therm. Eng. 2017, 117, 427–436.
- Joseph, A.; Sreekumar, S.; Kumar, C.S.S.; Thomas, S. Optimisation of thermo-optical properties of SiO2/Ag–CuO NF for direct absorption solar collectors. J. Mol. Liq. 2019, 296, 111986.
- Hormozi, F.; ZareNezhad, B.; Allahyar, H.R. An experimental investigation on the effects of surfactants on the thermal performance of hybrid NFs in helical coil heat exchangers. Int. Commun. Heat Mass Transf. 2016, 78, 271–276.
- Giwa, S.O.; Sharifpur, M.; Meyer, J.P. Experimental investigation into heat transfer performance of water-based magnetic hybrid NFs in a rectangular cavity exposed to magnetic excitation. Int. Commun. Heat Mass Transf. 2020, 116, 104698.
- Khooshechin, M.; Fathi, S.; Salimi, F.; Ovaysi, S. The influence of surfactant and ultrasonic processing on improvement of stability and heat transfer coefficient of CuO nanoparticles in the pool boiling. Int. J. Heat Mass Transf. 2020, 154, 119783.
- Gulzar, O.; Qayoum, A.; Gupta, R. Photo-thermal characteristics of hybrid NFs based on Therminol-55 oil for concentrating solar collectors. Appl. Nanosci. 2019, 9, 1133–1143.
- Cacua, K.; Buitrago-Sierra, R.; Herrera, B.; Pabón, E.; Murshed, S.M. NFs’ stability effects on the thermal performance of heat pipes: A critical review. J. Therm. Anal. Calorim. 2019, 136, 1597–1614.
- Koçak Soylu, S.; Atmaca, İ.; Asiltürk, M.; Doğan, A. Improving heat transfer performance of an automobile radiator using Cu and Ag doped TiO2 based NFs. Appl. Therm. Eng. 2019, 157, 113743.
- Ghafurian, M.M.; Akbari, Z.; Niazmand, H.; Mehrkhah, R.; Wongwises, S.; Mahian, O. Effect of sonication time on the evaporation rate of seawater containing a nanocomposite. Ultrason. Sonochem. 2020, 61, 104817.
- Abbasi, S.M.; Rashidi, A.; Nemati, A.; Arzani, K. The effect of functionalisation method on the stability and the κ of NF hybrids of carbon nanotubes/gamma alumina. Ceram. Int. 2013, 39, 3885–3891.
- Zawrah, M.F.; Khattab, R.M.; Girgis, L.G.; El Daidamony, H.; Abdel Aziz, R.E. Stability and σ of water-base Al2O3 NFs for different applications. HBRC J. 2016, 12, 227–234.
- Adio, S.A.; Sharifpur, M.; Meyer, J.P. Influence of ultrasonication energy on the dispersion consistency of Al2O3–glycerol NF based on μ data, and model development for the required ultrasonication energy density. J. Exp. Nanosci. 2016, 11, 630–649.
- Giwa, S.O.; Sharifpur, M.; Goodarzi, M.; Alsulami, H.; Meyer, J.P. Influence of base fluid, temperature, and concentration on the thermophysical properties of hybrid NFs of alumina–ferrofluid: Experimental data, modeling through enhanced ANN, ANFIS, and curve fitting. J. Therm. Anal. Calorim. 2021, 143, 4149–4167.
- Giwa, S.O.; Sharifpur, M.; Meyer, J.P.; Wongwises, S.; Mahian, O. Experimental measurement of μ and σ of water-based γ-Al2O3/MWCNT hybrid NFs with various particle mass ratios. J. Therm. Anal. Calorim. 2021, 143, 1037–1050.
- Giwa, S.O.; Momin, M.; Nwaokocha, C.N.; Sharifpur, M.; Meyer, J.P. Influence of nanoparticles size, per cent mass ratio, and temperature on the thermal properties of water-based MgO–ZnO NF: An experimental approach. J. Therm. Anal. Calorim. 2021, 143, 1063–1079.
- Mousavi, S.M.; Esmaeilzadeh, F.; Wang, X.P. Effects of temperature and particles volume concentration on the thermophysical properties and the rheological behavior of CuO/MgO/TiO2 aqueous ternary hybrid NF: Experimental investigation. J. Therm. Anal. Calorim. 2019, 137, 879–901.
- Aparna, Z.; Michael, M.; Pabi, S.K.; Ghosh, S. Κ of aqueous Al2O3/Ag hybrid nano fluid at different temperatures and volume concentrations: An experimental investigation and development of new correlation function. Powder Technol. 2019, 343, 714–722.
- Vicki, W.V.; Abdullah, M.Z.; Gunnasegaran, P. Thermophysical properties of Al2O3-CuO hybrid NF at different nanoparticle mixture ratio: An experimetal approach. J. Mol. Liq. 2020, 1862, 183135.
- Xian, H.W.; Sidik, N.A.C.; Saidur, R. Impact of different surfactants and ultrasonication time on the stability and thermophysical properties of hybrid NFs. Int. Commun. Heat Mass Transf. 2020, 110, 104389.
- Nabil, M.F.; Azmi, W.H.; Hamid, K.A.; Mamat, R. Experimental investigation of heat transfer and friction factor of TiO2-SiO2 NFs in water:ethylene glycol mixture. Int. J. Heat Mass Transf. 2018, 124, 1361–1369.
- Michael, M.; Zagabathuni, A.; Sikdar, S.; Pabi, S.K.; Ghosh, S. Effect of dispersion behavior on the heat transfer characteristics of alumina NF: An experimental investigation and development of a new correlation function. Int. Nano Lett. 2020, 10, 207–217.
- Mousavi, S.M.; Esmaeilzadeh, F.; Wang, X.P. A detailed investigation on the thermo-physical and rheological behavior of MgO/TiO2 aqueous dual hybrid NF. J. Mol. Liq. 2019, 282, 323–339.
- Menbari, A.; Alemrajabi, A.A.; Ghayeb, Y. Investigation on the stability, μ and extinction coefficient of CuO-Al2O3/Water binary mixture NF. Exp. Therm. Fluid Sci. 2016, 74, 122–129.
- Kamalgharibi, M.; Hormozi, F.; Zamzamian, S.A.H.; Sarafraz, M.M. Experimental studies on the stability of CuO nanoparticles dispersed in different base fluids: Influence of stirring, sonication and surface active agents. Heat Mass Transf. Und. Stoffuebertragung. 2016, 52, 55–62.
- Abadeh, A.; Passandideh-Fard, M.; Maghrebi, M.J.; Mohammadi, M. Stability and magnetization of Fe3O4/water NF preparation characteristics using Taguchi method. J. Therm. Anal. Calorim. 2019, 135, 1323–1334.
- Tiwari, A.K.; Pandya, N.S.; Said, Z.; Öztop, H.F.; Abu-Hamdeh, N. 4S consideration (synthesis, sonication, surfactant, stability) for the κ of CeO2 with MWCNT and water based hybrid NF: An experimental assessment. Colloids Surf. A Physicochem. Eng. Asp. 2021, 610, 125918.
- Suhaimi, N.S.; Din, M.F.M.; Ishak, M.T.; Rahman, A.R.A.; Wang, J.; Hassan, M.Z. Performance and limitation of mineral oil-based carbon nanotubes NF in transformer application. Alex. Eng. J. 2022, 61, 9623–9635.
- Sharifpur, M.; Ghodsinezhad, H.; Meyer, J.P.; Rolfes, H. Investigation on Ultrasonicaton Energy Density Effect on Characterization of Zinc Oxide (ZnO) NanoParticle Size Distribution with Using Zeta-Sizer. In Proceedings of the 11th International Conference on Heat Transfer, Fluid Mechanics and Thermodynamics, Conference Centre in the Skukuza Rest Camp, Kruger National Park, South Africa, 20–23 July 2015; pp. 211–216.
- Tiwari, A.K.; Pandya, N.S.; Said, Z.; Chhatbar, S.H.; Al-Turki, Y.A.; Patel, A.R. 3S (Sonication, surfactant, stability) impact on the μ of hybrid NF with different base fluids: An experimental study. J. Mol. Liq. 2021, 329, 115455.
- Sajid, M.U.; Ali, H.M. Κ of hybrid NFs: A critical review. Int. J. Heat Mass Transf. 2018, 126, 211–234.
- Gupta, M.; Singh, V.; Kumar, S.; Kumar, S.; Dilbaghi, N.; Said, Z. Up to date review on the synthesis and thermophysical properties of hybrid NFs. J. Clean. Prod. 2018, 190, 169–192.
- Nabil, M.F.; Azmi, W.H.; Abdul Hamid, K.; Mamat, R.; Hagos, F.Y. An experimental study on the κ and dynamic μ of TiO2-SiO2 NFs in water: Ethylene glycol mixture. Int. Commun. Heat Mass Transf. 2017, 86, 181–189.
- Ranga Babu, J.A.; Kumar, K.K.; Srinivasa Rao, S. State-of-art review on hybrid NFs. Renew. Sustain. Energy Rev. 2017, 77, 551–565.
- Kumar, D.D.; Arasu, A.V. A comprehensive review of preparation, characterization, properties and stability of hybrid NFs. Renew. Sustain. Energy Rev. 2018, 81, 1669–1689.
- Sundar, L.S.; Venkata Ramana, E.; Graça, M.P.F.; Singh, M.K.; Sousa, A.C.M. Nanodiamond-Fe3O4 NFs: Preparation and measurement of μ, electrical and thermal conductivities. Int. Commun. Heat Mass Transf. 2016, 73, 62–74.
- Adio, S.A.; Mehrabi, M.; Sharifpur, M.; Meyer, J.P. Experimental investigation and model development for effective μ of MgO-ethylene glycol NFs by using dimensional analysis, FCM-ANFIS and GA-PNN techniques. Int. Commun. Heat Mass Transf. 2016, 72, 71–83.
- Kakavandi, A.; Akbari, M. Experimental investigation of κ of NFs containing of hybrid nanoparticles suspended in binary base fluids and propose a new correlation. Int. J. Heat Mass Transf. 2018, 124, 742–751.
- Sundar, L.S.; Shusmitha, K.; Singh, M.K.; Sousa, A.C.M. Σ enhancement of nanodiamond-nickel (ND-Ni) nanocomposite based magnetic NFs. Int. Commun. Heat Mass Transf. 2014, 57, 1–7.
- Askari, S.; Koolivand, H.; Pourkhalil, M.; Lotfi, R.; Rashidi, A. Investigation of Fe3O4/Graphene nanohybrid heat transfer properties: Experimental approach. Int. Commun. Heat Mass Transf. 2017, 87, 30–39.
- Nabati Shoghl, S.; Jamali, J.; Keshavarz Moraveji, M. Σ, μ, and density of different NFs: An experimental study. Exp. Therm. Fluid Sci. 2016, 74, 339–346.
- Said, Z. Thermophysical and optical properties of SWCNTs NFs. Int. Commun. Heat Mass Transf. 2016, 78, 207–213.
- Alirezaie, A.; Saedodin, S.; Esfe, M.H.; Rostamian, S.H. Investigation of rheological behavior of MWCNT (COOH-functionalized)/MgO-Engine oil hybrid NFs and modelling the results with artificial neural networks. J. Mol. Liq. 2017, 241, 173–181.
- Kole, M.; Dey, T.K. Effect of aggregation on the μ of copper oxide-gear oil NFs. Int. J. Therm. Sci. 2011, 50, 1741–1747.
- Wang, X.-J.; Zhu, D.-S.; Yang, S. Investigation of pH and SDBS on enhancement of κ in NFs. Chem. Phys. Lett. 2009, 470, 107–111.
- Yang, J.-C.; Li, F.-C.; Zhou, W.-W.; He, Y.-R.; Jiang, B.-C. Experimental investigation on the κ and shear μ of viscoelastic-fluid-based NFs. Int. J. Heat Mass Transf. 2012, 55, 3160–3166.
- Pastoriza-Gallego, M.J.; Lugo, L.; Legido, J.L.; Pineiro, M.M. Enhancement of κ and volumetric behavior of FexOy NFs. J. Appl. Phys. 2011, 110, 1–9.
- Ramadhan, A.I.; Azmi, W.H.; Mamat, R.; Hamid, K.A.; Norsakinah, S. Investigation on stability of tri-hybrid NFs in water-ethylene glycol mixture Investigation on stability of tri -hybrid NFs in water- ethylene glycol mixture. IOP Conf. Ser. Mater. Sci. Eng. 2019, 469, 012068.
- Midhun Mohan, V.; Sajeeb, A.M. Improving the Efficiency of DASC by Adding CeO2/CuO Hybrid Nanoparticles in Water. Int. J. Nanosci. 2018, 17, 1–8.
- Ebrahimi, S.; Saghravani, S.F. Influence of magnetic field on the κ of the water based mixed Fe3O4/CuO NF. J. Magn. Magn. Mater. 2017, 441, 366–373.
- Ghadimi, A.; Saidur, R.; Metselaar, H.S.C. A review of NF stability properties and characterization in stationary conditions. Int. J. Heat Mass Transf. 2011, 54, 4051–4068.
- Chen, L.F.; Cheng, M.; Yang, D.J.; Yang, L. Enhanced Κ of NF by Synergistic Effect of Multi-Walled Carbon Nanotubes and Fe2O3 Nanoparticles. Appl. Mech. Mater. 2014, 548–549, 118–123.
- Naddaf, A.; Zeinali Heris, S. Experimental study on κ and σ of diesel oil-based NFs of graphene nanoplatelets and carbon nanotubes. Int. Commun. Heat Mass Transf. 2018, 95, 116–122.
- Hemmat Esfe, M.; Abbasian Arani, A.A.; Rezaie, M.; Yan, W.M.; Karimipour, A. Experimental determination of κ and dynamic μ of Ag-MgO/water hybrid NF. Int. Commun. Heat Mass Transf. 2015, 66, 189–195.
- Chen, L.; Yu, W.; Xie, H. Enhanced κ of NFs containing Ag/MWNT composites. Powder Technol. 2012, 231, 18–20.
- Hajiyan, M.; Ebadi, S.; Mahmud, S.; Biglarbegian, M.; Abdullah, H. Experimental investigation of the effect of an external magnetic field on the κ and μ of Fe3O4–glycerol. J. Therm. Anal. Calorim. 2018, 1, 1–14.
- Esmaeili, E.; Ghazanfar Chaydareh, R.; Rounaghi, S.A. The influence of the alternating magnetic field on the convective heat transfer properties of Fe3O4-containing NFs through the Neel and Brownian mechanisms. Appl. Therm. Eng. 2017, 110, 1212–1219.
- Adio, S.A.; Sharifpur, M.; Meyer, J.P. Investigation Into Effective Μ, Σ, and pH of γ-Al2O3-Glycerol NFs in Einstein Concentration Regime. Heat Transf. Eng. 2015, 36, 1241–1251.
- Adio, S.A.; Sharifpur, M.; Meyer, J.P. Factors affecting the pH and σ of MgO-ethylene glycol NFs. Bull. Mater. Sci. 2015, 38, 1345–1357.
- Suresh, S.; Venkitaraj, K.P.; Selvakumar, P.; Chandrasekar, M. Synthesis of Al2O3-Cu/water hybrid NFs using two step method and its thermo physical properties. Colloids Surf. A Physicochem. Eng. Asp. 2011, 388, 41–48.
- Kumar, V.; Sarkar, J. Numerical and experimental investigations on heat transfer and pressure drop characteristics of Al2O3-TiO2 hybrid NF in minichannel heat sink with different mixture ratio. Powder Technol. 2019, 345, 717–727.
- Mahbubul, I.M.; Elcioglu, E.B.; Amalina, M.A.; Saidur, R. Stability, thermophysical properties and performance assessment of alumina–water NF with emphasis on ultrasonication and storage period. Powder Technol. 2019, 345, 668–675.
- Mahbubul, I.M.; Elcioglu, E.B.; Saidur, R.; Amalina, M.A. Optimization of ultrasonication period for better dispersion and stability of TiO2–water NF. Ultrason. Sonochem. 2017, 37, 360–367.
- Sreekumar, S.; Joseph, A.; Sujith Kumar, C.S.; Thomas, S. Investigation on influence of antimony tin oxide/silver NF on direct absorption parabolic solar collector. J. Clean. Prod. 2020, 249, 119378.
- Cacua, K.; Ordoñez, F.; Zapata, C.; Herrera, B.; Pabón, E.; Buitrago-Sierra, R. Surfactant concentration and pH effects on the zeta potential values of alumina NFs to inspect stability. Colloids Surf. A Physicochem. Eng. Asp. 2019, 583, 123960.
- Chakraborty, S.; Mukherjee, J.; Manna, M.; Ghosh, P.; Das, S.; Denys, M.B. Effect of Ag nanoparticle addition and ultrasonic treatment on a stable TiO2 NF. Ultrason. Sonochem. 2012, 19, 1044–1050.
- Kumar, V.; Sarkar, J. Experimental hydrothermal characteristics of minichannel heat sink using various types of hybrid NFs. Adv. Powder Technol. 2020, 31, 621–631.
- Xian-Ju, W.; Xin-Fang, L. Influence of pH on NFs’ Μ and Κ. Chin. Phys. Lett. 2009, 26, 056601.
- Momin, G.G. Experimental Investigation of Mixed Convection With Water-Al2O3 & Hybrid NF In Inclined Tube For Laminar Flow. Int. J. Sci. Technol. Res. 2013, 2, 195–202.
- Akilu, S.; Baheta, A.T.; Mior, M.A.; Minea, A.A.; Sharma, K.V. Properties of glycerol and ethylene glycol mixture based SiO2-CuO/C hybrid NF for enhanced solar energy transport. Sol. Energy Mater. Sol. Cells 2018, 179, 118–128.
- Qing, S.H.; Rashmi, W.; Khalid, M.; Gupta, T.C.S.M.; Nabipoor, M.; Hajibeigy, M.T. Κ and electrical properties of hybrid SiO2-graphene naphthenic mineral oil NF as potential transformer oil. Mater. Res. Express 2017, 4, 015504.
- Okonkwo, E.C.; Wole-Osho, I.; Kavaz, D.; Abid, M. Comparison of experimental and theoretical methods of obtaining the thermal properties of alumina/iron mono and hybrid NFs. J. Mol. Liq. 2019, 292, 111377.
- Menbari, A.; Alemrajabi, A.A.; Ghayeb, Y. Experimental investigation of stability and extinction coefficient of Al2O3-CuO binary nanoparticles dispersed in ethylene glycol-water mixture for low-temperature direct absorption solar collectors. Energy Convers. Manag. 2016, 108, 501–510.
- Menbari, A.; Alemrajabi, A.A.; Rezaei, A. Experimental investigation of thermal performance for direct absorption solar parabolic trough collector (DASPTC) based on binary NFs. Exp. Therm. Fluid Sci. 2017, 80, 218–227.
- Baby, T.T.; Ramaprabhu, S. Experimental investigation of the thermal transport properties of a carbon nanohybrid dispersed NF. Nanoscale 2011, 3, 2208.
- Zawawi, N.N.M.; Azmi, W.H.; Redhwan, A.A.M.; Sharif, M.Z.; Samykano, M. Experimental investigation on thermo-physical properties of metal oxide composite nanolubricants. Int. J. Refrig. 2018, 89, 11–21.
- Ganguly, S.; Sikdar, S.; Basu, S. Experimental investigation of the effective σ of aluminum oxide NFs. Powder Technol. 2009, 196, 326–330.
- Ijam, A.; Saidur, R.; Ganesan, P.; Moradi Golsheikh, A. Stability, thermo-physical properties, and σ of graphene oxide-deionized water/ethylene glycol based NF. Int. J. Heat Mass Transf. 2015, 87, 92–103.
- Sun, B.; Peng, C.; Zuo, R.; Yang, D.; Li, H. Investigation on the flow and convective heat transfer characteristics of NFs in the plate heat exchanger. Exp. Therm. Fluid Sci. 2016, 76, 75–86.
- Hadadian, M.; Goharshadi, E.K.; Youssefi, A. Σ, κ, and rheological properties of graphene oxide-based NFs. J. Nanoparticle Res. 2014, 16, 1–17.
- Yu, W.; Xie, H.; Chen, L.; Li, Y. Enhancement of κ of kerosene-based Fe3O4 NFs prepared via phase-transfer method. Colloids Surf. A Physicochem. Eng. Asp. 2010, 355, 109–113.
- Mahrood, M.R.K.; Etemad, S.G.; Bagheri, R. Free convection heat transfer of non Newtonian NFs under constant heat flux condition. Int. Commun. Heat Mass Transf. 2011, 38, 1449–1454.
- Arani, A.A.A.; Pourmoghadam, F. Experimental investigation of κ behavior of MWCNTS-Al2O3/ethylene glycol hybrid NF: Providing new κ correlation. Heat Mass Transf. Und. Stoffuebertragung. 2019, 55, 2329–2339.
- Babu, S.R.; Rao, G.S. Experimental investigation of natural convective heat transfer using water-alumina NF with taguchi design of experiments. Int. J. Mech. Eng. Technol. 2017, 8, 795–804.
More
