Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Tan Mao and Version 2 by Sirius Huang.
Perfluorooctanoic acid (PFOA) is a new type of organic pollutant in wastewater that is persistent, toxic, and accumulates in living organisms. The development of rapid and sensitive analytical methods to detect PFOA in environmental media is of great importance. Fluorescence detection has the advantages of high efficiency and low cost, in which fluorescent probes have excellent fluorescence properties, excellent bio-solubility, and remarkable photostability.
- PFOA
- fluorescence detection
- fluorescent probe
1. Introduction
The chemical formula of Perfluorooctanoic acid (PFOA) is C8HF15O2 and its structure is shown in Figure 1. PFOA contains 15 F atoms and 8 C atoms. The C-F bond of PFOA has strong polarity and bond energy. Fluorine surfactants of it has a higher surface activity, stronger hydrophobicity, and oleophobicity than traditional surfactants. They are widely used in the chemical, textile, leather, paper making, and cosmetics industries. The fluorinated surfactants of PFOA have the strongest surface activity and the most extensive application. PFOA is hard to degrade because of its strong chemical stability and biological inertness, and can withstand strong light, heat, chemistry, microbial action, and has a high biological metabolism. Therefore, PFOA has bioaccumulation and toxic effect, which will threaten the ecosystem and human health.
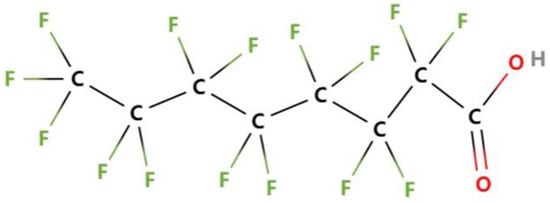
Figure 1.
The chemical structure of PFOA.
PFOA is the main active ingredient of waterproof, oil-proof, and stain-proof finishing agents, which were used in the coatings of various special garments, fabrics, and carpets. Adding PFOA into the coating can improve the wettability, the dispersibility, the uniformity of the color carrier, and prevent caking. The most familiar one is the “Non-stick pan” with a polytetrafluoroethylene (PTFE) surface coating. PFOA is one of the processing aids of PTFE [1]. Due to the applications of PFOA in many fields, PFOA enters the rivers, lakes, and even into drinking water. Li’s group [2] found that PFOA levels in many sewage discharges in Chongqing seriously exceeded the standard and was extremely difficult to deal with. Wang’s group [3] found that the contents of PFOA and PFOS in drinking water in some areas were too high. PFOA is accumulative and transferable, so it can be found in the air, soil, and water [4][5][6][7]. This will increase the possibility of PFOA entering the body.
The damage of PFOA to the human body is enormous. PFOA can enter the human body through the skin surface to cause PFOA to accumulate in the body, which may cause cancer. Sun’s group [8] found that exposure to PFOA leads to the accumulation of ROS in BRL-3A cells, which ultimately leads to the death of the experimental subject. In addition, PFOA has an impact on the human stomach, liver, and nervous system, even leading to cognitive abnormalities [9]. It also affects human sperm [10].
In 2006, the Environmental Protection Agency (EPA) of the United States proposed a reduction plan for PFOA [11]. In 2014, the Norwegian Environmental Protection Agency issued a national ban on the use of PFOA products. In 2016, the United States Toxic Substances Control Act (USTSCA) included PFOA on the list of toxic chemicals [12]. In 2015, China generated large quantities of fluoropolymers, and the consumption of PFOA reached 200 tons [13][14]. Frederick Pontius [15] analyzed in detail the requirements of PFOA and PFOS of various international organizations in recent years, and the regulatory agencies of more than 12 countries formulated the consultation of PFOA in drinking water or groundwater. PFOA was included on the third drinking water contaminant candidate list (CCL). US-EPA restricted the content of PFOA in healthy drinking water at 70 ng/L. PFOA has attracted worldwide attention since 2016. The requirements for PFOA content in drinking water in China are still improving [16][17][18].
The detection of PFOA has a great social significance to people’s lives, but the effectiveness of the detection methods is different. Therefore, the simplest, quickest, and most sensitive methods need to be explored [19][20].
2. Method of Detecting PFOA
Traditional methods of detecting PFOA include chromatography, mass spectrometry, and chromatography–mass spectrometry. These methods often need to use expensive instruments to detect due to the unique properties of PFOA [21][22][23][24][25]. The fluorescence detection method is a new way to detect trace elements, and PFOA as a trace element can be detected by it.
2.1. Liquid Chromatography-Mass Spectrometry
2.1.1. High Performance Liquid Chromatography–Tandem Mass Spectrometry (HPLC–MS/MS)
High performance liquid chromatography (HPLC) has the advantages of high accuracy, a wide separation range, and low destruction to the material structure. Figure 2 shows the detection flow chart of HPLC. The sensitivity of mass spectrometry (MS) is better than any other analytical method, it is more accurate in characterizing the structure of unknown compounds. Mass separation–mass spectra Characterization (MS–MS) allows further cleavage of the parent atom to obtain information on the cleavage process and molecular structure, often called as tandem mass spectrometry. HPLC–MS/MS combines the advantages of both. The chromatography can be used as a sampling system for the mass spectrometry, and the mass spectrometry as an identifier for the chromatography. HPLC–MS/MS has certain advantages in terms of selectivity and sensitivity [26][27].
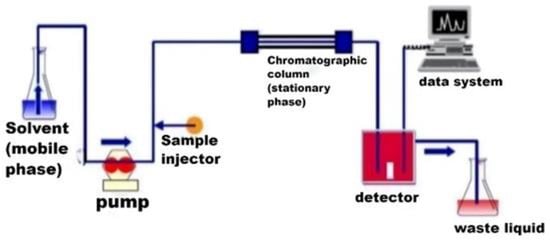
Figure 2.
Detection flow chart of HPLC.
2.1.2. High Performance Liquid Chromatography–Quadrupole-Time of Flight-Mass Spectrometry (HPLC–Q-TOF-MS)
The mass analyzer used for HPLC–MS/MS is a low-resolution mass spectrometer, it cannot effectively distinguish the interference when analyzing complex samples. Time of flight-mass spectrometry (TOF/MS) has certain advantages in terms of mass range and resolution, and it has a high mass accuracy and a fast analysis speed. Unlike low-resolution mass spectrometry, TOF/MS greatly improves the anti-interference ability in complex backgrounds and makes the detection results more accurate and reliable [28]. Therefore, HPLCQ–TOF-MS has a certain optimization to analyze samples when in a complex environment, increasing the correctness of detection [29][30].
In 2009, USEPA issued Method 537 to analyze 14 PFAS in drinking water. This method needs to use solid-phase extraction and liquid chromatography/tandem mass spectrometry (LC/MS/MS) [15]. However, this method needs skilled analysts and expensive instruments to get effective results.
2.2. Gas Chromatography–Mass Spectrometry
2.2.1. GC-MS
Because the partition coefficient of the sample in the chromatographic is different when it is in the gas or solid phase state. Therefore, GC–MS uses this principle to complete detection by multiple distributions of the sample. In GC–MS, the gas chromatography is used as the sampling system of mass spectrometry, while the mass spectrometer is used for gas chromatography detection. However, the GC–MS detection process is more cumbersome and therefore less used in applied methods [31].
2.2.2. Pre-Column Derivatization–Gas Chromatography
Pre-column Derivatization–Gas chromatography is an improvement on the shortcomings of GC–MS. The derivatization technology can reduce the temperature of the target analyte and improve the signal required to detect the target, which reduces the cost of the detection instrument and can effectively detect PFOA [32], such as silanization, esterification, and acylation [33][34].
2.3. Fluorescence Detection
Traditional methods of detecting PFOA have certain advantages, but still have the shortcomings of high cost and low universality. Fluorescence detection is the latest in current detection methods, which can detect target substances conveniently and quickly. Zheng’s group [35] sensitively detected perfluorinated pollutants through fluorescence detection.
The fluorescent probe uses fluorescent substances as indicators, and under the excitation of a certain wavelength of light, the indicator produces fluorescence. Then, the change of fluorescent strength is detected to achieve a qualitative or quantitative analysis of the detected substances. The signal emitted by the reporter fluorophore is absorbed by the quenching fluorophore when the probe is intact, but the two are separated when the probe detects the substance. In other words, the luminescent substance at the top of the fluorescent probe will specifically bind to the detection target when the fluorescent probe reacts with the detection substance, which can cause a fluorescence quenching reaction.
The probe can be divided into a Beacon probe and a FRET (fluorescence resonance energy transfer) probe. Beacon mode depends on the molecular beacon accumulating fluorescence in the presence of enzymatic digestion and cannot show the detection status in time, as shown in Figure 3a. However, the detection signal of the FRET mode is a real-time signal, which can display the detection status in time, as in Figure 3b. Therefore, the FRET mode will be selected when detecting trace elements. The FRET probe includes fluorescein probes, inorganic ion fluorescent probes, fluorescent quantum dots, and molecular beacons [36].
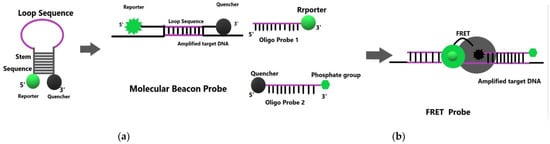
Figure 3.
(
a
) Detection principle of Beacon probe (
b
) Detection principle of FRET probe.
Fluorescence quenching can lower the intensity of emitted light from fluorescent molecules. Fluorescence quenching can cause some changes including fluorescence intensity, related excitation peak changes, and fluorescence peak position changes. The substances react with fluorescent materials causing these changes and these processes are called fluorescence quenchers. For example, electron acceptors are fluorescence quenchers in photosynthesis.
Fluorescence quenching can be divided into dynamic quenching and static quenching. Dynamic quenching occurs when the fluorescence quenching between the excited state fluorescent molecule and the quencher will cause an energy transfer or physical collision. However, for the excited state molecule, it is not necessary to complete the direct contact between the two acting molecules. They can create optical collision effects directly. Static quenching refers to the formation of a compound between the ground state fluorescent molecule and the quencher through weak binding, and the compound will completely quench the fluorescence.
The principle of fluorescence quenching is the result of fluorescence resonance energy transfer (FRET) [37][38][39]. As shown in Figure 4, FRET is a non-radiative process formed by the interaction between dipoles and dipoles, and the energy will be generated when donor and acceptor close to each other.
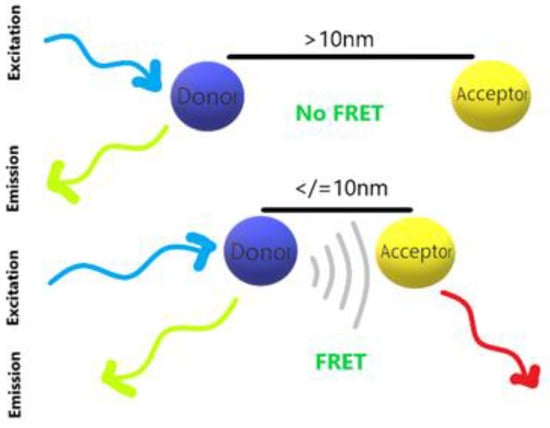
Figure 4.
Fluorescence resonance energy transfer (FRET).
A small molecule fluorescent probe is widely used to detect trace elements. It has the advantages of low cost, simplicity of handling, and a high detection sensitivity. A fluorescent probe made of fluorescent materials has the advantages of good hydrophilicity and multifunctional integration. It plays a significant role in detecting trace elements. A detailed comparison can be seen in Table 1.
Table 1.
Comparison table of fluorescent probes.
| Category | Advantages | Detection Limit | References |
|---|---|---|---|
| Small molecule fluorescent probes | Small molecule fluorescent probes have significant advantages such as high sensitivity, good membrane permeability, real-time in situ analysis, minimal biological damage, and easy handling. | 1.05 × 10−8~5.8 × 10−7 mol/L | [40][41] |
| Polymeric fluorescent probes | It has a long fluorescence lifetime, good biocompatibility, and high quantum yield. It can also enhance intramolecular electron transport and increase the sensitivity of the probe. | 0.17~2.3 μg/mL | [42][43] |
| [ | 44 | ] | [45] |
Fluorescent sensors use materials that have a fluorescence effect to label the detectors and detect substances through changes in the fluorescence signal. Fluorescent sensors have the advantages of high sensitivity and specificity. Kelsey L. Rodriguez’s group [46] analyzed in detail the various drawbacks of the conventional detection of PFASs such as GS–MS and so on, and analyzed the ability of various sensors to detect PFAs. Table 2 is a summary of the sensors involved in it. The advantages and disadvantages of HPLC–MS/MS, HPLC–Q-TOF-MS, GC–MS, and Pre-column Derivatization GC are also compared in Table 2.
Table 2.
Advantages and disadvantages of detection methods.
| Category | Advantages | Detection Limit | References |
|---|---|---|---|
| Electrochemical Sensors | Electrochemical sensors can be used to detect chemical and biological compounds. Molecularly imprinted polymers (MIPs) functionalized sensors are effective in detecting PFASs, but may have the disadvantage of long detection times and high costs, as well as the inability to differentiate between similar molecules. | 0.07~1.0 µg/L | [47][48][49] |
| Fluorescence Sensors | Fluorescent sensors occurred fluorescence quenching because of electron transfer between fluorescent dyes and PFASs, which can easily be detected using fluorescence spectrophotometers. However, this method can increase costs and cause environmental hazards | 2.5 ppt~120 ppb | [50][51] |
| Fluorescent probes based on nanomaterials | Compared with traditional fluorescent dyes, fluorescent nanomaterials not only have high fluorescence intensity and good light stability, but also have the characteristics of small size effect, quantum effect, and surface effect that are unique to nanomaterials, which can make up for the shortcomings of traditional fluorescent dyes. | 6.4 × 10−10~1.225 × 10−8 mol/L | |
| Optical Sensors/ Colorimetric sensors | Optical sensors/ Colorimetric sensors usually use organic dyes and can be detected by the naked eye based on visible color changes. Although it is not overly dependent on fluorescence spectrophotometry, co-existing atoms can interfere with the sensor. In addition, they require a good platform to improve sensor availability. | 1 ppb~5 ppt | [52][53] |
| HPLC–MS/MS | HPLC–MS/MS is based on the principle of using liquid chromatography monopole mass spectrometry or multistage mass spectrometry as the separation and detection system. It has the advantages of low detection limits, high resistance to matrix effect interference, convenience, and rapid detection. The detection limit is approximately 0.07~0.15 μg/kg. | The method is expensive, costly, and highly specialized and requires a high level of operator expertise | [54][55][56][57] |
| HPLC–Q-TOF-MS | HPLC–Q-TOF-MS has a certain optimization in the ability to analyze samples when in a complex environment, which increase the correctness of detection. The detection limit is approximately 20~30 ng/L. | The method is too costly to be suitable for universal detection. | [28] |
| GC–MS | GC–MS is a tandem gas chromatograph and mass spectrometer method that offers the advantages of simplicity and convenience. The detection limit is approximately 15~40 ng/L. | PFOA is less volatile and needs to be derivatized before it can be detected by injection. The application of GC–MS is narrow, and the experimental process is accompanied by toxic substances. | [58][59][60] |
| Pre-column Derivatization GC | It is a method that improves on the shortcomings of GC–MS. The derivatization technique reduces the temperature of the target, and improves the signal required to detect the target. The detection limit is approximately 0.01~0.05 mg/kg. | The formation of by-products may cause greater difficulties in chromatographic separation. Impurities or interfering peaks can easily be introduced or samples lost during derivatization. | [34] |
| Rare earth up-conversion fluorescent probe | Fluorescence burst reaction of the probe with the detected substance, offering the advantage of simple, efficient, and highly sensitive detection. The detection limit is approximately 0.01~1.2 nmol/L. | PFOA is a perfluorinated compound. The method is not selectively detectable for PFOA | [61] |
After comparing the detection methods, it was finally found that the fluorescence detection method was the most convenient and efficient, and the fluorescence probe method was more suitable to detect PFOA in wastewater.
As can be seen from Table 2, the detection effect of HPLC–MS/MS, HPLC–Q-TOF-MS, and GC–MS are not ideal and the cost is high. The detection effect of fluorescent sensors and other methods is good, in which the optical sensor is simple to operate, but the detection performance of rare earth up-conversion fluorescent probes is better.
References
- Guo, H.; Wang, J.H.; Yao, J.Z.; Sun, S.J.; Sheng, N.; Zhang X.W.; Guo, X.J.; Guo, Y.; Sun, Y.; Dai, J.Y; et al. Comparative Hepatotoxicity of Novel PFOA Alternatives(perfluoropolyether carboxylic acids)on Male Mice. Environ. Sci. Technol 2019, 53, 3929–3937, 10.1021/acs.est.9b00148.
- Guo, H.; Wang, J.H.; Yao, J.Z.; Sun, S.J.; Sheng, N.; Zhang X.W.; Guo, X.J.; Guo, Y.; Sun, Y.; Dai, J.Y; et al. Comparative Hepatotoxicity of Novel PFOA Alternatives(perfluoropolyether carboxylic acids)on Male Mice. Environ. Sci. Technol 2019, 53, 3929–3937, 10.1021/acs.est.9b00148.
- Wang, K.M.; Wu, X.Y.; Zhang, R.Z.; Hu, P. Determination of Perfluorooctanoate and Perfluorooctanesulfonate in Drinking Water by Solid Phase Extraction and High-Performance Liquid Chromatography Tandem Mass Spectrometry. Guangdong Chem. 2022, 49, 102–103.
- Rushing, B.R.; Hu, Q.; Franklin, J.N.; McMahen, R.; Dagnino, S.; Higgins, C.P.; Strynar, M.J.; DeWitt, J.C. Evaluation of the Immunomodulatory Effects of 2,3,3,3-Tetrafluoro-2-(heptafluoropropoxy)-Propanoate in C57BL/6 mice. Toxicol. Sci. 2017, 156, 179–189., 10.1093/toxsci/kfw251.
- Guo, H.; Wang, J.H.; Yao, J.Z.; Sun, S.J.; Sheng, N.; Zhang X.W.; Guo, X.J.; Guo, Y.; Sun, Y.; Dai, J.Y. Comparative Hepatotoxicity of Novel PFOA Alternatives(perfluoropolyether carboxylic acids)on Male Mice. Environ. Sci. Technol. 2019, 53, 3929–3937.
- Sheng, N.; Pan, Y.T.; Guo, Y.; Sun, Y.; Dai, J.Y. Hepatotoxic Effects of Hexafluoropropylene Oxide Trimer Acid(HFPO-TA),a Novel Perfluorooctanoic Acid (PFOA) Alternative on Mice. Environ. Sci. Technol. 2018, 52, 8005–8015., 10.1021/acs.est.8b01714.
- Shi, Y.L.; Vestergren, R.; Xu, L.; Zhou, Z.; Li, C.X.; Liang, Y.; Cai, Y.Q. Human Exposure and Elimination Kinetics of Chlorinated Polyfluoroalkyl Ether Sulfonic Acids(Cl-PFESAs). Environ. Sci. Technol. 2016, 50, 2396–2404.
- Sun, W.Q.; Ma, X.Z.; Zhou, Y.B.; Wang, L.; Liu, H. Effect of Perfluorooctanoic Acid on Lipid Accumulation of Rat Hepatocytes BRL-3A.. Med. Health Sci. Technol. 2022, 51, 107–112., 10.19813/j.cnki.weishengyanjiu.2022.01.018.
- Dong, G.H.; Zhang, Y.H.; Zhen, L.; Liang, Z.F.; He, Q.C. Effects of Perfluorooctane Sulfonic Acid on Proliferation and Cytokine Secretion of Mouse Lymphocytes in Vitro. J. Toxicol. 2011, 25, 125–128., 10.16421/j.cnki.1002-3127.2011.02.001.
- Zhang, L.M.; Rimal, B.P.; Nichols, R.G.; Tian, Y.; Smith, P.B.; Hatzakis, E.; Chang, S.C.; Butenhoff, J. L.; Peters, J.M.; Patterson, A.D.; et al. Perfluorooctane Sulfonate Alters Gut Microbiota-host Metabolic Homeostasis in Mice. Toxicology 2020, 413, 152365-152365, 10.1016/j.tox.2020.152365.
- Wei, J.P.; Xia, J.; Wang, X.J. Analysis of Detection Standards for PFOA and PFOS of Perfluorinated Compounds.. Org. Fluoride Ind. 2014, 162, 38–42. .
- Cennamo, N.; Zeni, L.; Tortora, P.; Regonesi, M.E.; Giusti, A.; Staiano, M.; D’Auria, S.; Varriale, A A High Sensitivity Biosensor to detect the presence of perfluorinated compounds in environment. Talanta 2018, 178, 955–961, 10.1016/j.talanta.2017.10.034.
- Xu, X.L. Production, Processing and Use status of PFOA Substances in China. Chem. Manag. 2017, 459(25), 60–61.
- Chen, H.; Zhang, Y.L. Current Situation and Safety Analysis of Food Packaging Materials in China. Packag. Food Mach. 2017, 35, 53–57.
- Pontius, F Regulation of Perfluorooctanoic Acid (PFOA) and Perfluorooctane Sulfonic Acid (PFOS) in Drinking Water: A Comprehensive Review. Water 2019, 11, 2003., 10.3390/w11102003.
- Baduel, C.; Paxman, C.J.; Mueller, J.F. Perfluoroalkyl Substances in a FireFigurehting Training Ground (FTG), Distribution and Potential Future Release.. J. Hazard. Mater. 2015, 296, 46–53., 10.1016/j.jhazmat.2015.03.007.
- Tsuda, S. Differential Toxicity between Perfluorooctane Sulfonate(PFOS)and Perfluorooctanoic Acid(PFOA). J. Toxicol. Sci. 2016, 41, 27–36.
- Lorenzo, M.; Campo, J.; Picó, Y. Analytical Challenges to Determine Emerging Persistent Organic Pollutants in Aquatic Ecosystems.. TrAC Trends Anal. Chem. 2018, 103, 137–155., 10.1016/j.trac.2018.04.003.
- Sher, M.; Javed, M.; Shahid, S.; Hakami, O.; Qamar, M.A.; Iqbal, S.; AL-Anazy, M.M.; Baghdadi, H.B. Designing of highly active g-C3N4/Sn doped ZnO heterostructure as a photocatalyst for the disinfection and degradation of the organic pollutants under visible light irradiation.. J. Photochem. Photobiol. A Chem. 2021, 418, 113393., 10.1016/J.JPHOTOCHEM.2021.113393.
- Qamar, M.A.; Javed, M.; Shahid, S.; Iqbal, S.; Abubshait, S.A.; Abubshait, H.A.; Ramay, S.M.; Mahmood, A.; Ghaithan, H.M. Designing of highly active g-C3N4/Co@ZnO ternary nanocomposites for the disinfection of pathogens and degradation of the organic pollutants from wastewater under visible light. J. Environ. Chem. Eng. 2021, 9, 105534.
- Leng, T.H.; Wang, L.; Zheng, Y. Determination of 12 Perfluorinated Compounds in Rice Powder for Infants and Young Children by High Performance Liquid Chromatography-tandem Mass Spectrometry. Food Saf. Qual. 2019, 10, 8087–8092. , 10.19812/j.cnki.jfsq11-5956/ts.2019.23.050.
- Liu, S.Y.; Jin, Q.; Ren, R. Determination of Perfluorocarboxylic Acids in Fish by Ultra Performance Liquid Chromatography Tandem Mass Spectrometry. Prev. Med. 2020, 32, 1204–1207.
- Guo, J.; Guo, M.M.; Wu, H.Y.;Zhuo, Y.X.; Mou, H.J.; Lu, L.N.; Tan, Z.J. Simultaneous Determination of Perfluorinatedacids and Their Precursors in Bivalve Shellfish by Double SPE Columns Purification and Ultra Fast Liquid Chromatography-tandem Mass Spectrometry. Food Sci. 2017, 38, 248–255.
- He, J.L.; Peng, T.; Xie, J.; Dai, H.H.; Chen, D.D.; Yue, Z.F; Fan, C.L.; Li, C. Development of a Qu ECh ERs Method for Determination of 20 Perfluorinated Compounds in Animal Liver by HPLC–MS/MS. Chin. J. Anal. Chem. 2015, 43, 40–48.
- Feng, S.; Lan, F.; Wu, X.P.; Shen, J.C.; Yue, Z.F; Xiong, B.B. Determination of 15 Perttuorinated Compounds in Chinese Spirit by Dispersive Solid Phase Extraction and HPLC-MS-MS. Food Sci. 2013, 34, 143–149.
- Huang, B.; Liu, W.J.; Fu, J.W.; Su, D.S.; Wu, J.H.; Ai Y.Z. Simultaneous Detection of 7 Neonicotinoid Insecticides in Camellia Oil by QuEChERS Method Coupled with Ultra High Performance Liquid Chromatography Tandem Mass Spectrometry. Qual. Saf. Agric. Prod. 2019, 97(01), 45–48.
- Yang, W.L.; Guo, J.; Du, W. Determination of Perfluorooctanoic Acid and Perfluorooctanesulfonates in Environmental Water and Soil Matrixes Using ultra High Performance Liquid Chromatography Coupled with State-of-art Tandem Quadrupole Mass Spectrometry. Environ. Chem. 2018, 37, 2820–2823.
- Yu, L.; Song, W.; Lv, Y.L.; Zhao, M.Y.; Zhao, M.Y.; Zhou, F.F; Hu, Y.Y.; Zheng, P. Rapid Determination of 204 Pesticide Residues in Tea by Ultra Performance Liquid Chromatography Coupled with Quadrupole-time of Flight Mass Spectrometry. Colour Spectr 2015, 33, 597–612., 10.3724/SP.J.1123.2015.02056.
- Zabaleta, I.; Negreira, N.; Bizkarguenaga, E. Screening and Identification of Per- and Polyfluoroalkyl Substances in Microwave Popcorn Bags.. Food Chem 2017, 230, 497., 10.1016/j.foodchem.2017.03.074.
- Huang, M.W.; Wu, H.; Yu, W.; Wang, Y.; Wang, F.C.; Zhang, C.C; Zhou, L.S.; Li, Z.G. Rapid Identification of Chemical Constituents of Qiyu Sanlong Decoction by Ultra Performance Liquid Chromatography-Quadrupole-Time of Flight Mass Spectrometry. Chromatogr. J. 2021, 39, 730–743.
- Buszewska-Forajta, M.; Bujak, R.; Yumba-Mpanga, A.; Siluk, D.; Kaliszan, R. GC/MS Technique and AMDIS Software Application in Identification of Hydrophobic Compounds of Grasshoppers’ Abdominal Secretion (Chorthippus spp.).. J. Pharm. Biomed. Anal. 2015, 102, 331–339. , 10.1016/j.jpba.2014.09.039.
- Wei, L.L. Rapid Determination of Perfluorooctanoic Acid in Food Contact Materials by Pre-column Derivatization and Gas Chromatography. Packag. Food Mach. 2019, 37, 69–72. .
- Luo, S.P.; Shang, G.Q.; Chen, M. Research Progress of Detection Methods of Perfluorinated Compounds in Food Contact Materials. J. Food Saf. Qual. Insp. 2013, 4, 993–998., 10.19812/j.cnki.jfsq11-5956/ts.2013.04.004.
- Wang, X.Y.; Shen, W.J.; Wang, H.; Yu, K.Y.; Wu, B.; Hu, G.S.; Yang, G.J. Determination of 10 Perfluorocarboxylic Acid Compounds in Water by Gas Chromatography-negative Chemical Source-mass Spectrometry. Chromatography 2019, 37, 32–39.
- Zheng, Z.; Yu, H.J.; Geng, W.C.; Hu, X.Y.; Wang, Y.Y.; Li, Z.H.; Wang, Y.F.; Guo, D.S. Guanidinocalix[5]arene for sensitive fluorescence detection and magnetic removal of perfluorinated pollutants. . Nat. Commun. 2019, 10, 5762., 10.1038/s41467-019-13775-1.
- Tian, F. Application of Rhodamine Fluorescent Probes in the Detection of Heavy Metals and Transition Metal atoms. Petrochem. Technol. 2019, 26, 2693–2399..
- Chen, Y.Y.; Ding, M.M.; Li, J.Q.; Sheng, W.; Liu, B.; Zhang, Y.; Wang, S. Fluorescence quenching immunoaffinity test column with quantum dots as fluorescence donors for the quick detection of malachite green and crystal violet in aquatic products. Food Anal. Methods 2018, 11, 3362–3370., 10.1007/s12161-018-1312-0.
- Hua, G.S.; Sheng, W.; Li, J.M.;Zhang, Y.; Wang, J.P.; Wang, S. Fluorescent quenching immune chromatographic strips with quantum dots and up-conversion nanoparticles as fluorescent donors for visual detection of sulfaquinoxaline in foods of animal origin. Anal. Chim. Acta 2017, 982, 185–192.
- Zhang, Z.H.; Zhou, H.L.; Wu, F.; Zhang, Y. Temperature Sensing Characteristics of Up-conversion Luminescence in Tm3+/Yb3+Co-dopeed LuYO3Phospho. J. Lumin. 2021, 42(12), 1872-1881.
- Chen, J.Y.; Su, W.; Wang, E.J. Highly selective ratiometric fluorescent probe for Hg2+ based on fluorescence resonance energy transfer between 1,8-naphthalimide and rhodamine B. Chem. J. Chin. Univ. 2016, 37, 232–238.
- Li, M.; Jiang, X.; Wu, H.; Jiang, X.J.; Wu, H.H.; Xu, H.; Zang, S.Q. A dual functional probe for “turn-on” fluorescence response of Pb2+and colorimetric detection of Cu2+based on a rhodamine derivative in aqueous media.. Dalton Trans. 2015, 44, 17326–17334., 10.1039/C5DT02731D.
- Balamurugan, A.; Kumar, V.; Jayakannan, M. Triple action polymer probe: carboxylic distilbene fluorescent polymer chemosensor for temperature, metal-ions and biomolecules.. Chem. Commun 2014, 50, 842. , 10.1039/c3cc45274c.
- Duret, D.; Haftek-Terreau, Z.; Carretier, M.; Berki, Z.; Ladavière, C.; . Monier, K.; Bouvet, P.; Marvel, J.; Leverrier, Y.; Charreyre, M.T.; et al. Labeling of native proteins with fluorescent RAFT polymer probes: application to the detection of a cell surface protein using flow cytometry. Polym. Chem. 2020, 9, 1857.
- Zhang, Y.; Jiang, H.; Wang, X. Cytidine-stabilized gold nanocluster as a fluorescence turn-on and turn-off probe for dual functional detection of Ag+and Hg2+.. Anal. Chim. Acta 2015, 870, 1–7., 10.1016/j.aca.2015.01.016.
- Peng, Y.; Wang, M.M.; Wu, X.X.; Wang, F.; Liu, L. Methionine-capped gold nanoclusters as a fluorescenceenhanced probe for cadmium(II)sensing. Sensors 2018, 18, 658–668.
- Kelsey, L.R.; Hwang, J.-H.; Esfahani, A.R.; Esfahani, A.R.; Anwar S. A.H.M.; Hyoung, W.Lee Recent Developments of PFAS-Detecting Sensors and Future Direction: A Review.. Micromachines 2020, 11, 667., 10.3390/MI11070667.
- Karimian, N.; Stortini, A.M.; Moretto, L.M; Costantino, C.; Bogialli, S.; Ugo, P. Electrochemosensor for trace analysis of perfluorooctanesulfonate in water based on a molecularly imprinted poly (o-phenylenediamine) polymer.. ACS Sens. 2018, 3, 1291–1298., 10.1021/acssensors.8b00154.
- Cheng, Y.H.; Barpaga, D.; Soltis, J.A.; Shutthanandan, V.; Kargupta, R.; Han, K.S.; McGrail, B.P.; Motkuri, R.K.; Basuray, S.; Chatterjee, S. Metal–Organic Framework-Based Microfluidic Impedance Sensor Platform for Ultrasensitive Detection of Perfluorooctanesulfonate. ACS Appl. Mater. Interfaces 2020, 12, 10503–10514.
- Feng, H.; Wang, N.Y.; Tran.T, T.T.; Yuan, L.J.; Li, J.Z; Cai, Q.Y. Surface molecular imprinting on dye–(NH2)–SiO2 NPs for specific recognition and direct fluorescent quantification of perfluorooctane sulfonate. Sens. Actuators B Chem. 2014, 195, 266–273., 10.1016/j.snb.2014.01.036.
- Cennamo, N.; D’Agostino, G.; Sequeira, F.; Mattiello, F.; Porto, G.; Biasiolo, A.; Nogueira, R.; Bilro, L.; Zeni, L. A simple and low-cost optical fiber intensity-based configuration for perfluorinated compounds in water solution.. Sensors 2018, 18, 3009., 10.3390/s18093009.
- Takayose, M.; Akamatsu, K.; Nawafune, H; Murashima, T.; Matsui, J. Colorimetric detection of perfluorooctanoic acid (PFOA) utilizing polystyrene-modified gold nanoparticles. Anal. Lett. 2012, 45, 2856–2864.
- Chen, S.H.; Li, A.M.; Zhang, L.Z.; Gong, J.M. Molecularly imprinted ultrathin graphitic carbon nitridenanosheets–Based electrochemiluminescence sensing probe for sensitive detection of perfluorooctanoic acid.. Anal. Chim. Acta 2 2015, 896, 68–77., 10.1016/j.aca.2015.09.022.
- Xia, W.; Wan, Y. J.; Wang, X.L.; Li, Y.Y.; Wang, W.J.; Wang, C.X.; Xu, S.Q. Sensitive bioassay for detection of PPARα potentially hazardous ligands with gold nanoparticle probe. Hazard. Mater. 2011, 192, 1148–1154.
- Yang, H. Monitoring of perfluorinated compounds from the imported fishery products in Korea by liquid chromatography-tandem mass spectrometry (LC-MS/MS).. Env. Epidemiol 2019, 3, 452–453., 10.1097/01.ee9.0000611108.47699.2e.
- Lertassavakorn, T.; Pholphana, N.; Rangkadilok, N.; Suriyo, T.; Satayavivad, J. Determination of perfluorooctane sulphonate and perfluorooctanoic acid in seafood and water from Map Ta Phut Industrial Estate area, Thailand. Food Addit. Contam. Part A 2021, 38, 11–16.
- Gan, C.D.; Gan, Z.W.; Cui, S.F.; Fan, R.J.; Fu, Y.Z.; Peng, M.Y.; Yang, J.Y. Agricultural activities impact on soil and sediment fluorine and perfluorinated compounds in an endemic fluorosis area.. Sci. Total Environ. 2021, 771, 144809., 10.1016/j.scitotenv.2020.144809.
- Wang, Z.; Qiao, H.Q.; Yang, J.; Fu, Y.Q.; Wang, H.; Yao, J.H; Gao, T.Q.; Deng, J.G. Simultaneous determination of fluoroalkanes in aquatic products by ultra tandem high performance liquid chromatography mass spectrometry. J. Food Saf. Qual. 2022, 13, 1132–1140.
- Wang, Y.K.; Chen, W.Y.; Wen, S.G.; Meng, K.Q.; Xie, S.Z.; Zhen, J.X.; Qiu, C.S. Headspace gas chromatography detection method for perfluorohydrocarbon tracer. Fine Spec. Chem. 2020, 28(03), 10–13., 10.19482/j.cn11-3237.2020.03.03.
- Gebbink, W.A.; Letcher, R.I. Linear and branched perfluorooctane sulfonate isomer patterns in herring gull eggs from colonial sites across the Laurentian Great Lakes. Env. Sci. Technol. 2010, 44, 3739–3745.
- Su, C.Y.; Zheng, N.; Li, S.L.; Qu, X.Y.; Zhou, X.W.; Ma, T.; Yang, H.J.; Wang, J.Q. Research progress on detection methods of perfluorinated compounds in milk. China Dairy Ind. 2018, 46, 29–33.
- Wang, J.; Chen, H.Y.; Ru, F.; Zhang, Z.; Mao, X.; Shan, D.L.; Chen, J.; Lu, X.Q. Encapsulation of dual-emitting fluorescent magnetic nanoprobe in metal-organic frameworks for ultrasensitive ratiometric detection of Cu2+. Chem. A Eur. J. 2018, 24, 3499–3505., 10.1002/chem.201704557.
More
