Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Conner Chen and Version 1 by José Pérez de la Lastra.
Classically, superoxide anion O2•− and reactive oxygen species ROS play a dual role. At the physiological balance level, they are a by-product of O2 reduction, necessary for cell signalling, and at the pathological level they are considered harmful, as they can induce disease and apoptosis, necrosis, ferroptosis, pyroptosis and autophagic cell death.
- reactive species
- ROS
- reactive stress
1. Introduction
In medicine, a great interest in the study of cellular stress and free radicals has emerged in recent years, focused on deepening our knowledge of the mechanisms of cellular self-control that allow us to improve the quality of human life and understand the origin of a large number of diseases [1].
Oxidative stress is a component of many diseases, including atherosclerosis, chronic obstructive pulmonary disease, Alzheimer’s disease and cancer, among others [2]. Simultaneously, ROS are essential for a variety of biological functions, such as cell survival, growth, proliferation and differentiation, and immune response. However, one of the major obstacles to understanding the role of these species is the lack of adequate methods to detect ROS/RNS in vivo, mainly due to their very short lifetimes and the presence of several antioxidants in cells [3]. In fact, radicals are continuously generated by most organisms as a result of the use of O2 as a terminal electron acceptor in the mitochondrial electron transport chains and in cytochrome P450 [4].
The term reactive species refers to two types of molecules: free radicals and non-radicals [5]. This set of molecules is formed as a result of cellular metabolism and is represented in biological systems by reactive oxygen species ROS and reactive nitrogen species RNS, which arise in both normal physiological and pathological processes. Not excluding that, there are also reactive species from other elements, such as chlorine RClS and bromine RBrS, although ROS and RNS are the two major groups involved in redox biology [6].
The superoxide anion is a primary oxygen radical that is formed when an oxygen molecule acquires an electron. The initial formation of O2•− triggers a cascade of ROS, some of which, such as H2O2, behave as key molecules in cell signalling, and others, such as HO, are damaging. Ultimately, the biological impact of these molecules will be determined by the amount of ROS, cellular defences and the capacity for cellular adaptation [7].
O2•− is one of the most important reactive oxygen species ROS responsible for oxidative stress in bio-organisms and is generated as a by-product of the mitochondrial respiratory chain [8]. Because of its charge, superoxide has a low membrane permeability, it passes through anion channels, but this is inefficient, and superoxide reacts to a large extent in the physiological compartment where it is generated.
Reactive oxygen species (ROS) are a group of highly reactive oxygen-containing chemicals produced exogenously or endogenously from the reduction of oxygen and include both radicals and non-radicals, one of which is superoxide. ROS present in the body are mostly of endogenous origin, although they can also be generated in response to external stimuli, such as ultraviolet light, ionising radiation, pollution, alcohol and tobacco consumption, drugs and toxic agents [9], Figure 1.
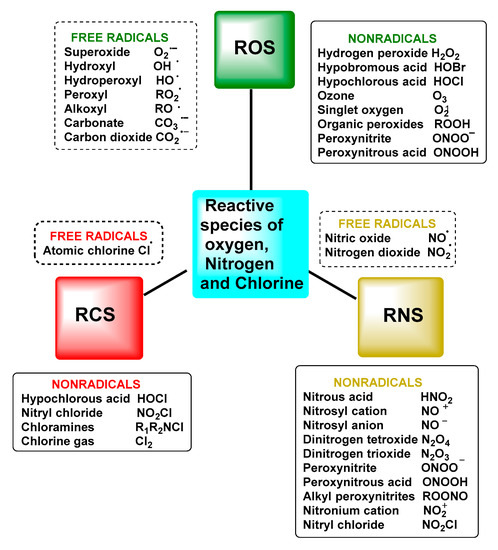
Figure 1.
Nomenclature of reactive species and free radicals and other reactive oxygen, nitrogen and chlorine species.
To control ROS, the body uses several antioxidant mechanisms, including enzymatic and non-enzymatic antioxidants [10]. Non-enzymatic low-molecular-weight antioxidant compounds include cellular glutathione, vitamins C and E, β-carotene, polyphenols and uric acid. Antioxidant enzymes include superoxide dismutase, catalase, glutathione reductase and glutathione peroxidase, among others. SOD catalyses the dismutation of superoxide to H2O2. Mammalian cells contain three forms of SOD: Mn-SOD, cytosolic Cu, Zn-SOD and extracellular Cu, Zn-SOD. MnSOD is most abundant in the mitochondria, whereas Cn, Zn-SOD predominates in the cytoplasm [11]. Catalase is an important antioxidant enzyme that catalyses the reduction of H2O2 to H2O. Glutathione peroxidase is another important enzyme for the decomposition of H2O2. Polyphenols, ingested regularly through the fruit and vegetable diet, are a large family of natural organic compounds characterized by multiple hydroxyl phenolic units, with a polyphenolic structure, (several hydroxyl groups on aromatic rings), including four main classes: phenolic acids, flavonoids, stilbenes and lignans [12]. Evidence and research to date supports the role of polyphenols in the prevention of cancer, cardiovascular and neurodegenerative diseases [13]. A significant part of their beneficial effects are based on the modulation of cell signalling pathways [14].
2. Superoxide Radical Anion O2•−
O2•− is a reduced form of molecular oxygen O2, consisting of two oxygen atoms with 17 electrons and a negative electrical charge, Figure 2. Superoxide is the first species produced in the respiratory chain by the reduction of oxygen by the transfer of an electron and is one of the first species generated by various cellular systems. O2•− is formed in all living aerobic organisms, and can act as a signalling agent, a toxic specie or a harmless intermediate that spontaneously decomposes. Its levels are limited in vivo by two different types of enzymes, superoxide reductase SOR and superoxide dismutase SOD.
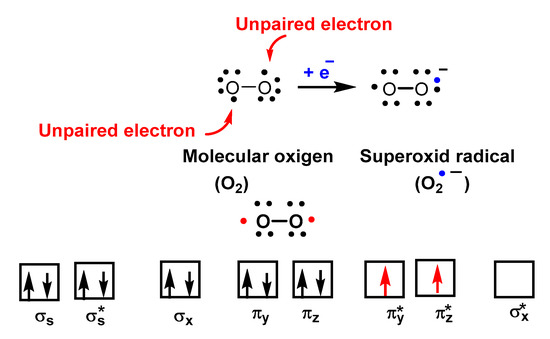
Figure 2.
Molecular orbital diagram of O
2
showing its biradical nature.
Despite being a “free biradical”, oxygen has a low reactivity because the unpaired electrons of each oxygen atom have parallel spins, Figure 3.
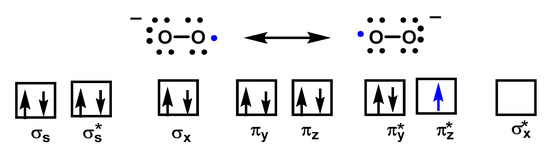
Figure 3. The molecular orbital of O2•− shows one unpaired electron and is delocalized between the π* orbitals of the two oxygen atoms.
Superoxide is considered both a radical and a −1 charged anion. It is a relatively unstable molecule, with a half-life of milliseconds, a reasonably strong oxidant, in which case it is reduced to hydrogen peroxide, and can also act as a reductant and convert to oxygen. There are two standard redox potentials for O2•− showing that it can act as a reducing agent E′(O2/O2•−) = 0.33 V or as an oxidizing agent E′(O2•−/H2O2) = 0.93 V [15]), Figure 4.
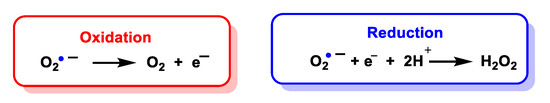
Figure 4.
Oxidation and reduction of O
2•−
to form oxygen or hydrogen peroxide, respectively.
O2•− is a relatively small anion, highly soluble in water, where it is solvated by four water molecules strongly bound by hydrogen bonds [16] and reacts with a proton or proton donor to form HO2•, Figure 5. Various organic and inorganic compounds can act as a source of a proton in a large number of reactions [17].
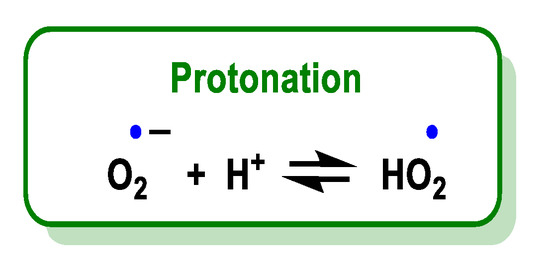
Figure 5.
Protonation of O
2•−
leads to the formation of HO
2•
.
The superoxide radical is the conjugate base of a weak acid, the hydroperoxide radical HOO•, whose pKa is 4.88 [18]. The pH controls the distribution between HO2• and O2•.
Near the membrane, where this radical is produced, the pH is much lower than in the cytoplasm, so the acid form or hydroperoxide radical will predominate. Due to its non-ionic nature, it can enter the cell membrane and trigger lipid peroxidation processes [19]. The hydroperoxide radical is much more reactive, more oxidising than the superoxide radical, but in aqueous solution at physiological pH the non-protonated form, i.e., the superoxide radical, predominates. Perhydroxyl constitutes less than 1% of superoxide at neutral pH so its impact is more limited. Superoxide absorbs light in the ultraviolet range with a maximum at 245 nm and an extinction coefficient of 2350 M−1 cm−1, whereas hydroperoxyl absorbs at 225 nm with an extinction coefficient of 1400 M−1 cm−1 [20].
O2•− is toxic, mainly because it damages proteins containing Fe-S centres, such as aconitase, succinate dehydrogenase and NADH-ubiquinone oxidoreductase, among others. However, it can also be the generator of other reactive species even more toxic than itself, the iron released from iron and sulphur proteins can give rise to secondary products, such as hydroxyl radicals, and these, plus peroxynitrite, are thought to be the main contributors to superoxide toxicity. Superoxide dismutase SOD is the enzyme responsible for transforming this reactive species into one of a lower toxicity, such as hydrogen peroxide H2O2, Figure 6.
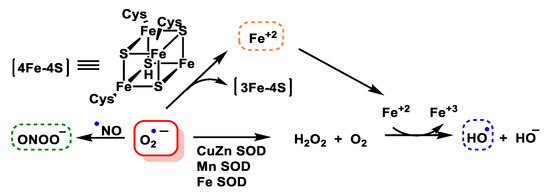
Figure 6.
Generation of hydroxyl radical, peroxynitrite and hydrogen peroxide by the O
2•−
anion.
References
- Corrales, L.C.; Muñoz Ariza, M.M. Estrés oxidativo: Origen, evolución y consecuencias de la toxicidad del oxígeno. Nova 2012, 10, 213–225.
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709.
- Vona, R.; Pallotta, L.; Cappelletti, M.; Severi, C.; Matarrese, P. The impact of oxidative stress in human pathology: Focus on gastrointestinal disorders. Antioxidants 2021, 10, 201.
- Napolitano, G.; Fasciolo, G.; Venditti, P. The ambiguous aspects of oxygen. Oxygen 2022, 2, 382–409.
- Lozada, S.M.; García, L. Estrés oxidativo y antioxidantes: Cómo mantener el equilibrio. Rev. Asoc. Colomb. Dermatol. Cirugía Derm. 2009, 17, 172–179.
- Kohen, R.; Nyska, A. Invited review: Oxidation of biological systems: Oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol. Pathol. 2002, 30, 620–650.
- Hancock, J.T. Oxygen Is Instrumental for Biological Signaling: An Overview. Oxygen 2021, 1, 3–15.
- Turrens, J.F.; Boveris, A. Generation of superoxide anion by the NADH dehydrogenase of bovine heart mitochondria. Biochem. J. 1980, 191, 421–427.
- Li, R.; Jia, Z.; Trush, M.A. Defining ROS in biology and medicine. React. Oxyg. Species 2016, 1, 9.
- Storey, K.B. Oxidative stress: Animal adaptations in nature. Braz. J. Med. Biol. Res. 1996, 29, 1715–1733.
- Fridovich, I. Superoxide radical and superoxide dismutases. Annu. Rev. Biochem. 1995, 64, 97–112.
- Brglez Mojzer, E.; Knez Hrnčič, M.; Škerget, M.; Knez, Ž.; Bren, U. Polyphenols: Extraction methods, antioxidative action, bioavailability and anticarcinogenic effects. Molecules 2016, 21, 901.
- Williams, R.J.; Spencer, J.P.; Rice-Evans, C. Flavonoids: Antioxidants or signalling molecules? Free Radic. Biol. Med. 2004, 36, 838–849.
- Dong, Z.; Surh, Y.-J. Dietary Modulation of Cell Signaling Pathways; CRC Press: Boca Raton, FL, USA, 2008.
- Wardman, P. Reduction potentials of one-electron couples involving free radicals in aqueous solution. J. Phys. Chem. Ref. Data 1989, 18, 1637–1755.
- Narayana, P.; Suryanarayana, D.; Kevan, L. Electron spin-echo studies of the solvation structure of superoxide ion (O2-) in water. J. Am. Chem. Soc. 1982, 104, 3552–3555.
- Afanas’ ev, I.B. Superoxide Ion: Chemistry and Biological Implications; CRC Press: Boca Raton, FL, USA, 1991; Volume 2.
- Aikens, J.; Dix, T. Perhydroxyl radical (HOO.) initiated lipid peroxidation. The role of fatty acid hydroperoxides. J. Biol. Chem. 1991, 266, 15091–15098.
- Bielski, B.; Arudi, R.L.; Sutherland, M.W. A study of the reactivity of HO2/O2-with unsaturated fatty acids. J. Biol. Chem. 1983, 258, 4759–4761.
- Bielski, B.H.; Cabelli, D.E.; Arudi, R.L.; Ross, A.B. Reactivity of HO2/O− 2 radicals in aqueous solution. J. Phys. Chem. Ref. Data 1985, 14, 1041–1100.
- Liochev, S.I.; Fridovich, I. Superoxide and iron: Partners in crime. IUBMB life 1999, 48, 157–161.
- Huie, R.E.; Padmaja, S. The reaction of NO with superoxide. Free. Radic. Res. Commun. 1993, 18, 195–199.
More
