Microbial Surfactants are the class of surfactants produced by microbes like bacteria, fungi or yeast. Due to their easy biodegradability, and less toxicity, this class is gaining huge interests of scientists, researchers, environmentalists and industrialists. Current article throws some light on the introduction and classification of microbial surfactants. Some properties og microbial surfactants has also been discussed in the same.
Industrila Applications of Microbial surfactants will be discussed in upcoming article
- Biosurfactant
- Microbial Surfactants
- Bioemulsifiers
- Microbial Surfactants, sustainable surfactants, green surfactants, biodegradable surfactants
1. Introduction
Surfactants' amphiphilic nature makes them essential molecules to be used in a wide range of industrial applications, including detergents, polymers, insecticides, paper, paints, textiles, medicines, and personal care items (Shaban et al., 2020). Surfactants are used in essential processes like oil recovery and mining because of their capacity to reduce interfacial tension and maintain emulsions and foams. For example, benzyl-alkonium chlorides and alkyl benzene straight chain sulfonates are used to produce personal care products like textile softeners and laundry detergents (Dutta, et al., 2022). Molecules will be used for massive industrial processes. Surfactants' high demand and excessive unavoidable use led to a 42.1 billion US dollar rise in output on the global market. By 2025, this is probably going to rise to $52.4 billion (Badmus et al., 2021). The majority of the residual surfactants are disseminated in soil, water, or sediment when they are released into the environment after use through sewage systems or directly into surface waters. This caused surfactants to have a negative effect on the environment, particularly on aquatic life.
A class of adaptable amphiphilic compounds known as microbial surfactants are created by microorganisms through green enzymatic processes. Recently, environmentalists throughout the world consider biosurfactants highly due to their biodegradability and lack of toxicity, but their high production costs caused by the employment of complex biotechnological processes and the issue of product recovery and purification prevents their commercial acceptance.
2. Classification:
These molecules are divided into two categories based on their molecular weight. In comparison to another class of microbial surfactants, biosurfactants are among the varieties with a low molecular weight. As opposed to their high molecular weight counterparts, which make up a different class of bio-emulsifiers. Detailed Classification of Microbial surfactants can be understood by Figure 1
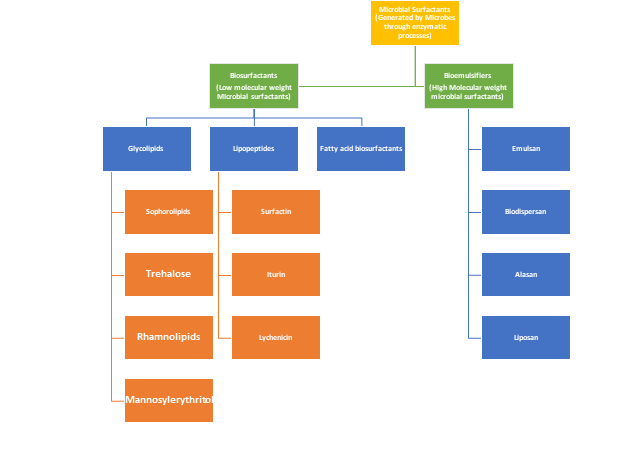 Figure 1: Classification of Microbial Surfactants
Figure 1: Classification of Microbial Surfactants
2.1. Biosurfactants:
On the basis of the micro-organism, through which they are produced, biosurfactants (low molecular weight microbial surfactants) are further divided into Rhamnolipids, Glycolopids, Sophorolipids, Trehalose, lipopeptides and mannosylerythritol broad subclasses.
2.1.1. Glycolipids contains mono to tetra saccharides linked with long chains of aliphatic or hydroxy aliphatic acids. General head groups present in glycolipids are glucose, galactose, mannose,glucuronic acid, and rhamnose. Subclasses of Glycolipids are Rhamnolipids, Trehaloselipids and Sophorolipids.
2.1.1.1. Rhamnolipids: In Rhamnolipids biosurfactants, two monomers of rhamnose sugars are connected with beta-hydroxydecanoic acid chain. Microorganism that is frequently exploited to produce Rhamnolipid are P. aeruginosa.
2.1.1.2. Sophorolipids: As the name indicated, Sophorolipids are the types of biosurfactants comprises of gemini of carbohydrate sophorose, which is linked with a long fatty alkyl chain are and of yeasts origin. Common sophorolipids producing yeasts are T. apicola, T. bombicola and W. domeriqiae. Sophorolipids are potential candidate to reduce the water surface tension from 72.8 mN/m down to 30mN.m and have manifold better CMC than their synthetic counterparts.
2.1.1.3. Trehalose: Trehalose is a type of disaccharide in which two α-glucose are connected via 1,1-glycosidic bond. Common trehalose producing microbes are Mycobacterium, Corynebacterium and Nocardia.
2.1.1.4. This glycolipid biosurfactant contains mannosylerythritol and produced by yeast like C. antarctica and Candida sp. These glycolipids have potential to reduce the surface tension of water down to 29 dyne.cm-1 at CMC of 10mg.l-1.
2.1.2. Lipopeptides: This class of biosurfactants are further divided into surfactin, Iturin, Fengycin and Lichenysin.
2.1.2.1. Surfactin: Surfactin is a type of cyclic lipopeptide molecules that are one of the most active microbial surfactants that have potential to reduce down the surface tension of water to 27.9 nM.m-1. First such type was produced by B. subtilis.
2.1.2.2. Lichenysin: The structure and properties of Lichenysin are very much similar to that of surfactin. These surfactants are generally produced by B. lichenyformis. These molecules are thermostable and pH stable over broad range of temperature and pH, this property make them potential stable dispersants.
2.1.2.3. Fengycin: Fengycin have structure of lipo-deca-peptide that contains beta hrdroxy fatty acid type hydrophobic moeity.
2.1.3. Fatty acid biosurfactants: Fatty acid biosurfactants are produced by degradation of extracellular C12-C14 free fatty acids through microbes. Common fatty acid bisurfactants producing microbes are P. Aeruginosa and Arthobacter.
2.2. Bioemusifiers: Chemically these microbial surfactants are the high molecular weight polymeric biomolecules that have amphiphiliic nature. Good tensile strength, high viscosity and resistance to shear are the aided characteristics that make them suitable as bioemulsifiers, biodispersants etc. This class has further division into biodispersan, emulsan, liposan and alasan.
2.2.1. Biodispersan: Biodispersan is an excracellularly generated biosurfactants produced by A. caloaceticus. Polar head group of this surfactant is a negatively charged heteropolysaccharide with average molecular weight of 51500, which is consists of four different reducing sugars namely galactosamine, glucosamine uronic acid, methylamino hexose and one more non identified aminosugar. These molecules are very effective biodispersants.
2.2.2. Emulsan: Like biodispersan, Emulsan is also produced by A. caloaceticus extracellularly. It is made up of the repeating subunits of acetyl galactosamine, acetyl galactosamine uronic acid and a nonidentified aminosugar.
2.2.3. Liposan: Liposan is an extracellularly synthesize polar emulsifier made up of carbohydrate and protein in the ratio of 87: 17 (w/v). Sugar units present in liposan are galactose, Glucose, galactosamine and galacturonic acid.
2.2.4. Alasan: Alasan is a negatively charged A. radioresistens produced bioemulsifiers reported as a potential candidate to degrade polyaromatic hydrocarbons after solublizing them efficiently.
3. Properties
3.1. Thermal Stability and ionic strength:Several biosurfactants can be used at high temperatures and pH ranges between 2 and 12. Temperature, pH, and Ionic Strength Tolerance Biosurfactants may withstand salt concentrations of up to 10%, unlike synthetic surfactants that need to be inactivated by 2% NaCl.
3.2. Interfacial activity and Surface Tension: It is essential for a surfactant to be both effective and efficient. Efficiency is measured by the CMC, whereas effectiveness is related to surface and interfacial tensions. The CMC of biosurfactants ranges from 1 to 2000 mg/L, whereas the surface tension (oil/water) and interfacial (oil/water) tensions are around 1 and 30 mN/m, respectively. A good surfactant may reduce the surface tension of water from 72 to 35 mN/m and the interfacial tension of n-hexadecane from 40 to 1 mN/m.
3.3. Specificity, biocompatibility, and digestibility: Because biosurfactants are complex compounds with distinctive functional groups, they typically behave in a specific way. The detoxification of various pollutants, the de-emulsification of industrial emulsions, and specific food, medicinal, and cosmetic purposes are of particular interest in this. Because of their biocompatibility and digestible properties, biomolecules can be used in a variety of industries, but they are especially useful in the food, pharmaceutical, and cosmetic industries.
Biosurfactants are suitable for bioremediation and waste treatment because microorganisms in soil and water can quickly break them down.
3.4. Low Toxicity: Biosurfactants can be employed in foods, cosmetics, and medications due to their low level of toxicity. Applications in the environment also require low toxicity. Biosurfactants can be produced from a number of readily accessible source materials, including industrial waste.
Biofilms are a buildup of bacteria or other organic materials that have colonised or collected on a surface.
Biofilms are resistant to several anti-adhesive agents.Bacterial adherence to a surface is the first step in the formation of a biofilm.
This process is influenced by a wide range of variables, including the kind of microorganism, the hydrophobicity and electrical charges of the surface, the atmospheric conditions, and the capacity of the microorganisms to produce extracellular polymers that facilitate cell attachment to surfaces.
The hydrophobicity of a surface can be altered by the use of biosurfactants, which in turn affects how effectively bacteria stick to it. A surfactant from Streptococcus thermophilus slows down the colonisation of other thermophilic strains of Streptococcus over the steel that induce fouling. A biosurfactant from Pseudomonas fluorescens also inhibited Listeria monocytogenes from sticking to steel surfaces.
Emulsion breaking and framing:
3.5. Biosurfactants have emulsifying and de-emulsifying properties. The impermeable liquid droplets that make up an emulsion typically have a diameter greater than 0.1 mm and are dispersed over another liquid. The two most popular types are water-in-oil (w/o) and oil-in-water (o/w) emulsions.
It is commonly accepted that biosurfactants have the potential to be used in the production of commercial goods. The widespread use of these chemicals favours novel inquiries on various technical fronts. Biosurfactants are utilised in a wide range of commercial sectors, from the oil business for the recovery of oils and habitat bioremediation to the medical and pharmaceutical industries, due to their surface-active, antibacterial, anti-adhesive, and anti-biofilm properties. The ability to create metabolites in sufficient numbers for product production and commercialization has only been demonstrated by a small number of species of bacteria that produce biosurfactants of the glycolipid class. Low yields and high production costs continue to be the main obstacles to large-scale manufacturing.
By-products from the agro-industrial sector are used to simplify production, reduce costs, and enhance environmental sustainability. The relevance of increasing productivity and yields brought about by bioengineering techniques, adjustments to fermentation processes, and statistical experiment design has been underlined in research breakthroughs. The financial viability of biosurfactant production can therefore be improved by using low-cost substrates, optimising the environment for the production of different bioproducts, developing new purification techniques, obtaining high-yield strains, and producing refined products for larger niche markets.
References
- Sharrel, K. A. Aju, M. Sathish and S. Jisha, Environ. Chem. Lett., 2014, 12, 275.
- I .M. Banat, A. Franzetti, I. Gandolfi, G. Bestetti, M. G. Martinotti, L. Fracchia, T. J. Smyth and R. Marchant, Appl. Microbiol. Biotechnol., 2010, 87(2), 427.
- Sachdev, D. P., & Cameotra, S. S. (2013). Biosurfactants in agriculture. Applied microbiology and biotechnology, 97(3), 1005-1016.
- Erum, A. Faiza, B. Uzma, A. Jameela and I. Samina, Nat. Appl. Sci., 2013, 4, 243.
- Gayathiri, Ekambaram & Prakash, Palanisamy & Karmegam, Natchimuthu & Varjani, Sunita & Awasthi, Mukesh & Balasubramani, Ravindran. (2022). Biosurfactants: Potential and Eco-Friendly Material for Sustainable Agriculture and Environmental Safety—A Review. Agronomy. 12. 662. 10.3390/agronomy12030662.
- Gregorich, E. G., Gillespie, A. W., Beare, M. H., Curtin, D., Sanei, H., & Yanni, S. F. (2015). Evaluating biodegradability of soil organic matter by its thermal stability and chemical composition. Soil Biology and Biochemistry, 91, 182-191.
- Chakrabarti, S. (2012). Bacterial biosurfactant: Characterization, antimicrobial and metal remediation properties (Doctoral dissertation).
- Sayyed, R. Z., El-Enshasy, H. A., & Hameeda, B. (Eds.). (2021). Microbial Surfactants: Volume I: Production and Applications. CRC Press.
- Vijayakumar S., Saravanan V. Biosurfactants-types, sources and applications. Res. J. Microbiol. 2015;10:181–192.
- Roy, Arpita. (2017). A Review on the Biosurfactants: Properties, Types and its Applications. Journal of Fundamentals of Renewable Energy and Applications. 8. 10.4172/2090-4541.1000248.
