The immunohistochemical detection of Cutibacterium acnes in sarcoid granulomas suggests its potential role in granuloma formation. C. acnes is the sole microorganism ever isolated from sarcoid lesions. Histopathologic analysis of some sarcoid lymph nodes reveals latent infection and intracellular proliferation of cell-wall-deficient C. acnes followed by insoluble immune-complex formation. Activation of T helper type 1 (Th1) immune responses by C. acnes is generally higher in sarcoidosis patients than in healthy individuals. Pulmonary granulomatosis caused by an experimental adjuvant-induced allergic immune response to C. acnes is preventable by antimicrobials, suggesting that the allergic reaction targets C. acnes commensal in the lungs. C. acnes is the most common bacterium detected intracellularly in human peripheral lungs and mediastinal lymph nodes. In predisposed individuals with hypersensitive Th1 immune responses to C. acnes, granulomas may form to confine the intracellular proliferation of latent C. acnes triggered by certain host-related or drug-induced conditions.
- Cutibacterium acnes
- Propionibacterium acnes
- sarcoidosis
1. C. acnes in Sarcoid Granulomas
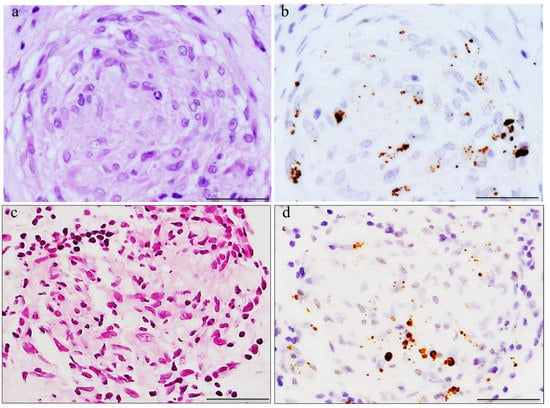
2. Histopathologic Analysis of C. acnes in Sarcoid Lymph Nodes
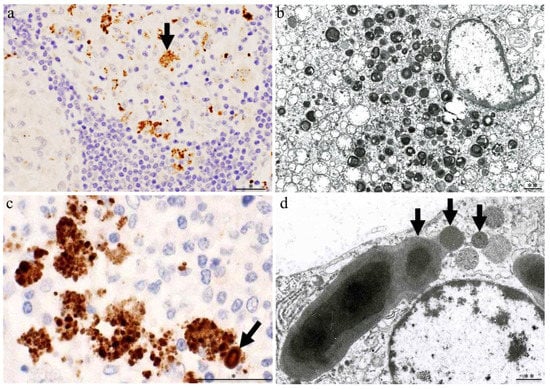
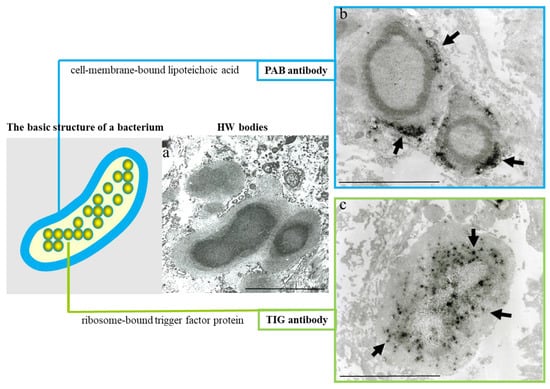
3. Latent Infection and Intracellular Proliferation of C. acnes
4. Humoral and Cellular Immune Responses to C. acnes
5. Allergic Cellular Immune Response to Autoantigens
6. Pathogenesis of Sarcoidosis Caused by C. acnes
The assumed pathogenesis of sarcoidosis caused by C. acnes is summarized in Figure 4. Commensal extracellular C. acnes causes asymptomatic intracellular infection via the respiratory tract. C. acnes is the most common bacterium detected intracellularly in the peripheral lungs and mediastinal lymph nodes in humans [2][20][46]. Susceptibility to latent C. acnes infection may be partly influenced by the NOD1 allele type of the host [47] or by catalase expression of the bacterium [48]. Latent C. acnes can be reactivated and proliferate intracellularly after certain triggering events, and this is not specific to sarcoidosis patients [2][20][49]. Autophagy is induced by an intracellular overload of C. acnes [50]; thus, intracellular C. acnes proliferation may induce the housekeeping function of autophagy, which plays a decisive role in determining the outcome of infection and immunologic balance [51]. It was recently proposed that dysfunction of mTOR, Rac1, and autophagy-related pathways not only hampers pathogen or nonorganic particle clearance, but also participates in T-cell and macrophage dysfunction, thereby driving granuloma formation [52]. The proposed mechanisms may contribute to the outcome of C. acnes infection and immunologic balance against intracellular C. acnes proliferation. Granuloma formation occurs only in individuals predisposed to a hypersensitive Th1 immune response against the intracellular proliferation of C. acnes. An allergic reaction to intracellular C. acnes proliferation seems to be caused by a different mechanism than the immunomodulatory effect of C. acnes itself [53]. Successful confinement of C. acnes that is proliferating intracellularly via granuloma formation prevents further spread of infective C. acnes to other cells, which resolves the granulomatous inflammation that leads to spontaneous remission in many sarcoidosis patients. Extracellular C. acnes that escapes granulomatous confinement or local phagocytosis has the potential to cause new latent infection in extrapulmonary organs (primarily in vascular endothelial cells) via dissemination through the lymphatic system and bloodstream, whereas local entry and subsequent latent infection of C. acnes through other than systemic spread may occur in some organs or patients. Latent infection in systemic organs can be simultaneously reactivated by additional triggering events, which leads to granuloma formation at all sites of latent infection in each organ of sarcoidosis patients.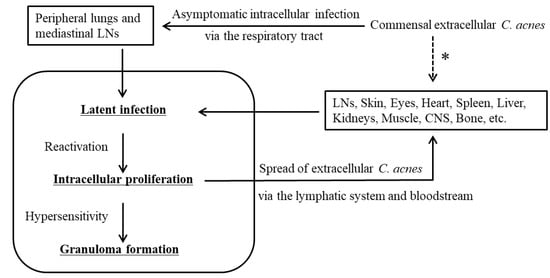
References
- Eishi, Y.; Miyamae, C.; Ando, N. Seeking a causative agent of sarcoidosis. Sarcoidosis 1994, 11, 265–268.
- Negi, M.; Takemura, T.; Guzman, J.; Uchida, K.; Furukawa, A.; Suzuki, Y.; Iida, T.; Ishige, I.; Minami, J.; Yamada, T.; et al. Localization of Propionibacterium acnes in granulomas supports a possible etiologic link between sarcoidosis and the bacterium. Mod. Pathol. 2012, 25, 1284–1297.
- Asakawa, N.; Uchida, K.; Sakakibara, M.; Omote, K.; Noguchi, K.; Tokuda, Y.; Kamiya, K.; Hatanaka, K.C.; Matsuno, Y.; Yamada, S.; et al. Immunohistochemical identification of Propionibacterium acnes in granuloma and inflammatory cells of myocardial tissues obtained from cardiac sarcoidosis patients. PLoS ONE 2017, 12, e0179980.
- Nagata, K.; Eishi, Y.; Uchida, K.; Yoneda, K.; Hatanaka, H.; Yasuhara, T.; Nagata, M.; Sotozono, C.; Kinoshita, S. Immunohistochemical detection of Propionibacterium acnes in the retinal granulomas in patients with ocular sarcoidosis. Sci. Rep. 2017, 7, 15226.
- Goto, H.; Usui, Y.; Umazume, A.; Uchida, K.; Eishi, Y. Propionibacterium acnes as a possible pathogen of granuloma in patients with ocular sarcoidosis. Br. J. Ophthalmol. 2017, 101, 1510–1513.
- Suzuki, T.; Fujita, A. Implication of immunohistochemistry for Propionibacterium acnes in differential diagnosis of necrotizing granuloma. J. Pulm. Respir. Med. 2016, 6, 2–4.
- Isshiki, T.; Matsuyama, H.; Sakamoto, S.; Honma, N.; Mikami, T.; Shibuya, K.; Eishi, Y.; Homma, S. Development of Propionibacterium acnes-associated sarcoidosis during etanercept therapy. Intern. Med. 2019, 58, 1473–1477.
- Sawahata, M.; Sakamoto, N.; Yamasawa, H.; Iijima, Y.; Kawata, H.; Yamaguchi, T. Propionibacterium acnes-associated sarcoidosis complicated by acute bird- related hypersensitivity pneumonitis. BMC Pulm. Med. 2020, 20, 288.
- Sawahata, M.; Fujiki, Y.; Nakano, N.; Ohtsuki, M.; Yamaguchi, T.; Uchida, K.; Eishi, Y.; Suzuki, T. Propionibacterium acnes-associated Sarcoidosis Possibly Initially Triggered by Interferon-alpha Therapy. Intern. Med. 2021, 60, 777–781.
- Asahina, A.; Miura, K.; Saito, I.; Oshikata, C.; Ishii, N.; Eishi, Y. Cutaneous sarcoidosis with livedoid lesions: Evidence of the involvement of Propionibacterium acnes. J. Dermatol. 2013, 40, 501–502.
- Hiroyuki, T.; Takeshi, Y.; Jun, M.; Al, E. Granulomatous pigmented purpuric dermatosis containing Propionibacterium acnes. Eur. J. Dermatol. 2018, 28, 540–542.
- Sasaki, S.; Kato, M.; Nakamura, K.; Namba, Y.; Nagashima, O.; Takahashi, K. Management of skin sarcoidosis with minocycline monotherapy. Respirol. Case Rep. 2019, 7, 4–7.
- Noda, S.; Maeda, A.; Komiya, Y.; Soejima, M. A patient with necrotizing vasculitis related to sarcoidosis, which was diagnosed via immunohistochemical methods using P. acnes-specific monoclonal antibodies. Intern. Med. 2020, 59, 2423–2425.
- Ishikawa, M.; Ohashi, T.; Eishi, Y.; Yamamoto, T. Palmoplantar pustulosis in a patient with sarcoidosis. Eur. J. Dermatol. 2020, 30, 325–326.
- Takama, H.; Ohshima, Y.; Ando, Y.; Yanagishita, T.; Takahashi, E.; Tsuzuki, T.; Uchida, K.; Eishi, Y.; Akiyama, M.; Watanabe, D. Annular sarcoidosis with geographic appearance in a patient with systemic sarcoidosis. Acta Derm. Venereol. 2020, 100, adv00182.
- Shimamura, S.; Yokogawa, N.; Murata, K.; Yamaguchi, T.; Uchida, K.; Eishi, Y. Saddle nose with sarcoidosis: “A great imitator” of relapsing polychondritis. Mod. Rheumatol. 2018, 28, 1053–1057.
- Yang, G.; Eishi, Y.; Raza, A.; Rojas, H.; Achiriloaie, A.; De Los Reyes, K.; Raghavan, R. Propionibacterium acnes-associated neurosarcoidosis: A case report with review of the literature. Neuropathology 2018, 38, 159–164.
- Akimoto, J.; Nagai, K.; Ogasawara, D.; Tanaka, Y.; Izawa, H.; Kohno, M.; Uchida, K.; Eishi, Y. Solitary tentorial sarcoid granuloma associated with Propionibacterium acnes infection: Case report. J. Neurosurg. 2017, 127, 687–690.
- Yamaguchi, T.; Costabel, U.; Mcdowell, A.; Guzman, J.; Uchida, K.; Ohashi, K.; Eishi, Y. Immunohistochemical Detection of Potential Microbial Antigens in Granulomas in the Diagnosis of Sarcoidosis. J. Clin. Med. 2021, 10, 983.
- Isshiki, T.; Homma, S.; Eishi, Y.; Yabe, M.; Koyama, K.; Nishioka, Y.; Yamaguchi, T.; Uchida, K.; Yamamoto, K.; Ohashi, K.; et al. Immunohistochemical Detection of Propionibacterium acnes in Granulomas for Differentiating Sarcoidosis from Other Granulomatous Diseases Utilizing an Automated System with a Commercially Available PAB Antibody. Microorganisms 2021, 9, 1668.
- Beijer, E.; Seldenrijk, K.; Eishi, Y.; Uchida, K.; Damen, J.; Grutters, J.C.; Veltkamp, M. Presence of Propionibacterium acnes in granulomas associates with a chronic disease course in Dutch sarcoidosis patients. ERJ Open Res. 2020, 7, 00486–02020.
- Eishi, Y. Etiologic aspect of sarcoidosis as an allergic endogenous infection caused by Propionibacterium acnes. BioMed Res. Int. 2013, 2013, 935289.
- Wesenberg, W. On acid-fast Hamazaki spindle bodies in sarcoidosis of the lymph nodes and on double-refractive cell inclusions in sarcoidosis of the lungs. Arch. Klin. Exp. Dermatol. 1966, 227, 101–107.
- Sieracki, J.C.; Fisher, E.R. The ceroid nature of the so-called “Hamazaki-Wesenberg bodies”. Am. J. Clin. Pathol. 1973, 59, 248–253.
- Moscovic, E.A. Sarcoidosis and mycobacterial L-forms. A critical reappraisal of pleomorphic chromogenic bodies (Hamazaki corpuscles) in lymph nodes. Pathol. Annu. 1978, 13 Pt 2, 69–164.
- Alavi, H.A.; Moscovic, E.A. Immunolocalization of cell-wall-deficient forms of Mycobacterium tuberculosis complex in sarcoidosis and in sinus histiocytosis of lymph nodes draining carcinoma. Histol. Histopathol. 1996, 11, 683–694.
- Esteves, T.; Aparicio, G.; Garcia-Patos, V. Is there any association between Sarcoidosis and infectious agents?: A systematic review and meta-analysis. BMC Pulm. Med. 2016, 16, 165.
- Ishige, I.; Usui, Y.; Takemura, T.; Eishi, Y. Quantitative PCR of mycobacterial and propionibacterial DNA in lymph nodes of Japanese patients with sarcoidosis. Lancet 1999, 354, 120–123.
- Zhou, Y.; Wei, Y.R.; Zhang, Y.; Du, S.S.; Baughman, R.P.; Li, H.P. Real-time quantitative reverse transcription-polymerase chain reaction to detect propionibacterial ribosomal RNA in the lymph nodes of Chinese patients with sarcoidosis. Clin. Exp. Immunol. 2015, 181, 511–517.
- Yamada, T.; Eishi, Y.; Ikeda, S.; Ishige, I.; Suzuki, T.; Takemura, T.; Takizawa, T.; Koike, M. In situ localization of Propionibacterium acnes DNA in lymph nodes from sarcoidosis patients by signal amplification with catalysed reporter deposition. J. Pathol. 2002, 198, 541–547.
- Eishi, Y.; Suga, M.; Ishige, I.; Kobayashi, D.; Yamada, T.; Takemura, T.; Takizawa, T.; Koike, M.; Kudoh, S.; Costabel, U.; et al. Quantitative analysis of mycobacterial and propionibacterial DNA in lymph nodes of Japanese and European patients with sarcoidosis. J. Clin. Microbiol. 2002, 40, 198–204.
- McLaughlin, J.; Watterson, S.; Layton, A.M.; Bjourson, A.J.; Barnard, E.; McDowell, A. Propionibacterium acnes and acne vulgaris: New insights from the integration of population genetic, multi-omic, biochemical and host-microbe studies. Microorganisms 2019, 7, 128.
- Miura, Y.; Ishige, I.; Soejima, N.; Suzuki, Y.; Uchida, K.; Kawana, S.; Eishi, Y. Quantitative PCR of Propionibacterium acnes DNA in samples aspirated from sebaceous follicles on the normal skin of subjects with or without acne. J. Med. Dent. Sci. 2010, 57, 65–74.
- Uchida, K.; Furukawa, A.; Yoneyama, A.; Furusawa, H.; Kobayashi, D.; Ito, T.; Yamamoto, K.; Sekine, M.; Miura, K.; Akashi, T.; et al. Propionibacterium acnes-derived circulating immune complexes in sarcoidosis patients. Microorganisms 2021, 9, 2194.
- Furusawa, H.; Suzuki, Y.; Miyazaki, Y. Th1 and Th17 immune responses to viable Propionibacterium acnes in patients with sarcoidosis. Respir. Investig. 2012, 50, 104–109.
- Ebe, Y.; Ikushima, S.; Yamaguchi, T.; Kohno, K.; Azuma, A.; Sato, K.; Ishige, I.; Usui, Y.; Takemura, T.; Eishi, Y. Proliferative response of peripheral blood mononuclear cells and levels of antibody to recombinant protein from Propionibacterium acnes DNA expression library in Japanese patients with sarcoidosis. Sarcoidosis Vasc. Diffus. Lung Dis. 2000, 17, 256–265.
- Yorozu, P.; Furukawa, A.; Uchida, K.; Akashi, T.; Kakegawa, T.; Ogawa, T.; Minami, J.; Suzuki, Y.; Awano, N.; Furusawa, H.; et al. Propionibacterium acnes catalase induces increased Th1 immune response in sarcoidosis patients. Respir. Investig. 2015, 53, 161–169.
- Schupp, J.C.; Tchaptchet, S.; Lützen, N.; Engelhard, P.; Müller-Quernheim, J.; Freudenberg, M.A.; Prasse, A. Immune response to Propionibacterium acnes in patients with sarcoidosis—In vivo and in vitro. BMC Pulm. Med. 2015, 15, 75.
- Nakata, Y.; Ejiri, T.; Kishi, T.; Mori, Y.; Hioka, T.; Kataoka, M.; Ohnoshi, T.; Kimura, I. Alveolar lymphocyte proliferation induced by Propionibacterium acnes in sarcoidosis patients. Acta Med. Okayama 1986, 40, 257–264.
- Mori, Y.; Nakata, Y.; Kataoka, M.; Ejiri, T.; Hioka, T.; Maeda, T.; Hosoya, S.; Ohnoshi, T.; Kimura, I. Interleukin-2 production and receptor expression of alveolar lymphocytes stimulated by Propionibacterium acnes in sarcoidosis. Nihon Kyobu Shikkan Gakkai Zasshi 1989, 27, 42–50.
- Tuohy, V.K.; Yu, M.; Yin, L.; Kawczak, J.A.; Kinkel, R.P. Spontaneous regression of primary autoreactivity during chronic progression of experimental autoimmune encephalomyelitis and multiple sclerosis. J. Exp. Med. 1999, 189, 1033–1042.
- Kong, Y.-C.M.; Morris, G.P.; Brown, N.K.; Yan, Y.; Flynn, J.C.; David, C.S. Autoimmune thyroiditis: A model uniquely suited to probe regulatory T cell function. J. Autoimmun. 2009, 33, 239–246.
- Eishi, Y.; McCullagh, P. PVG rats, resistant to experimental allergic thyroiditis, develop high serum levels of thyroglobulin after sensitization. Clin. Immunol. Immunopathol. 1988, 49, 101–106.
- Eishi, Y.; McCullagh, P. The relative contributions of immune system and target organ to variation in susceptibility of rats to experimental allergic thyroiditis. Eur. J. Immunol. 1988, 18, 657–660.
- Eishi, Y.; McCullagh, P. Regulation of Experimental Allergic Thyroiditis. Scand. J. Immunol. 1988, 27, 629–634.
- Ishige, I.; Eishi, Y.; Takemura, T.; Kobayashi, I.; Nakata, K.; Tanaka, I.; Nagaoka, S.; Iwai, K.; Watanabe, K.; Takizawa, T.; et al. Propionibacterium acnes is the most common bacterium commensal in peripheral lung tissue and mediastinal lymph nodes from subjects without sarcoidosis. Sarcoidosis Vasc. Diffus. Lung Dis. 2005, 22, 33–42.
- Tanabe, T.; Ishige, I.; Suzuki, Y.; Aita, Y.; Furukawa, A.; Ishige, Y.; Uchida, K.; Suzuki, T.; Takemura, T.; Ikushima, S.; et al. Sarcoidosis and NOD1 variation with impaired recognition of intracellular Propionibacterium acnes. Biochim. Biophys. Acta 2006, 1762, 794–801.
- Yamamoto, K.; Uchida, K.; Furukawa, A.; Tamura, T.; Ishige, Y.; Negi, M.; Kobayashi, D.; Ito, T.; Kakegawa, T.; Hebisawa, A.; et al. Catalase expression of Propionibacterium acnes may contribute to intracellular persistence of the bacterium in sinus macrophages of lymph nodes affected by sarcoidosis. Immunol. Res. 2019, 67, 182–193.
- Beijer, E.; Seldenrijk, K.; Meek, B.; Damen, J.; Quanjel, M.J.R.; Grutters, J.C.; Veltkamp, M. Detection of Cutibacterium acnes in granulomas of patients with either hypersensitivity pneumonitis or vasculitis reveals that its presence is not unique for sarcoidosis. ERJ Open Res. 2021, 7, 00930–2020.
- Nakamura, T.; Furukawa, A.; Uchida, K.; Ogawa, T.; Tamura, T.; Sakonishi, D.; Wada, Y.; Suzuki, Y.; Ishige, Y.; Minami, J.; et al. Autophagy induced by intracellular infection of Propionibacterium acnes. PLoS ONE 2016, 11, e0156298.
- Sharma, V.; Verma, S.; Seranova, E.; Sarkar, S.; Kumar, D. Selective autophagy and xenophagy in infection and disease. Front. Cell Dev. Biol. 2018, 6, 147.
- Pacheco, Y.; Lim, C.X.; Weichhart, T.; Valeyre, D.; Bentaher, A.; Calender, A. Sarcoidosis and the mTOR, Rac1, and Autophagy Triad. Trends Immunol. 2020, 41, 286–299.
- Kraaijvanger, R.; Veltkamp, M. The Role of Cutibacterium acnes in Sarcoidosis: From Antigen to Treatable Trait? Microorganisms 2022, 10, 1649.
