The molluscan hemocyanins have complex carbohydrate structures with predominant N-linked glycans. Determination of glycans and glycopeptides was performed with the most common used methods for the analysis of biomolecules, including peptides and proteins like MALDI-TOF-TOF (time of flight), LC/ESI-MS, LC-Q-trap-MS/MS nano-ESI-MS and others. A novel acidic glycan structures with specific glycosylation positions were observed in Rapana venosa, Helix lucorum, Haliotis tuberculata, e.g. hemocyanins that enable a deeper insight into the glycosylation process.
- Glycoproteins, Hemocyanins,
- garden snail helix lucorum,
- marine snail rapana venosa
1. Introduction
Glycoproteins are of great importance for the optimal and proper functioning of many bioactivities in the human body. Absence or alteration of glycoproteins can cause a wide range of diseases, e.g., the glycated form of haemoglobin is responsible for the onset of diabetes[1] [1]. Glycans also play an important role in cardiovascular disease, microbial and viral pathogenesis, development of tumors, tissue repair, strengthening of the immune system, and others[2][3][4] [2–4]. As demonstrated by various studies[5][6] [5,6], hemocyanins have gained a large amount scientific interest due to their interesting glycoprotein structures.
Hemocyanins are type-3 copper-binding proteins found freely dissolved in the hemolymph in two main phyla (mollusca and arthropoda), which have the same physiological function, but show significant differences in their structural organization and carbohydrate content.
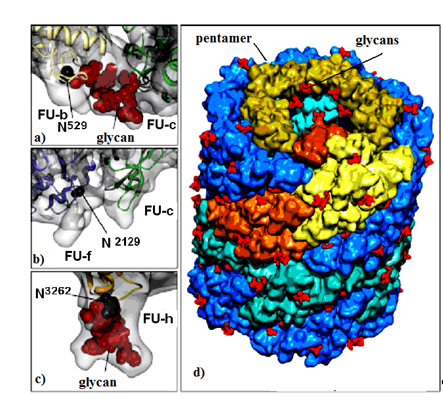
Molluscan hemocyanins are among the largest known glycoproteins with huge cylindrical multimeric forms and molecular masses ranging from 3.3 to 13.5 MDa. They form decamers or multi-decamers of 330- to 550-kDa subunits organised by more than seven different functional units (FUs) with molecular masses ranging from 45 to 65 kDa. Structural subunits assemble to di-decamers as presented for keyhole limpet hemocyanin (KLH1) in (Figure 1)[7] [7].
Figure 1. KLH1 subunit model. Location of Asp residue in the glycosylation site of: (a) FU-b and FU-с; (b) FU-f and FU-с; (c) FU-h; (d) di-decamer KLH1 model and the position of 120 glycans (marked in red)[7] [7].
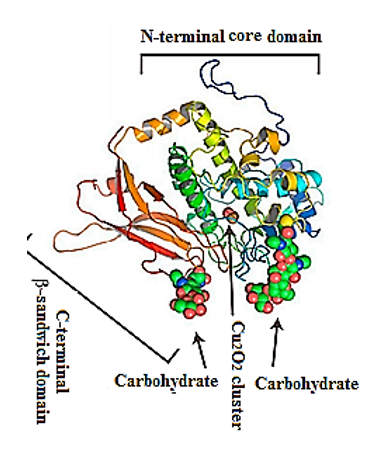
Their structure has been investigated using a combination of electron cryo-microsopy and X-ray crystallography (Figure 2). The represented X-ray crystal structure of the intact 3.8-MDa molecule of Todarodes pacificus squamous hemocyanin (TpH) shows the complex oligosaccharide structures of the glycoprotein[8] [8]. Chemical structure analysis of Todarodes pacificus hemocyanin revealed that the two most abundant sequences, HexNAcMan3GlcNAc2 and HexHexNAcMan3GlcNAc2, accounted for more than 95% of all oligosaccharides tested in all individuals. The Cu2O2 cluster and the carbohydrates of functional unit “d” of TpH are represented in the 3D-model of Figure 2. The authors demonstrated the influence of glycans on the dissociation and re-association behavior of the decameric forms of TpH[9] [9].
Figure 2. Representative ribbon diagram of FU-d of Todarodes pacificus squamous hemocyanin, colored as a ramp from blue (N-terminus) to red (C-terminus). The Cu2O2 cluster and carbohydrates [9].
Therefore, the great scientific interest in molluscan hemocyanins is due not only to their complex quaternary structures, but also to their great diversity in oligosaccharide structures, high carbohydrate content (2–9%), specific monosaccharide composition[10][11][12][13] [10–13], and their well-established link with the immunostimulatory effect[14][15] [14,15].
The studies on carbohydrate structures of hemocyanins from molluscs occupy an important part of this review, demonstrating significant differences in carbohydrate structures of molluscan hemocyanins.
2. Carbohydrate Structures of Hemocyanins
The first analyses of the carbohydrate structures of hemocyanins from the earth snail Helix pomatia (HpH) [10], freshwater snail Lymnaea stagnalis (LsH)[16] [16], and the California mussel (keyhole limpet) Megathura crenulata (KLH)[13][17] [13,17] present complex carbohydrate structures composed predominantly of the monosaccharides Xyl, Fuc, Man, Gal, GalNAc, and GlcNAc. Along with common monosaccharides, other monosaccharides such as Xyl and O-methyl-Gal have also been identified in HpH glycans [10][11][18][10,11,18]. Though the Xyl residue is less common in glycoproteins from animal organisms, their presence is essential for immunogenic effects[10] [10].
Other specifically-modified structures have been identified that bind (β1-2)Xyl residues to β-Man and α-Fuc to 1,6-GlcNAc from the core of the glycan. Traces of large neutral N-glycans ending with 3-O-MeGal, Xyl, and/or Fuc monosaccharides have been found in Planorbarius corneus, Achatina fulica, and Arion lusitanicus[12][19] [12,19]. The presented complete N-glycan spectrum of the slug Arion lusitanicus includes a combination of carbohydrate structures found in mammals, plants, insects, and the like[12][19] [12,19]. Complex structures have also been published for some glycans of Rapana thomasiana hemocyanin (RtH), later renamed Rapana venosa (RvH)[11][20] [11,20].
Furthermore, the studies on the carbohydrate structure of KLH, used in medicine, present two new N-linked glycans, Fuc(α1-3)GalNAc(β1-4)[Fuc(α1-3)]GlcNAc and Gal(β1-4)Gal(β1-4)Fuc(α1-6), as part of the huge protein molecule[13][17] [13,17]. In one glycan, a Gal monosaccharide is linked to the final GalNAc, for which the clinical success of KLH in the treatment of bladder cancer is found. The carbohydrate chain Gal(β1-4)Gal(β1-4)Fuc(α1-6) is linked to the nucleus of another glycan, which also contributes to the use of KLH in immunotherapy.
The structural diversity of N-glycans in snails has been investigated very thoroughly by a number of authors who report predominantly high-mannose carbohydrate chains. Exceptions were found for some carbohydrate structures that bind sialic acid, α1-6- and α1-3-Fuc, β1-2-Xyl, MeMan, MeGal, GlcNAc, GalNAc, and other monosaccharides, but significantly fewer glycans end up with a 3-O-MeMan residue[10][11][18] [10,11,18].
A great variety of oligosaccharide structures has also shown for side chains linked to carbohydrate structures of hemocyanins from the abdominal mollusc Lymnaea stagnalis, the California mussel KLH, and others [12][14][16][19][21][12,14,16,19,21]. Specific enzymes, responsible for the formation of these complex structures, have been found in various organs and tissues of Limax maximus, Lymnaea stagnalis, and other representatives of the molluscs[12] [12].
Important information for the positions of glycosylated Asn residues for three FU-s (FU-b, FU-f and FU-h) is provided by the di-decameric model of KLH1 (Figure 1a–d). The model shows 120 potential N-glycan motifs (NXT and/or NXS), located mainly on the surface of the molecule (Figure 1d). FU-c is of special interest due to its absence of a residual glycan (Figure 1b)[22] [22]. This corresponds to KLH-c with only one O-glycosylation site, lacking an attached, glycan moiety[23] [23]. The absence of glycan in FU-c was also observed in hemocyanins of Haliotis tuberculata (HtH), Octopus dofleini (OdH), and Aplysia californica (AcH)[24] [24]. Published literature mostly presents O-glycosylated hemocyanins of the arthropodan species, while very few O-linked glycans have been found in hemocyanins of the Molluscs.
Another interesting feature for molluscan and arthropodan hemocyanins is the position of the putative glycosylation sites, located mainly on the surface of the molecule. Two N-linked sites of FU HtH1-h are located at rather unusual positions and are not found in other FUs, including HtH2-h. These glycans probably perform an important role related to the function of glycosylated FU-h, suggesting that they prevent binding of decamers to di-decamers and formation of multi-decamers in hemocyanins. The assumption is confirmed by the established absence of multi-decamers in the hemocyanins of AcH and HtH1[24][25] [24,25].
In this review, the oligosaccharide structures of three hemocyanins are presented: from the Black Sea snail Rapana venosa, the garden snail Helix lucorum, and the abalone Haliotis tuberculata, which normally exist under different living conditions. They have been compared with the well-studied oligosaccharide structure of keyhole limpet hemocyanin (KLH) from a mussel inhabiting the northern coast of America, and other hemocyanins with complex carbohydrate structures.
Mass spectral methods and techniques are appropriate for analysis of glycoproteins and identification of their structure. To determine molecular masses of amino acid sequences (AAS) of proteins and peptides, mass spectrometry is used as the nucleus of proteomics. It is one of the most widely used methods for the analysis of biomolecules like peptides, proteins, and glycoproteins, with a series of sophisticated instrumentations such as Matrix Assisted Laser Desorption/Ionisation–Time of Flight (MALDI-TOF-TOF), Liquid Chromatography-Electrospray Ionization-Mass Spectrometry (LC/ESI-MS), Liquid Chromatography (LC-Q-trap-MS/MS) or Nano- Electrospray Ionization-Mass Spectrometry (nano-ESI-MS).
3. Carbohydrate Structure of Hemocyanins from the Marine snail Rapana venosa
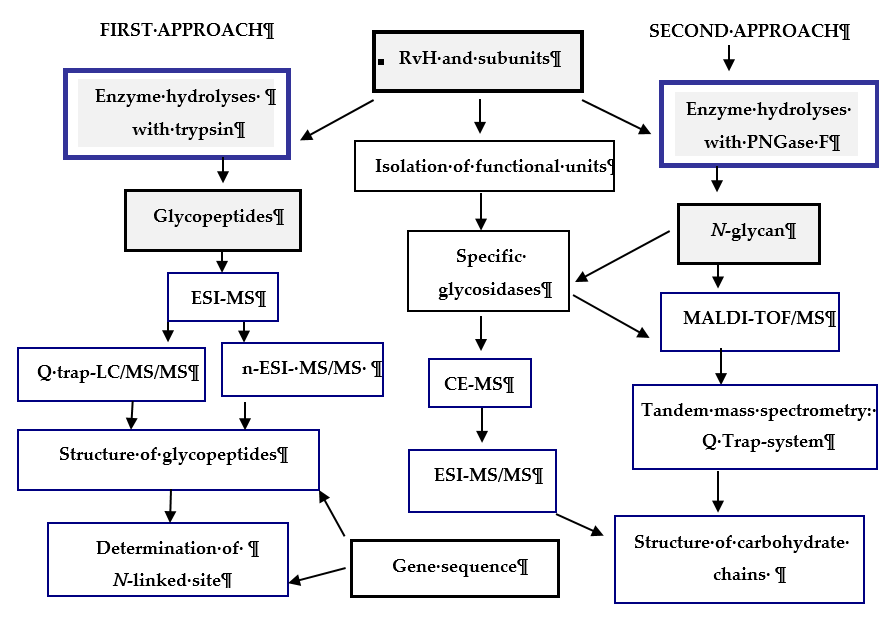
Recent research reported on the glycosylated nature of RvH, with a carbohydrate content of 8.9%, which differs for the two subunits RvH1 (12.4%) and RvH2 (4.4%)[26][27] [26,27]. A new strategy for analysis and determination of the carbohydrate structure of subunits and FUs of RvH is presented here (Figure 3).
Figure 3. Scheme for analysis and determination of the carbohydrate structure of RvH1, RvH2 and FUs. On the basis of this strategy, two main approaches are involved.
In the first approach, functional units (FUs) of the two subunits RvH1 and RvH2 are treated with trypsin and the obtained glycopeptides are identified through LC/ESI-MS, LC-Q-trap-MS/MS or nano-ESI-MS[28][29][30][31][32][33] [28–33]. The second approach includes isolation of glycans after hydrolysis of the subunits of RvH with specific glycosidases (PNGase F) and analysis of the glycan structures with MALDI-TOF-MS, СЕ-MS, and Q-trap instrumental systems[28][28][34] [28,29,34].