One of the hallmarks of Cu metabolism in mammals is that tissue and fluid levels are normally maintained within a very narrow range of concentrations. This results from the ability of the organism to respond to variations in intake from food and drink by balancing excretion, which occurs mainly via the bile and feces. Although this sounds straightforward and we have already learned a great deal about aspects of this process, the balance between overall intake and excretion occurs over a high background of Cu recycling, which has generally been ignored. In fact, most of the Cu absorbed from the GI tract actually comes from digestive fluids and is constantly “re-used”. A great deal more recycling of Cu probably occurs in the interior, between cells of individual tissues and the fluid of the blood and interstitium. This review presents what is known that is pertinent to understanding these complexities of mammalian Cu homeostasis and indicates where further studies are needed.
- copper
- homeostasis
- excretion
- secretion
- mammals
- transport
- blood
- bile
- urine
- small copper carriers
The main and best established points about how mammalian Cu homeostasis occurs through a balance between absorption, transport and excretion, and the important roles of secretions are summarized in
. The figure depicts the amounts of Cu entering the digestive tract from various sources and how they travel from the intestine to key organs (like liver and kidney) and the rest of the organism, as well as how Cu is excreted. Values indicated are average mg Cu per day, based on the current and earlier literature, as previously discussed. Starting with Cu in food and drink entering the mouth (top left; “Diet”), as well as that in salivary, gastric, biliary and pancreatic secretions, plus some Cu from the intestinal mucosal cells of the small intestine, provide Cu to the GI tract. Most of that is absorbed in the duodenum of the small intestine.

Overview and Summary of the homeostatic processes described in this review for the normal human adult. This figure diagrams amounts of copper entering the digestive tract from various sources and how they travel from the intestine to key organs (like liver and kidney) and the rest of the organism, as well as how copper is excreted. Values indicated are average mg Cu per day. Dietary Cu is indicated by purple lines; absorption and distribution to liver, kidney and other cells is in darker blue; involvement of ceruloplasmin (Cp) is in lighter blue; involvement of Cp and hephaestin (Hp) in Fe efflux from liver and intestine, respectively, is in the light blue and orange; secretions that lead to Cu efflux into the GI tract, feces and urine are in red. Modified and updated from Linder [1].
Transfer of absorbed Cu from the intestinal cells to the blood is accomplished mainly by the Cu ATPase (ATP7A). Based upon tracer studies with radioactive Cu, Cu that enters the hepatic portal circulation binds tightly to proteins that compose the exchangeable Cu pool of the blood plasma, which consists mainly of albumin and alpha-2-macroglobulin (transcuprein), but may include other protein or non-protein components. The bulk of this Cu then first enters cells of the liver and partly also those of the kidney (
), where Cu is supplied to endogenous Cu-dependent proteins via various chaperones (ATOX1, CCS, Cox17). Most travels to the transmembrane Cu “pump”, ATP7B, in the trans Golgi network, from where a significant portion is incorporated into ceruloplasmin (Cp) for secretion into the blood via exocytosis, where it joins Cu on albumin and transcuprein/macroglobulin for delivery to cells in other organs all over the body (including the intestine). Another portion of Cp interacts with Fe (orange section of the diagram) to facilitate Fe release and transport on its blood carrier, transferrin. Hephaestin (Hp), a membrane-bound homolog of Cp, plays a similar role in Fe release from enterocytes to the blood. Excess Cu in the liver cells finds its way into the bile after entering the bile canaliculi, with the help of ATP7B and the endo-lysosomal compartment (see
3). Most excretion of Cu is via the bile, as biliary Cu is not as readily re-absorbed, and so ends up in the feces. Cells regularly sloughed off the intestinal mucosa and degraded in the large intestine may also contribute to fecal excretion. Normally, only a small amount of Cu is excreted in the urine, which probably derives not only from tubular secretions but also the filtration of blood plasma. However, urinary secretion markedly increases when biliary secretion is compromised, leading to increases in small Cu carriers (SCC) in blood plasma that greatly increase levels of SCC in the urine. A great deal is still unknown about the additional forms of Cu that may be involved in its flow, especially through various secretory pathways leading to the blood, bile and urine, and the contributions of these processes and components to mammalian Cu homeostasis.
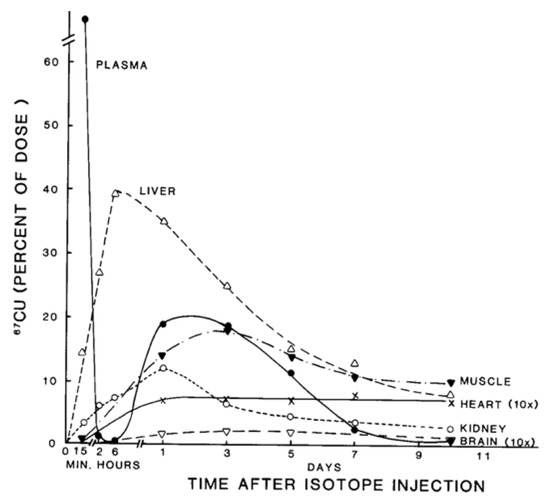
Figure 2. Distribution of 67Cu in rat organs and blood plasma at various times after tracer administration. Data for each time point are mean values for mostly 5–7 rats, given as % of 67Cu dose. Data for brain and heart have been multiplied 10× to make them more visible. Reprinted with permission from reference Weiss, K.C.; et al. [2].
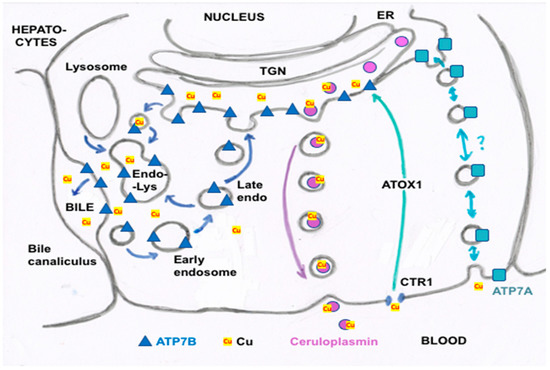
Figure 3. Summary of proposed steps involved in the movement of cu into bile and secreted by hepatocytes. Based mainly on the data, reviews and figures provided by Polishchuk and Polishchuk [3] and Stewart et al. [4]. Cu ions enter hepatocytes from plasma proteins via copper transporter 1 (CTR1) and at least one other as yet unidentified transporter, and are carried via the chaperone ATOX1 to ATP7B (blue triangles) in the transGolgi network (TGN). In the lumen of the TGN, Cu will be incorporated into apoceruloplasmin (apoCp) (violet circles), forming holoCp, and be released into the blood plasma by exocytosis (center right of the figure) across the “basolateral” hepatocyte membrane. In the absence of excessive Cu not otherwise incorporated into Cp and endogenous Cu-dependent proteins and mitochondria, TGN Cu in the lumen will be exported to the bile canaliculi that are formed between hepatocyte apical membranes, leading to the gall bladder and bile duct. This happens through the budding of vesicles from the TGN that contain Cu and may also contain ATP7B. These fuse with late endosomes also containing ATP7B as well as lysosomes (to form the endo-lysosomal compartment), which then fuses with the apical hepatocyte membrane at a canaliculus (left side of figure). This releases Cu that was present in the vesicular bodies and also can lead to ATP7B being part of the apical membrane. The latter is particularly important in the presence of excess Cu, which can then flow from ATOX1 directly to ATP7B that pumps it into the bile canaliculi. Small amounts of the Cu efflux “pump”, ATP7A (blue squares), are also expressed in hepatocytes (far right of the figure), and may also contribute to Cu that exits hepatocytes and enters the blood plasma, to bind to proteins like albumin in the exchangeable plasma Cu pool. Based on what is known in other cells, the much less abundant ATP7A in hepatocytes would either transfer some Cu in the TGN into secretory vesicles (for exocytosis), and/or traffic it in vesicles to the basolateral membrane to pump Cu in the cytosol (on ATOX1) into the blood plasma, following which ATP7A would recycle back to the TGN.
References
- Linder, M.C. Nutritional biochemistry of copper, with emphasis on the perinatal period. In Biochemical Aspects of Human Nutrition; Avigliano, L.A., Rossi, L., Eds.; Transworld Research Network: Trivandrum, Kerala, India, 2010; pp. 143–179. ISBN 978-81-7895-478-3
- Weiss, K.C.; Linder, M.C. Copper transport in rats involving a new plasma protein. Am. J. Physiol. Endocrinol. 1985, 249, E77–E88.
- Polishchuk, R.S.; Polishchuk, E.V. From and to the Golgi—Defining the Wilson disease protein road map. FEBS Lett. 2019, 593, 2341–2350.
- Stewart, D.J.; Short, K.K.; Maniaci, B.N.; Burkhead, J.L. COMMD1 and PtdIns (4,5) P2 interaction maintain ATP7B copper transporter trafficking fidelity in HepG2 cells. J. Cell Sci. 2019, 132.
