Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Mark Obrenovich and Version 2 by Camila Xu.
Ginkgo biloba, and its main constituent compounds, the ginkgolides, have become something of a celebrity as far as natural drug candidates go.
- natural products
- Ginkgo biloba
- vascular dementia
1. Pathogenesis of Alzheimer’s Disease/Vascular Dementia and Effects of Ginkgo biloba
Alzheimer’s disease is characterized by the formation of neurofibrillary tangles [1][23] and the accumulation of abnormal amyloid beta (Aβ) peptide or smaller soluble Aβ oligomers and their deposition in senile and neuritic plaques [2][24] and in brain parenchyma and cerebral capillaries [3][25]. AD is largely a protracted disease, but the exact etiology is largely unknown. Nevertheless, age is implicated to be the strongest risk factor and the presence of a first-order family member also increases risk significantly for developing AD [4][9].
However, these hallmark lesions are mostly end-stage changes and their protracted nature is indicative of the disease, but are not sufficient and necessary for dementia to occur, which suggests earlier mechanisms are involved well before any prodromal stages [5][6][17,26]. The role of natural compounds such as Ginkgo biloba on immunomodulation, inflammation, and cardiovascular homeostasis have been demonstrated to include the antagonist activity of the platelet activating factor, by reducing the infiltration of eosinophils and neutrophils and alleviating neuroinflammatory injury by inhibiting astrocytic Lipocalin-2 expression after ischemic brain injury and stroke [7][27]. Taken together, this provides more evidence to support AD as a vascular disorder [8][28].
One of the major potential therapeutic indications for the experimental use of Ginkgo biloba extract EGb761 is its ability to protect against Aβ-induced neurotoxicity and neuroinflammation, a key to pathology in Alzheimer’s disease, which also includes cerebral amyloid angiopathy [9][29]. Moreover, there is a vascular and cerebrovascular–brain axis in AD including VaD since vascular dementia and cardiovascular disease implicate shared vascular mechanisms in the development and/or progression of AD [10][11][12][13][30,31,32,33]. In the development of this disease, the Aβ deposition in the brain causes a reactionary stimulation of the body’s defense system. In this defense, resident microglia/infiltration macrophages become activated to clear the deposition and concomitantly produce a range of inflammatory mediators that are potentially neurotoxic, which contributes to the pathogenesis of AD [14][15][16][34,35,36]. In a study analyzing the abnormal amyloid beta peptide formation, it was found that Ginkgo biloba has an anti-amyloid aggregation effect due to its effect on increasing transthyretin RNA levels, an amyloid beta peptide transporter that decreases amyloid brain deposition [4][9]. Moreover, amyloid oligomers and downstream fibril formation can be affected or arrested in many ways that are not immediately evident in a clinical study, due to reseaourchers inability to image such changes in vivo [17][37]. Many studies in rats and in vitro reveal an alternate method of fighting Aβ accumulation by modulating alpha-secretase, the enzyme responsible for cutting the amyloid beta peptide, and preventing the final fragments from being made [18][38]. Further, in human subjects, Ginkgo biloba was found to have utility in the treatment of cerebral insufficiency due to inflammatory neurotoxicity-induced dementia [19][39], whether due to degenerative processes or vascular insufficiency [20][40].
Another hallmark lesion in AD is the hyperphosphorylation of the microtubule-associated protein tau [21][41]. To mimic AD-like pathological conditions and memory deficits, hyperhomocysteinemia (HHcy) rat models were employed. Low folate raises blood HHcy concentrations, which are associated with poor cognitive performance in the general population. Elevated HHcy has neurotoxic and vasotoxic effects in AD and dementia and can be considered an early marker of cognitive decline [22][42]. HHcy is implicated in many age-related diseases, including neurologic diseases AD and VaD, cardiovascular disease, and ocular disorders such as macular degeneration and diabetic retinopathy, but has a less defined role in vascular dementia. Further, HHcy induces inflammatory-mediated blood–brain barrier (BBB) dysfunction through the activation of glutamate receptor N-methyl-d-Aspartate receptor [23][43]. HHcy-induced brain inflammation is a mechanism of BBB dysfunction and other immune-privileged barriers [23][43]. HHcy can also induce oxidative stress, which contributes to endothelial and arterial damage, increases tau microtubule-associated protein phosphorylation, and promotes blood clot formation, all of which are comorbidities of aging. While these models do not result in senile plaque deposition or intracellular inclusions or recapitulate the human condition, rodent models are the best approximation for in vivo changes. DNA damage with apoptosis is another protracted hallmark of AD pathobiology [24][25][26][44,45,46].
A hallmark lesion in AS is the hyperphosphorylation of tau protein. To explore the phosphorylation condition, rats were injected with EGb761 or saline as a sham control [27][47] and the status of oxidative damage, spatial/learning memory, level of memory-related proteins, tau phosphorylation, level/activity of tau kinase (GSK-β), and protein phosphatase 2A (PP2A) were all measured and reported. This particular group [27][47] showed that EGb761 could significantly antagonize HHcy-induced oxidative damage and recover PP2Ac and GSK3 β activities deregulated by HHcy. Not only did EGb761 protect against Aβ-induced neurotoxicity, but this researchtudy gave promising results with EGb761 for treating AD by decreasing hyperphosphorylation and demonstrating antioxidative activity [27][47]. When analyzing the abnormal amyloid beta peptide formation, it was found that Ginkgo biloba has an anti-amyloid aggregation effect due to its ability to increase transthyretin RNA levels, an amyloid beta peptide transporter that decreases amyloid brain deposition [1][4][9,23]. In vitro, Ginkgo biloba extract (GLE) provided protection from abnormal Aβ peptide accumulation with deposition in the brain parenchyma and cerebral capillaries [3][25]. However, native amyloid precursor protein is not sufficient or necessary for AD pathology alone [28][48] and the microtubule-associated protein tau is another mechanism believed to cause AD pathology [29][49]. The effects of Ginkgo biloba on beta secretase or the cholinergic system as well as increasing neurotransmitters should be further explored as they are both associated with AD [30][31][50,51]. In that regard, in studies aimed at improving cognitive function and control, Ginkgo biloba extracts were found to increase levels of acetylcholine, dopamine, and 5-hydroxytryptamine as well as to inhibit the activation of acetylcholinesterase, which is responsible for degrading acetylcholine [32][52]. The use of cholinergic agents, such as acetylcholinesterase (AChE) inhibitors, is currently being explored and these agents have shown efficacy and considerable benefit in AD and VaD therapy [33][53]. AChE inhibitors include donepezil, rivastigmine, and galantamine, as well as active compounds from the herb huperzine [33][53]. This accumulating evidence reveals that cholinergic deficiency contributes to vascular dementia and disease, which can be modulated by drugs such as Z-ligustilide [34][35][54,55]. Other natural products such as Hwangryunhaedok-tang (HT) [36][56], black tea, green tea, and coffee contain compounds that inhibit acetylcholinesterase and green tea metabolites also inhibit beta secretase, which could prevent the release of Aβ peptides from amyloid precursor protein. However, coffee is a less effective inhibitor of acetylcholinesterase, having no butyrylcholinesterase- or beta secretase-inhibiting activity [37][57].
The popularity for the use of Ginkgo biloba stems in part from the finding that it largely has no major adverse events or reactions or known side effects, to date, other than those due to the antagonism of clotting mechanisms, which should be a contraindication in some trials. Moreover, one study showed that when compared to donepezil monotherapy, the adverse event rate was lower in the Ginkgo biloba group and even under the combination group when compared to a placebo [38][58]. Contrary to the expectation that taking two drugs simultaneously would increase adverse events, this researchtudy had the opposite effect. In fact, improvement in adverse reactions with donepezil was observed when taken in conjunction with Ginkgo biloba extracts. These findings would be expected to lead to renewed interest in this area toward developing Ginkgo-derived compounds as therapeutics for the treatment of dementia. Nutrition companies looking towards commercializing this new discovery can help fund clinical trials and help this newly developed research accumulate more evidence-based research to support the claims of treatment success.
Amyloid β deposition is associated with the pathogenesis of AD, in particular tissue damage in the brain and associated parenchymal cells and neurons. Oxidative stress and neuroinflammation are associated with the damage caused by this pathology, which contributes significantly to dementia and the deleterious symptoms of AD. One of the cytokines found to increase with AD is CD38. This cytokine, expressed by neurons, astrocytes, and microglial cells, is responsible for regulating the repair and inflammatory processes within the brain [2][24], among other actions, degrades nicotine adenine dinucleotide (NAD), and regulates the migration of inflammatory cells during neuroinflammation [39][40][59,60]. One study examined CD38 expression on AD pathology in CD38-deficient mice and in vitro cultures. All treatments decreased secretions of Aβ from neuronal cultures, decreased the activity of β- and γ-secretase, the enzymes that cleave amyloid precursor protein, and significantly reduced Aβ plaque load and levels of insoluble Aβ [2][24]. This resulted in the attenuation of AD pathology and improved spatial learning and learning performance. The neuroprotective effects of CD38 inhibition suggest a novel therapeutic approach for AD with GLE. One mechanism of action for GLE and other flavonoids in AD involves attenuating CD38 neuroinflammation. Studies examining select flavonoids reported that, at low molecular levels, CD38 was inhibited by flavonoids, such as luteolinidin, kuromanin, and luteolin [41][61]. Other studies exploring kuromanin and luteolin found it could inhibit CD38 directly by affecting NAD levels in glycolysis [42][62]. Taken together, these findings suggest alternative roles for flavanols, such as those found in GLE, which have alternative mechanisms in modifying the hallmark lesions associated with AD pathology, including effects on neuronal metabolism and glyco-oxidative stress. Moreover, the microbiota also plays a key role in flavanol metabolism and must be considered when assigning any effect of GLE on the experimental model.
Also implicated in AD are mitogen-activated protein kinase (MAPK) signaling pathways. MAPK pathways represent a promising therapeutic target as they are implicated in inflammatory and apoptotic processes during cerebral ischemia and reperfusion injury as well [43][44][63,64]. Inhibitors of MAPK pathways are being explored as a therapeutic strategy for ischemic stroke. Moreover, bilobalide, a predominant sesquiterpene trilactone constituent of Ginkgo biloba leaves, has been shown to exert powerful neuroprotective properties, which are closely related to both anti-inflammatory and anti-apoptotic pathways [43][63]. This group investigated the neuroprotective roles of bilobalide in middle cerebral artery occlusion and reperfusion and oxygen–glucose deprivation and reoxygenation models of cerebral ischemia/reperfusion injury. They attempted to confirm the hypothesis that the protective effects of bilobalide were through the modulation of pro-inflammatory mediators and MAPK pathways. Their data indicated that the neuroprotective effects of bilobalide on their cerebral injury model are associated with the inhibition of pro-inflammatory mediator production and the downregulation of JNK1/2 and p38 MAPK activation [43][63].
2. Safety of Ginkgo biloba, GLE Extracts, and Similar Compounds
As the oldest extant deciduous tree, Ginkgo biloba, with its bilobed fan-shaped leaves, is known for its beauty, longevity, and resistance to pathogens [45][65]. In terms of longevity, it can live for over 1000 years [46][66]. Ginkgo biloba has a rich history for medicinal use dating back 5000 years [45][65]. Currently, it is a treatment option for acute ischemic stroke in China [19][39]. It was not until the mid-1960s that it was introduced into Western medicine by German physician–pharmacist Dr. Willmar Schwabe [45][65]. Standardized Ginkgo biloba leaf extract (EGb761) is typically prepared from whole dried green leaves [45][65], which contain many bioactive compounds. Two main components stand out and are largely responsible for GLE’s putative pharmacological effects (see Figure 1), namely, flavonoid glycosides and terpene trilactones, which represent 24% and 6% of the overall plant content, respectively [1][19][23,39]. Other potentially beneficial small molecules and phenolic acids, including aging, are present in Ginkgo biloba extracts, which are useful for the treatment of vascular cognitive impairment, vertigo, tinnitus, early diabetic retinopathy, and senile macular degeneration [19][45][39,65]. Ginkgo biloba leaf extract is also taken for the treatment of inflammation, asthma, and bronchitis [47][48][67,68].
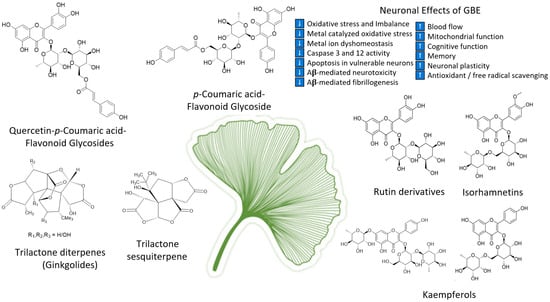
Figure 1. Structures of ginkgolides A, B, C (diterpenes), and bilobalide (sesquiterpene) and function of compounds found in G. biloba extract and EGb 761; proanthocyanidins, quercetins, coumarins, flavonoid glycosides, ginkgolides A, B, C-J, isorhamnetins, bilobalide, and rutin derivatives.
An important consideration for all of the superfood-related treatments in this work is bleeding risk, which is often associated with potential adverse interactions between some of the used herbal supplements and analgesic drugs with natural and prosaic foods, derivatives, and some of the so-called superfoods—in particular, ginkgo, garlic, ginger, bilberry, dong quai, ginseng, turmeric, and willow bark [49][69], as well as with those containing coumarin, i.e., chamomile, horse chestnut, fenugreek, and red clover. That said, taking the herbal or whole food extracts can limit the risk. Nevertheless, the whole food of Ginkgo biloba and its GLE extracts together with the interaction of Ginkgo biloba with non-steroidal anti-inflammatories could complicate the bleeding risk, since the combined use may induce increased bleeding risk to sensitive patients. However, these risks were not exclusory in the studies elucidated. Further, it is known that the use of the whole Ginkgo biloba extract and not simply the fractions can be the better form for consumption due to the synergic effect that occurs when consuming the different fractions together. Interestingly, the hibernating ground squirrel has a defect in clotting, which may offer clues into the neuroprotective mechanisms or mimic natural compounds such as those discussed [50][51][70,71].
Commercial Ginkgo biloba standardized extracts are available in the US market as coated tablets for oral administration [45][65] with a recommended daily dose of 240 mg, either taken in one or two doses. One of the most important contraindications when supplementing orally with Ginkgo biloba is counter indicated for anyone receiving anticoagulation, antiplatelet therapy, or for those with bleeding disorders [45][65]. This is due to the ability of Ginkgo biloba to form free platelets by antagonizing platelet-activating factors [19][39] and inhibiting platelet aggregation [45][65]. Administration is contraindicated in children and during pregnancy and nursing because of the potential adverse effects [45][65].
The safety and efficacy of ginkgolides in AD and VaD can be found in a Ginkgo biloba randomized 400 patient clinical trial of 50 years or older with VaD or AD who were given a special extract EGb761 or placebo for 22 weeks [52][72]. Patients who scored below 36 on the test for the early detection of dementia, with discrimination from depression, and who also scored between 9 and 23 on the Short Syndrome Test battery and at least 5 on the Neuropsychiatric Inventory were chosen for this particular trial. The drug was well tolerated and the adverse events were no greater than those of the placebo treatment according to this clinical trial, which focused on safety. Moreover, the treatment was found to improve cognitive functioning and behavior symptoms in patients with neuropsychiatric features and age-associated cognitive impairment or mild to moderate dementia. Since the treatment results were essentially similar for the AD and VaD subgroups, the data supports the safety and efficacy of EGb761 in the treatment of cognitive and non-cognitive symptoms of dementia overall, notwithstanding any interference with clotting [52][72]. A clinical trial examined the efficacy of Ginkgo biloba on the neuropsychological functioning of cognitively intact adults aged 60 years or older assigned randomly to receive either 180 mg/day of GLE or a placebo daily for 6 weeks. Compared to the placebo group, the group taking GLE rated their overall abilities as “improved”. This researchtudy harmonizes evidence for the potential efficacy of GLE.
However, the findings on Ginkgo are mixed. A larger study of three thousand and sixty-nine community volunteers 75 years or older conducted from a total of five academic medical centers in the United States revealed that 120 mg of GLE twice a day was not effective in reducing the overall incidence of dementia. In this researchtudy, five hundred and twenty-three individuals developed dementia with 92% classified as possible or probable AD or VaD with evidence of vascular disease of the brain, offering hope for the vasculopathies in AD and VaD [53][73]. AWe do not know enough about why the outcomes were mixed is not known enough, but poor diet in the elderly and the co-metabolism of the host with select gut microbiota can contribute or modulate the pathogenesis of AD and VaD, which could explain any variant results. Nevertheless, an association was found between gut microbial composition and arterial stiffness and the GM has an influence on the aging of the vasculature [54][74]. Therefore, one must consider the impact a poor diet has on the gut microbiota composition and overall health in the elderly [55][75]. It was observed that in the elderly population over 100 years of age, centenarians, the structure of their human gut microbiota greatly differs from that of young adults and people 70 years of age. Centenarians were described as having an increased number of facultative anaerobes leading to an increased inflammatory status [56][76].
