Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Catherine Yang and Version 1 by Yu-Chen Chen.
Neurogenic lower urinary tract dysfunction, common in patients with chronic spinal cord injury, inevitably results in urological complications. To address neurogenic lower urinary tract dysfunction after spinal cord injury, proper and adequate bladder management is important in spinal cord injury rehabilitation, with the goal and priorities of the protection of upper urinary tract function, maintaining continence, preserving lower urinary tract function, improvement of spinal cord injury (SCI) patients’ quality of life, achieving compatibility with patients’ lifestyles, and decreasing urological complications.
- spinal cord injuries
- urinary catheterization
- bladder
1. Urological Complications in Chronic SCI Patients
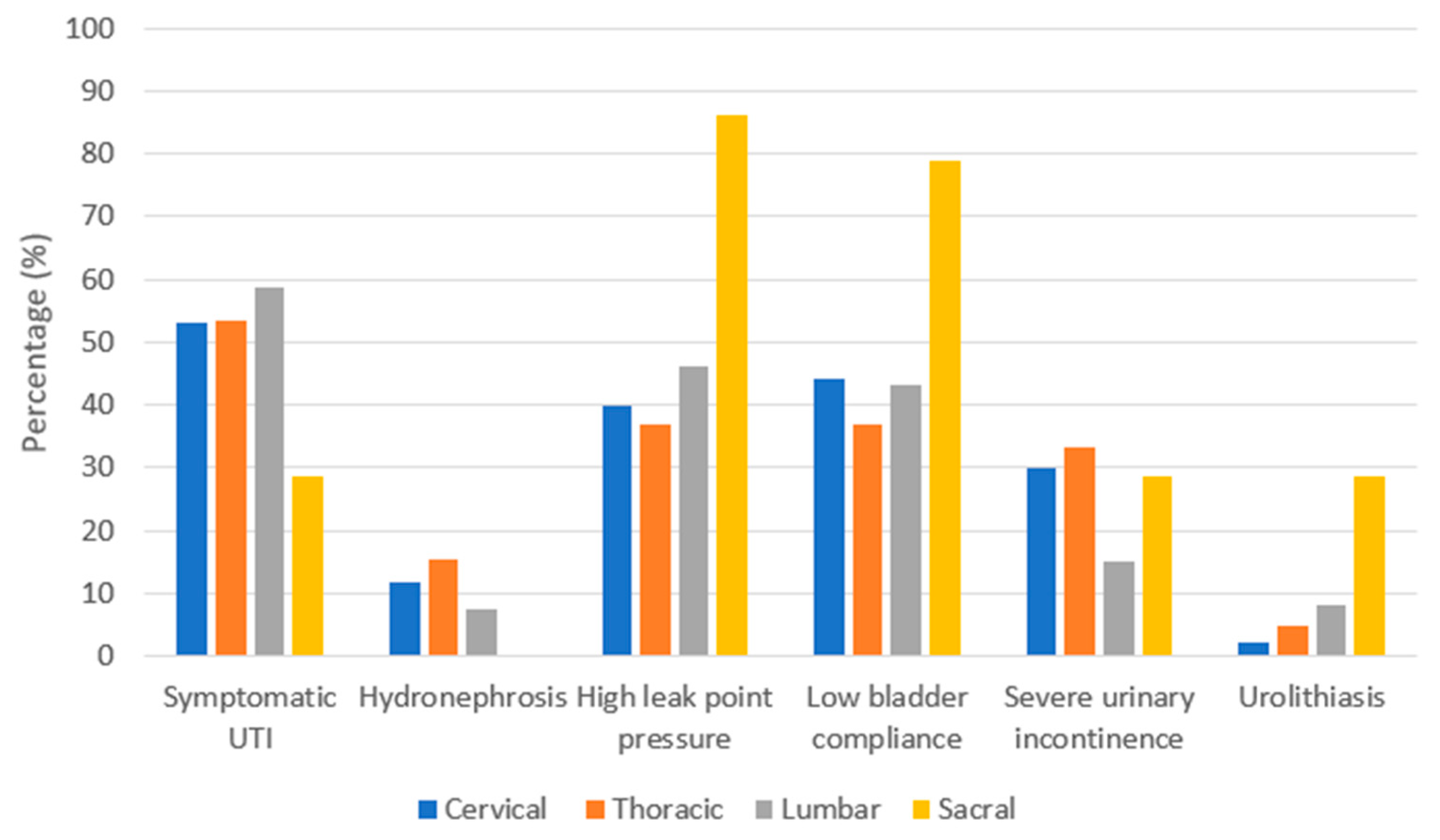
As summarized in Figure 1, NLUTD gradually and inevitably results in urological complications [16[1][2][3],17,18], which are closely related to each other. The rate of urological complications remains high in patients with chronic SCI [12,19][4][5]. Furthermore, the level of SCI and urological complications are closely associated [20,21][6][7]. As summarized in Figure 2, Chen et al. analyzed urological complications based on different SCI levels and reported that severe UI occurred significantly in patients with cervical and thoracic SCI, whereas urolithiasis was found to be more significant in patients with sacral SCI than in other levels of SCI [12][4]. Weld et al. analyzed bladder dysfunction based on urodynamic findings, and high rates of poor bladder compliance and high detrusor leak points were found in patients with sacral injuries [22][8]. However, patients with combined suprasacral and sacral injuries may have relatively unpredictable urodynamic findings and different estimated voiding dysfunction. Therefore, it is important to screen high-risk patients with SCI, especially when the detrusor leak-point pressure is higher than 40 cm H2O, indicating that the upper urinary tract is endangered [23,24][9][10].
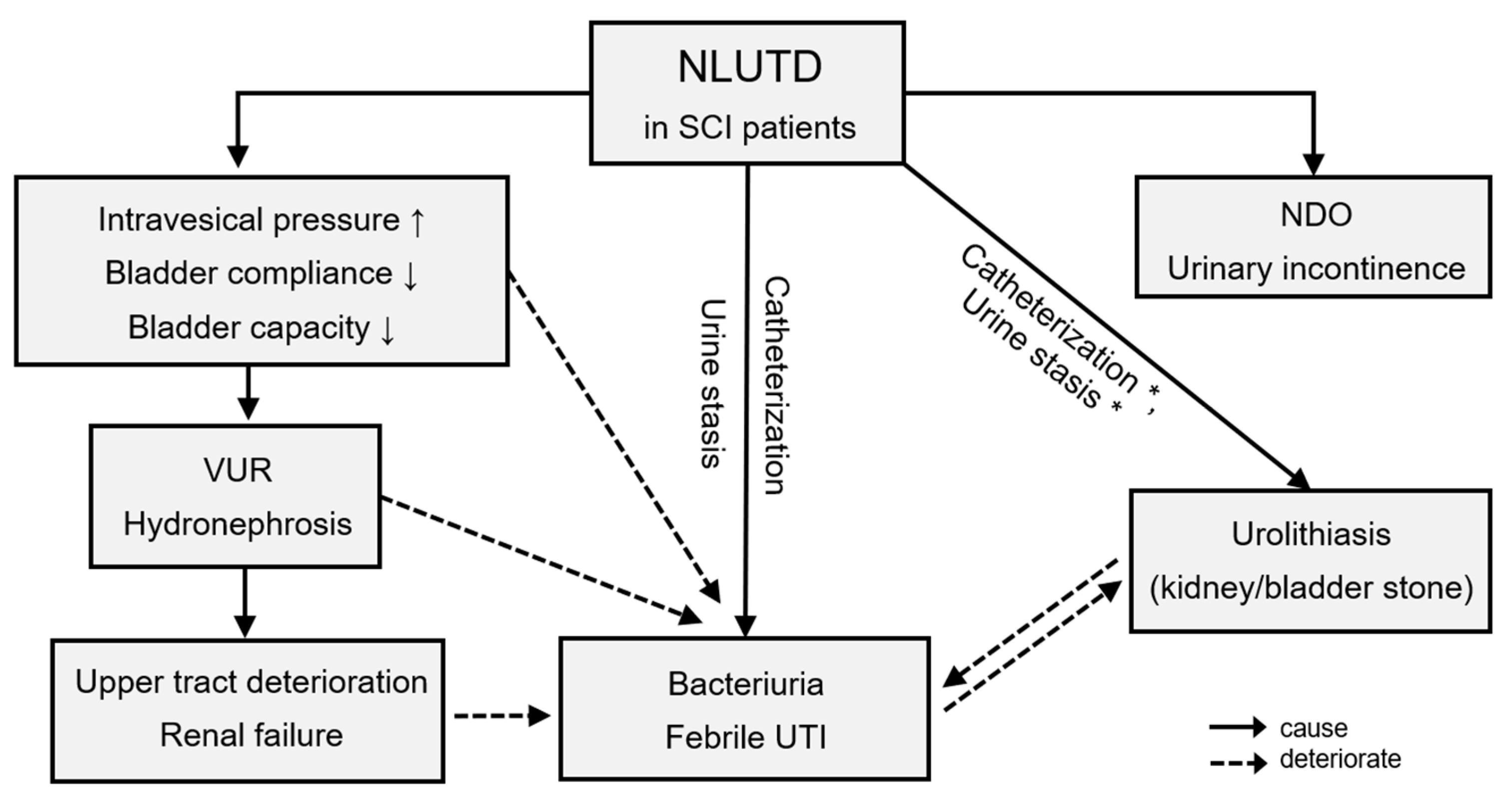
Figure 1. Urological complications in chronic spinal cord injury patients. * Catheterization and urine stasis were mainly related to bladder stones. Abbreviations: SCI, spinal cord injury; NLUTD, neurogenic lower urinary tract dysfunction; VUR, vesicoureteral reflux; NDO, neurogenic detrusor overactivity; UTI, urinary tract infection.
A UTI is the most common reason for SCI patients presenting to the emergency department and being re-hospitalized [25][11]. UTIs were reported to occur in 100% of patients with SCI in one study with at least a 40-year follow-up [26][12]. The incidence of UTI was reported to peak in the 1st and 10th five-year intervals [26][12]. Pickelsimer et al. conducted a 10-year follow-up study, in which UTI, hydronephrosis, and urolithiasis were the three main complications of NLUTD [27][13]. However, Chen et al. compared the urological complications at different time periods after SCI and found that there was no significant difference in the occurrence rate of urological complications among different SCI durations [12][4]. Another retrospective study found that the percentage of patients with urolithiasis was 20% and 80% before and after 20 years after SCI, respectively [22][8]. Overall, most complications initially occurred during the first 25 years after SCI. Close follow-up of UTIs, renal condition, and bladder function is important for all SCI patients and at any disease duration.
2. Bladder Management of Urge Urinary Incontinence (UUI) in Chronic SCI Patients
In an assessment of 236 patients with a mean follow-up of 24 years, 43% of patients reported UUI, with paraplegics reporting daily incontinence more frequently than tetraplegics (presumably because of catheter dependence in the latter group) [48][14]. Only 19% of patients used some form of medication for assistance in managing their incontinence. Surprisingly, CIC was associated with higher rates of UUI than other types of bladder management. In a study by Blanes et al., which included 60 patients with traumatic paraplegia, the complication rate of UUI was found to be more than twice that found in a previous report [49][15]. Current evidence shows that the effects on bladder function depend on the different levels and locations of SCI [20][6], which may potentially explain the different rates reported by these two studies. The appropriate management of NLUTD in patients with SCI is a major challenge for urologists. In most patients with suprasacral SCI who have neurogenic detrusor overactivity (NDO) with or without detrusor sphincter dyssynergia (DSD), bladder management by patients themselves depends on good hand dexterity, powerful abdominal muscle strength, intact bladder sensation, and coordination of the urethral sphincter during stimulation to voiding [22][8]. Regarding the medication for NDO, antimuscarinics are the most common treatment and are suggested as the first-line treatment by current guidelines [32][16]. The role of Beta-3-adrenergic receptor agonists, which are not yet approved by the FDA for the treatment of neurogenic bladder, is still unclear [50][17]. In a recent systemic review, mirabegron was shown to improve the storage symptoms of NLUTD and urologic QoL with very few side effects [51][18]. Improved maximum cystometric capacity and bladder compliance were demonstrated after treatment with mirabegron in only two studies with short follow-ups [52,53][19][20]; however, other studies showed no significant changes in the urodynamic parameters [54,55][21][22]. Vibegron, another novel Beta-3-adrenergic receptor agonist, was reported to improve bladder capacity and bladder compliance without apparent adverse effects in SCI patients with NLUTD in two recent studies with limited cases [56,57][23][24]. Further prospective studies are necessary for the role of Beta-3-adrenergic receptor agonists in the treatment of NDO. Currently, it is possible to use a botulinum toxin A (BoNT-A) injection over the bladder detrusor to decrease detrusor contractility [58,59][25][26]; a BoNT-A injection over the urethral sphincter to decrease urethral resistance [60,61][27][28]; or combine detrusor and urethral BoNT-A injections to spontaneously improve bladder storage and emptying [62][29]. Although the efficacy of BoNT-A over the sphincter was reported to be high with few side effects, this treatment is not licensed and the necessity of reinjection is its main disadvantage [60,63,64][27][30][31]. In addition, because of limited evidence from small studies, further randomized studies are still needed to comment on the effectiveness of BoNT-A and the optimal dose [65][32].3. Long-Term Complications and Satisfaction of Augmentation Enterocystoplasty (AE) in Chronic SCI Patients
Currently, the first-line treatment for NLUTD in SCI is anticholinergic agents, timely voiding schedules, and CIC in relatively good circumstances, followed by detrusor BoNT-A injections when the effects of conservative treatment are inadequate [32][16]. Repeat BoNT-A injections every 6–9 months are necessary to maintain the therapeutic effects of NDO, especially in patients with chronic SCI [58,96][25][33]. If the outcome is still refractory to repeated BoNT-A injections, intravesical pressure will remain high, which eventually leads to hydronephrosis, renal failure, and UUI, so more aggressive surgical treatment should be considered to obtain life-long therapeutic effects instead of periodic BoNT-A injections [71][34]. AE should be considered in patients with reduced bladder capacity and poor compliance due to refractory NLUTD [97,98][35][36]. This procedure is recommended for reconstructing the bladder and increasing the bladder capacity and, therefore, has been used to treat bladder dysfunction in adults and pediatric patients with myelomeningocele [99][37]. AE can effectively reduce intravesical pressure during bladder storage and increase bladder capacity in patients with end-stage bladder diseases or refractory detrusor overactivity [100][38]. Although AE is a procedure with long-term durability and high satisfaction, some major complications still exist [2][39]. Overall, 86.9% of 76 patients who underwent AE were well or moderately satisfied with the treatment outcomes, and the postoperative UI rate was only 16.5% in a large cohort by Wu et al. [101][40]. Moreover, 76% of patients required CIC, whereas others could void spontaneously with the Credé maneuver. Among the patients who needed CIC, some finally chose an indwelling transurethral catheter or cystostomy for convenient bladder emptying. In addition, AE is associated with a risk of bladder malignancy. A recent systemic review reported that the estimated incidence of developing a malignant tumor after AE ranged from 0 to 272.3 per 100,000 patients/year [102,103,104,105,106,107,108,109,110][41][42][43][44][45][46][47][48][49] and that 51.6% of the malignancy was adenocarcinoma. Up to 90% of bladder malignancies were diagnosed more than 10 years after AE. Although the exact mechanism of carcinogenesis after AE is still unclear, several factors, such as bacteriuria, chronic inflammation, and urinary hyperosmolality conditions, are reported to be possibly involved [111,112,113,114,115,116][50][51][52][53][54][55]. The follow-up time for regular surveillance after AE is controversial; however, an annual cystoscopy starting 10 years after AE was recommended by most studies [102,105,117,118][41][44][56][57]. AE is usually performed during the final step of NDO treatment due to the relatively high rates of postoperative complications. In a small prospective study comparing the QoL between SCI patients who underwent AE (n = 16) and those who underwent repeat BoNT-A injections (n = 14), the continence rate and QoL index were both significantly higher in the AE group (continence rate: 87.5% vs. 42.3%, p = 0.0187; QoL index: 1.625 vs. 1.077, p = 0.037). The overall outcome was good and no patients post-AE had poor bladder compliance or higher intravesical pressure at the filling phase [119][58].References
- Lai, E.C.; Kao Yang, Y.H.; Kuo, H.C. Complication rate of neurogenic lower urinary tract dysfunction after spinal cord injury in Taiwan. Int. Urol. Nephrol. 2014, 46, 1063–1071.
- Lee, B.B.; A Cripps, R.; Fitzharris, M.; Wing, P.C. The global map for traumatic spinal cord injury epidemiology: Update 2011, global incidence rate. Spinal Cord 2014, 52, 110–116.
- Simpson, L.A.; Eng, J.J.; Hsieh, J.T.; Dalton, L. The health and life priorities of individuals with spinal cord injury: A systematic review. J. Neurotrauma 2012, 29, 1548–1555.
- Chen, S.; Jiang, Y.; Jhang, J.; Lee, C.; Kuo, H. Bladder management and urological complications in patients with chronic spinal cord injuries in Taiwan. Tzu Chi Med. J. 2014, 26, 25–28.
- Stillman, M.D.; Barber, J.; Burns, S.; Williams, S.; Hoffman, J.M. Complications of Spinal Cord Injury Over the First Year After Discharge From Inpatient Rehabilitation. Arch. Phys. Med. Rehabil. 2017, 98, 1800–1805.
- Jeong, S.J.; Cho, S.Y.; Oh, S.J. Spinal cord/brain injury and the neurogenic bladder. Urol. Clin. N. Am. 2010, 37, 537–546.
- Weld, K.J.; Graney, M.J.; Dmochowski, R.R. Clinical significance of detrusor sphincter dyssynergia type in patients with post-traumatic spinal cord injury. Urology 2000, 56, 565–568.
- Weld, K.J.; Dmochowski, R.R. Association of level of injury and bladder behavior in patients with post-traumatic spinal cord injury. Urology 2000, 55, 490–494.
- McGuire, E.J.; Cespedes, R.D.; O’Connell, H.E. Leak-point pressures. Urol. Clin. N. Am. 1996, 23, 253–262.
- Stöhrer, M.; Goepel, M.; Kondo, A.; Kramer, G.; Madersbacher, H.; Millard, R.; Rossier, R.; Wyndaele, J.-J. The standardization of terminology in neurogenic lower urinary tract dysfunction with suggestions for diagnostic procedures. Neurourol. Urodyn. 1999, 18, 139–158.
- Cardenas, D.D.; Hoffman, J.M.; Kirshblum, S.; McKinley, W. Etiology and incidence of rehospitalization after traumatic spinal cord injury: A multicenter analysis. Arch. Phys. Med. Rehabil. 2004, 85, 1757–1763.
- Gao, Y.; Danforth, T.; Ginsberg, D.A. Urologic Management and Complications in Spinal Cord Injury Patients: A 40- to 50-year Follow-up Study. Urology 2017, 104, 52–58.
- Pickelsimer, E.; Shiroma, E.J.; Wilson, D.A. Statewide investigation of medically attended adverse health conditions of persons with spinal cord injury. J. Spinal Cord Med. 2010, 33, 221–231.
- Hansen, R.B.; Biering-Sørensen, F.; Kristensen, J.K. Urinary incontinence in spinal cord injured individuals 10–45 years after injury. Spinal Cord 2010, 48, 27–33.
- Blanes, L.; Lourenco, L.; Carmagnani, M.I.; Ferreira, L.M. Clinical and socio-demographic characteristics of persons with traumatic paraplegia living in Sao Paulo, Brazil. Arq. Neuropsiquiatr. 2009, 67, 388–390.
- Blok, B.F.; Castro-Diaz, D.; Del Popolo, G.; Groen, J.; Hamid, R.; Karsenty, G.; Kessler, T.M.; Pannek, J. EAU Guidelines on Neuro-Urology; European Association of Urology: Arnhem, The Netherlands, 2022.
- Lin, C.T.; Chiang, B.J.; Liao, C.H. Perspectives of medical treatment for overactive bladder. Urol. Sci. 2020, 31, 91–98.
- El Helou, E.; Labaki, C.; Chebel, R.; El Helou, J.; Abi Tayeh, G.; Jalkh, G.; Nemr, E. The use of mirabegron in neurogenic bladder: A systematic review. World J. Urol. 2020, 38, 2435–2442.
- Park, J.S.; Lee, Y.S.; Lee, C.N.; Kim, S.H.; Kim, S.W.; Han, S.W. Efficacy and safety of mirabegron, a β3-adrenoceptor agonist, for treating neurogenic bladder in pediatric patients with spina bifida: A retrospective pilot study. World J. Urol. 2019, 37, 1665–1670.
- Krhut, J.; Borovička, V.; Bílková, K.; Sýkora, R.; Míka, D.; Mokriš, J.; Zachoval, R. Efficacy and safety of mirabegron for the treatment of neurogenic detrusor overactivity-Prospective, randomized, double-blind, placebo-controlled study. Neurourol. Urodyn. 2018, 37, 2226–2233.
- Welk, B.; Hickling, D.; McKibbon, M.; Radomski, S.; Ethans, K. A pilot randomized-controlled trial of the urodynamic efficacy of mirabegron for patients with neurogenic lower urinary tract dysfunction. Neurourol. Urodyn. 2018, 37, 2810–2817.
- Wöllner, J.; Pannek, J. Initial experience with the treatment of neurogenic detrusor overactivity with a new β-3 agonist (mirabegron) in patients with spinal cord injury. Spinal Cord 2016, 54, 78–82.
- Aoki, K.; Momose, H.; Gotoh, D.; Morizawa, Y.; Hori, S.; Nakai, Y.; Miyake, M.; Anai, S.; Torimoto, K.; Tanaka, N.; et al. Video-urodynamic effects of vibegron, a new selective β3-adrenoceptor agonist, on antimuscarinic-resistant neurogenic bladder dysfunction in patients with spina bifida. Int. J. Urol. 2022, 29, 76–81.
- Matsuda, K.; Teruya, K.; Uemura, O. Urodynamic effect of vibegron on neurogenic lower urinary tract dysfunction in individuals with spinal cord injury: A retrospective study. Spinal Cord 2022, 60, 716–721.
- Schurch, B.; Stöhrer, M.; Kramer, G.; Schmid, D.M.; Gaul, G.; Hauriet, D. Botulinum-A toxin for treating detrusor hyperreflexia in spinal cord injured patients: A new alternative to anticholinergic drugs? Preliminary results. J. Urol. 2000, 164, 692–697.
- Reitz, A.; Stöhrer, M.; Kramer, G.; Del Popolo, G.; Chartier-Kastler, E.; Pannek, J.; Burgdörfer, H.; Göcking, K.; Madersbacher, H.; Schumacher, S.; et al. European experience of 200 cases treated with botulinum-A toxin injections into the detrusor muscle for urinary incontinence due to neurogenic detrusor overactivity. Eur Urol. 2004, 45, 510–515.
- Schurch, B.; Hauri, D.; Rodic, B.; Curt, A.; Meyer, M.; Rossier, A.B. Botulinum-A toxin as a treatment of detrusor-sphincter dyssynergia: A prospective study in 24 spinal cord injury patients. J. Urol. 1996, 155, 1023–1029.
- Kuo, H.C. Botulinum A toxin urethral injection for the treatment of lower urinary tract dysfunction. J. Urol. 2003, 170, 1908–1912.
- Schulte-Baukloh, H.; Weiss, C.; Stolze, T.; Herholz, J.; Sturzebecher, B.; Miller, K.; Knispel, H.H. Botulinum-A toxin detrusor and sphincter injection in treatment of overactive bladder syndrome: Objective outcome and patient satisfaction. Eur. Urol. 2005, 48, 984–990.
- Dykstra, D.D.; Sidi, A.A. Treatment of detrusor-sphincter dyssynergia with botulinum A toxin: A doubleblind study. Arch. Phys. Med. Rehabil. 1990, 71, 24.
- Huang, M.; Chen, H.; Jiang, C.; Xie, K.; Tang, P.; Ou, R.; Zeng, J.; Liu, Q.; Li, Q.; Huang, J.; et al. Effects of botulinum toxin A injections in spinal cord injury patients with detrusor overactivity and detrusor sphincter dyssynergia. J. Rehabil. Med. 2016, 48, 683.
- Utomo, E.; Groen, J.; Blok, B.F. Surgical management of functional bladder outlet obstruction in adults with neurogenic bladder dysfunction. Cochrane Database Syst. Rev. 2014, 24, CD004927.
- Kuo, H.C. Urodynamic evidence of effectiveness of botulinum A toxin injection in treatment of detrusor overactivity refractory to anticholinergic agents. Urology 2004, 63, 868–872.
- Goldwasser, B.; Webster, G.D. Augmentation and substitution enterocystoplasty. J. Urol. 1986, 135, 215–224.
- Chen, J.L.; Kuo, H.C. Long-term outcomes of augmentation enterocystoplasty with an ileal segment in patients with spinal cord injury. J. Formos. Med. Assoc. 2009, 108, 475–480.
- Krebs, J.; Bartel, P.; Pannek, J. Functional outcome of supratrigonal cystectomy and augmentation ileocystoplasty in adult patients with refractory neurogenic lower urinary tract dysfunction. Neurourol. Urodyn. 2016, 35, 260–266.
- Lendvay, T.S.; Cowan, C.A.; Mitchell, M.M.; Joyner, B.D.; Grady, R.W. Augmentation cystoplasty rates at children’s hospitals in the United States: A Pediatric Health Information System database study. J. Urol. 2006, 176, 1716–1720.
- Reyblat, P.; Ginsberg, D.A. Augmentation cystoplasty: What are the indications? Curr. Urol. Rep. 2008, 9, 452–458.
- Ku, J.H. The management of neurogenic bladder and quality of life in spinal cord injury. BJU Int. 2006, 98, 739–745.
- Wu, S.Y.; Kuo, H.C. A real-world experience with augmentation enterocystoplasty-High patient satisfaction with high complication rates. Neurourol. Urodyn. 2018, 37, 744–750.
- Ali-El-Dein, B.; El-Tabey, N.; Abdel-Latif, M.; Abdel-Rahim, M.; El-Bahnasawy, M.S. Late uro-ileal cancer after incorporation of ileum into the urinary tract. J. Urol. 2002, 167, 84–87.
- Husmann, D.A.; Rathbun, S.R. Long-term follow up of enteric bladder augmentations: The risk for malignancy. J. Pediatr. Urol. 2008, 4, 381–385.
- Higuchi, T.; Granberg, C.; Fox, J.; Husmann, D. Augmentation cystoplasty and risk of neoplasia: Fact, fiction and controversy. J. Urol. 2010, 184, 2492–2496.
- Kälble, T.; Hofmann, I.; Riedmiller, H.; Vergho, D. Tumor growth in urinary diversion: A multicenter analysis. Eur. Urol. 2011, 60, 1081–1086.
- Castellan, M.; Gosalbez, R.; Perez-Brayfield, M.; Healey, P.; McDonald, R.; Labbie, A.; Lendvay, T. Tumor in bladder reservoir after gastrocystoplasty. J. Urol. 2007, 178, 1771–1774.
- Vemulakonda, V.M.; Lendvay, T.S.; Shnorhavorian, M.; Joyner, B.D.; Kaplan, H.; Mitchell, M.E.; Grady, R.W. Metastatic adenocarcinoma after augmentation gastrocystoplasty. J. Urol. 2008, 179, 1094–1097.
- Soergel, T.M.; Cain, M.P.; Misseri, R.; Gardner, T.A.; Koch, M.O.; Rink, R.C. Transitional cell carcinoma of the bladder following augmentation cystoplasty for the neuropathic bladder. J. Urol. 2004, 172, 1649–1652.
- Kispal, Z.; Balogh, D.; Erdei, O.; Kehl, D.; Juhász, Z.; Vastyan, A.M.; Farkas, A.; Pinter, A.B.; Vajda, P. Complications after bladder augmentation or substitution in children: A prospective study of 86 patients. BJU Int. 2011, 108, 282.
- Biardeau, X.; Chartier-Kastler, E.; Rouprêt, M.; Phé, V. Risk of malignancy after augmentation cystoplasty: A systematic review. Neurourol. Urodyn. 2016, 35, 675–682.
- Higgy, N.A.; Verma, A.K.; Ertürk, E.; Oberley, T.D.; El-Aaser, A.A.; El-Merzabani, M.M.; Bryan, G.T. Escherichia coli infection of the urinary bladder: Induction of tumours in rats receiving nitrosamine precursors and augmentation of bladder carcinogenesis by N-nitrosobutyl (4-hydroxybutyl) amine. IARC Sci. Publ. 1987, 84, 380–383.
- Nurse, D.E.; Mundy, A.R. Cystoplasty infection and cancer. Neurourol. Urodyn. 1989, 8, 343–344.
- Vajda, P.; Kaiser, L.; Magyarlaki, T.; Farkas, A.; Vastyan, A.M.; Pinter, A.B. Histological findings after colocystoplasty and gastrocystoplasty. J. Urol. 2002, 168, 698–701.
- Austen, M.; Kalble, T. Secondary malignancies in different forms of urinary diversion using isolated gut. J. Urol. 2004, 172, 831–838.
- Malone, M.J.; Izes, J.K.; Hurley, L.J. Carcinogenesis: The fate of intestinal segments used in urinary reconstruction. Urol. Clin. N. Am. 1997, 24, 723–728.
- Dixon, B.P.; Chu, A.; Henry, J.; Kim, R.; Bissler, J.J. Increased cancer risk of augmentation cystoplasty: Possible role for hyperosmolal microenvironment on DNA damage recognition. Mutat. Res. 2009, 670, 88–95.
- Hamid, R.; Greenwell, T.; Nethercliffe, J.M.; Freeman, A.; Venn, S.N.; Woodhouse, C.R.J. Routine surveillance cystoscopy for patients with augmentation and substitution cystoplasty for benign urological conditions: Is it necessary? BJU Int. 2009, 104, 392–395.
- Shaw, J.; Lewis, M.A. Bladder augmentation surgery—What about the malignant risk? Eur. J. Pediatr. Surg. 1999, 9, 39–40.
- Anquetil, C.; Abdelhamid, S.; Gelis, A.; Fattal, C. Botulinum toxin therapy for neurogenic detrusor hyperactivity versus augmentation enterocystoplasty: Impact on the quality of life of patients with SCI. Spinal Cord 2016, 54, 1031–1035.
More
