Once fundamental difficulties such as active sites and selectivity are fully resolved, metal-free catalysts such as 3D graphene or carbon nanotubes (CNT) are very cost-effective substitutes for the expensive noble metals used for catalyzing CO
2
. A viable method for converting environmental wastes into useful energy storage or industrial wealth, and one which also addresses the environmental and energy problems brought on by emissions of CO
2
, is CO
2
hydrogenation into hydrocarbon compounds. The creation of catalytic compounds and knowledge about the reaction mechanisms have received considerable attention. Numerous variables affect the catalytic process, including metal–support interaction, metal particle sizes, and promoters. CO
2
hydrogenation into different hydrocarbon compounds like lower olefins, alcoholic composites, long-chain hydrocarbon composites, and fuels, in addition to other categories.
- 3D nanomaterial
- carbon nanotube
- graphene
- catalyst
1. Introduction
2. Characteristics and Applications of Carbon Nanotubes-based Catalyst
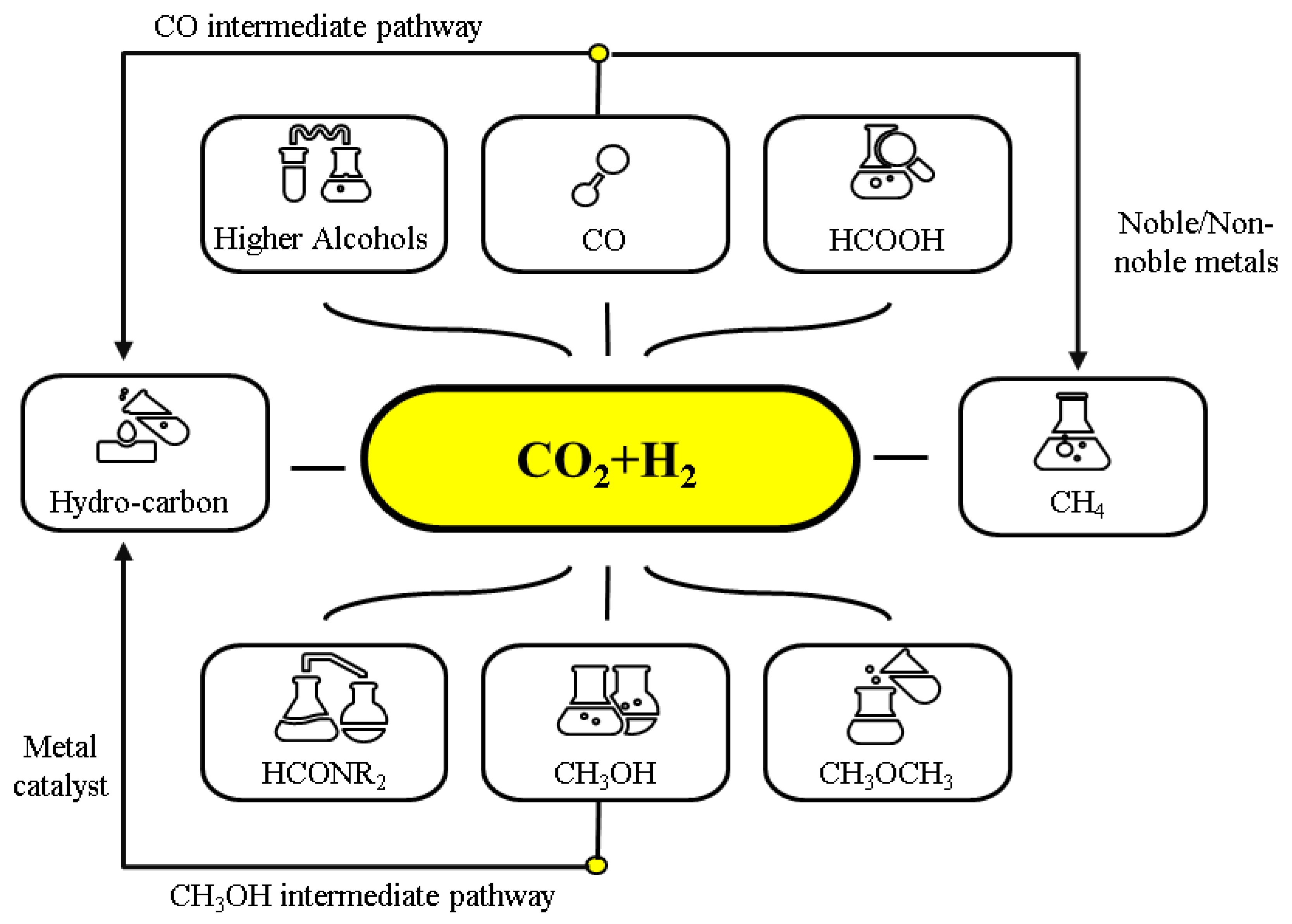
3. CO2 Hydrogenation into Hydrocarbons and Oxygenated Hydrocarbons
| Catalysts | Metal/Metal-free | Preparation Method | Process Type | Conversion | Selectivity | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cu-Zn-Al oxide and HB zeolite | Metal | Co-precipitation method | The production of C | 2+ | hydrocarbons by CO | 2 | hydrogenation. | 2.8% | 12.6 C-mol% | ||||||
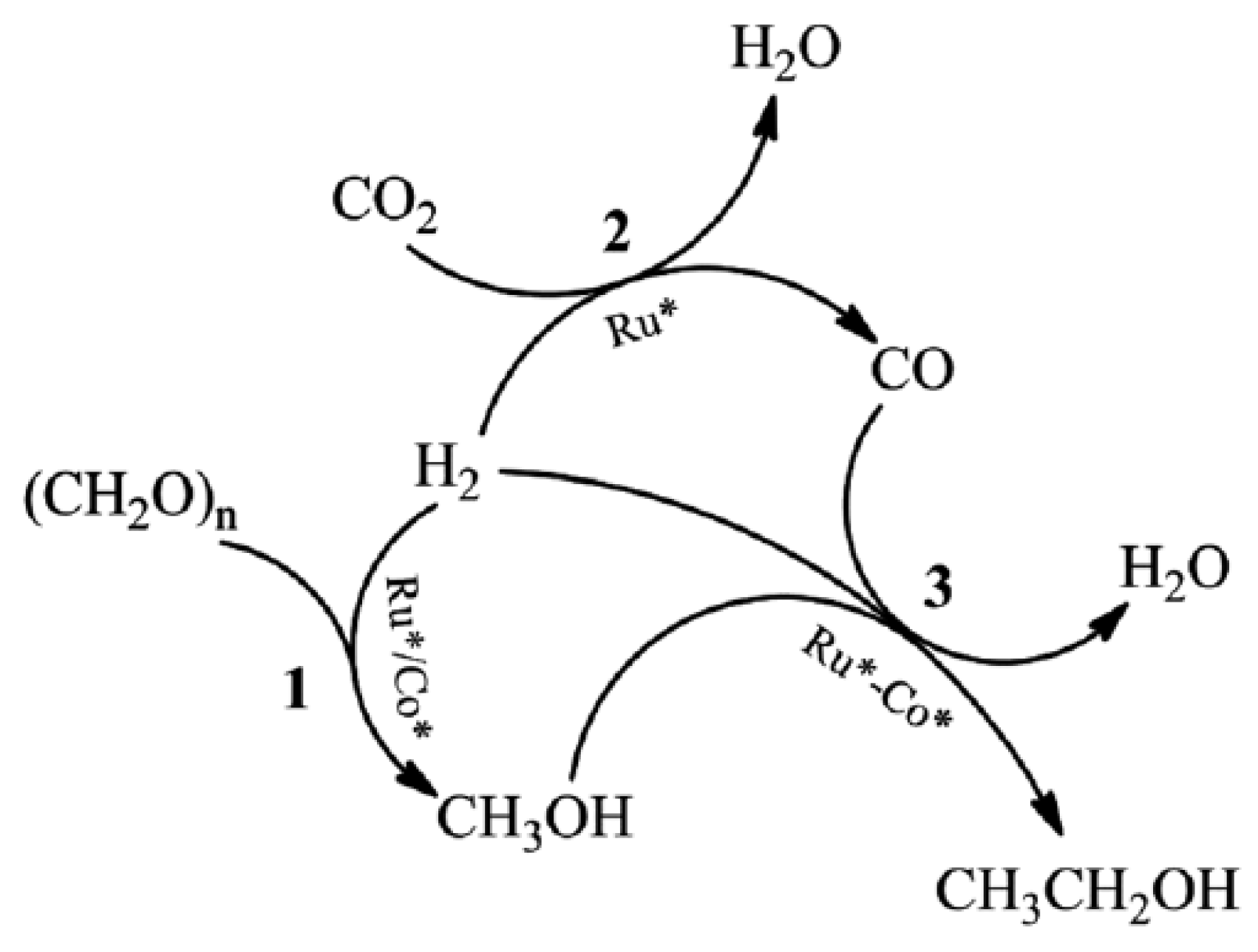
| Ru-Co | Metal | - | The production of ethanol from paraformaldehyde, CO | 2 | , and H | 2 | - | 50.9 C-mol% | |||||||
| WSe | 2 | -graphene | Metal | Ultra-sonication method | Photocatalytic reduction of CO | 2 | into CH | 3 | OH | 5.0278 μmol g | −1 | h | −1 | . | - |
| WSe | 2 | -graphene-TiO | 2 | Hybrid | Ultra-sonication method | CO | 2 | reduction to CH | 3 | OH | 6.3262 μmol g | −1 | h | −1 | - |
| hydroxide-mediated Cu | Metal | Hydroxide-mediated abrupt reaction interface | CO | 2 | conversion to ethylene | 70% | 65% | ||||||||
| CoPc/CNT | Hybrid | CO | 2 | reduction to methanol | Dispersion process | 40% | - | ||||||||
| Fe | 2 | O | 3 | @K | 2 | CO | 3 | Metal | CO | 2 | conversion to olefins | Mortar mixing | 40% | 60% |
References
- Ma, R.; Xu, B.; Zhang, X. Catalytic partial oxidation (CPOX) of natural gas and renewable hydrocarbons/oxygenated hydrocarbons—A review. Catal. Today 2019, 338, 18–30.
- Hasan, S.Z.; Ahmad, K.N.; Isahak, W.N.R.W.; Pudukudy, M.; Masdar, M.S.; Jahim, J.M. Synthesis, Characterisation and Catalytic Activity of NiO supported Al2O3 for CO2 Hydrogenation to Carboxylic Acids: Influence of Catalyst Structure. IOP Conf. Ser. Earth Environ. Sci. 2019, 268, 012079.
- Palmeri, N.; Chiodo, V.; Freni, S.; Frusteri, F.; Bart, J.; Cavallaro, S. Hydrogen from oxygenated solvents by steam reforming on Ni/Al2O3 catalyst. Int. J. Hydrogen Energy 2008, 33, 6627–6634.
- Kahn, B. Earth’s CO2 Passes the 400 PPM Threshold—Maybe Permanently. Sci. Am. 2016. Available online: https://www.scientificamerican.com/article/earth-s-co2-passes-the-400-ppm-threshold-maybe-permanently/ (accessed on 1 November 2022).
- Capros, P.; Tasios, N.; De Vita, A.; Mantzos, L.; Paroussos, L. Model-based analysis of decarbonising the EU economy in the time horizon to 2050. Energy Strat. Rev. 2012, 1, 76–84.
- European Commission. A Roadmap for Moving to a Competitive Low Carbon Economy in 2050. COM(2011) 112 Final; European Commission: Brussels, Belgium, 2011; Volume 34, pp. 1–34.
- Dimitriou, I.; García-Gutiérrez, P.; Elder, R.H.; Cuéllar-Franca, R.M.; Azapagic, A.; Allen, R.W.K. Carbon dioxide utilisation for production of transport fuels: Process and economic analysis. Energy Environ. Sci. 2015, 8, 1775–1789.
- Ma, X.; Wang, X.; Song, C. “Molecular Basket” Sorbents for Separation of CO2 and H2S from Various Gas Streams. J. Am. Chem. Soc. 2009, 131, 5777–5783.
- Du, G.; Lim, S.; Yang, Y.; Wang, C.; Pfefferle, L.; Haller, G.L. Methanation of carbon dioxide on Ni-incorporated MCM-41 catalysts: The influence of catalyst pretreatment and study of steady-state reaction. J. Catal. 2007, 249, 370–379.
- Duyar, M.S.; Treviño, M.A.A.; Farrauto, R.J. Dual function materials for CO2 capture and conversion using renewable H2. Appl. Catal. B Environ. 2015, 168–169, 370–376.
- Lee, C.-Y.; Zhao, Y.; Wang, C.; Mitchell, D.R.G.; Wallace, G.G. Rapid formation of self-organised Ag nanosheets with high efficiency and selectivity in CO2 electroreduction to CO. Sustain. Energy Fuels 2017, 1, 1023–1027.
- Li, K.; Peng, B.; Peng, T. Recent Advances in Heterogeneous Photocatalytic CO2 Conversion to Solar Fuels. ACS Catal. 2016, 6, 7485–7527.
- Welch, A.J.; DuChene, J.S.; Tagliabue, G.; Davoyan, A.R.; Cheng, W.-H.; Atwater, H.A. Nanoporous Gold as a Highly Selective and Active Carbon Dioxide Reduction Catalyst. ACS Appl. Energy Mater. 2019, 2, 164–170.
- Xie, H.; Wang, T.; Liang, J.; Li, Q.; Sun, S. Cu-based nanocatalysts for electrochemical reduction of CO2. Nano Today 2018, 21, 41–54.
- Huang, H.; Jia, H.; Liu, Z.; Gao, P.; Zhao, J.; Luo, Z.; Yang, J.; Zeng, J. Understanding of Strain Effects in the Electrochemical Reduction of CO2: Using Pd Nanostructures as an Ideal Platform. Angew. Chem. Int. Ed. 2017, 56, 3594–3598.
- Ma, M.; Trześniewski, B.J.; Xie, J.; Smith, W.A. Selective and Efficient Reduction of Carbon Dioxide to Carbon Monoxide on Oxide-Derived Nanostructured Silver Electrocatalysts. Angew. Chem. Int. Ed. 2016, 55, 9748–9752.
- Su, P.; Xu, W.; Qiu, Y.; Zhang, T.; Li, X.; Zhang, H. Ultrathin Bismuth Nanosheets as a Highly Efficient CO2 Reduction Electrocatalyst. Chemsuschem 2018, 11, 848–853.
- Li, Q.; Fu, J.; Zhu, W.; Chen, Z.; Shen, B.; Wu, L.; Xi, Z.; Wang, T.; Lu, G.; Zhu, J.-J.; et al. Tuning Sn-Catalysis for Electrochemical Reduction of CO2 to CO via the Core/Shell Cu/SnO2 Structure. J. Am. Chem. Soc. 2017, 139, 4290–4293.
- Hu, X.-M.; Rønne, M.H.; Pedersen, S.U.; Skrydstrup, T.; Daasbjerg, K. Enhanced Catalytic Activity of Cobalt Porphyrin in CO2 Electroreduction upon Immobilization on Carbon Materials. Angew. Chem. Int. Ed. 2017, 56, 6468–6472.
- Chen, Q.; Tsiakaras, P.; Shen, P. Electrochemical Reduction of Carbon Dioxide: Recent Advances on Au-Based Nanocatalysts. Catalysts 2022, 12, 1348.
- Brouzgou, A.; Song, S.; Liang, Z.-X.; Tsiakaras, P. Non-Precious Electrocatalysts for Oxygen Reduction Reaction in Alkaline Media: Latest Achievements on Novel Carbon Materials. Catalysts 2016, 6, 159.
- Alaba, P.A.; Abbas, A.; Daud, W.M.W. Insight into catalytic reduction of CO2: Catalysis and reactor design. J. Clean. Prod. 2017, 140, 1298–1312.
- Centi, G.; Perathoner, S. CO2-based energy vectors for the storage of solar energy. Greenh. Gases Sci. Technol. 2011, 1, 21–35.
- Porosoff, M.D.; Yan, B.; Chen, J.G. Catalytic reduction of CO2 by H2 for synthesis of CO, methanol and hydrocarbons: Challenges and opportunities. Energy Environ. Sci. 2016, 9, 62–73.
- Gao, P.; Li, S.; Bu, X.; Dang, S.; Liu, Z.; Wang, H.; Zhong, L.; Qiu, M.; Yang, C.; Cai, J.; et al. Direct conversion of CO2 into liquid fuels with high selectivity over a bifunctional catalyst. Nat. Chem. 2017, 9, 1019–1024.
- Song, H.; Zhang, N.; Zhong, C.; Liu, Z.; Xiao, M.; Gai, H. Hydrogenation of CO2 into formic acid using a palladium catalyst on chitin. New J. Chem. 2017, 41, 9170–9177.
- Visconti, C.G.; Martinelli, M.; Falbo, L.; Infantes-Molina, A.; Lietti, L.; Forzatti, P.; Iaquaniello, G.; Palo, E.; Picutti, B.; Brignoli, F. CO2 hydrogenation to lower olefins on a high surface area K-promoted bulk Fe-catalyst. Appl. Catal. B Environ. 2017, 200, 530–542.
- Larmier, K.; Liao, W.-C.; Tada, S.; Lam, E.; Verel, R.; Bansode, A.; Urakawa, A.; Comas-Vives, A.; Copéret, C. CO2-to-Methanol Hydrogenation on Zirconia-Supported Copper Nanoparticles: Reaction Intermediates and the Role of the Metal-Support Interface. Angew. Chem. Int. Ed. 2017, 56, 2318–2323.
- Bai, S.; Shao, Q.; Wang, P.; Dai, Q.; Wang, X.; Huang, X. Highly Active and Selective Hydrogenation of CO2 to Ethanol by Ordered Pd–Cu Nanoparticles. J. Am. Chem. Soc. 2017, 139, 6827–6830.
- Sorcar, S.; Hwang, Y.; Lee, J.; Kim, H.; Grimes, K.M.; Grimes, C.A.; Jung, J.-W.; Cho, C.-H.; Majima, T.; Hoffmann, M.R.; et al. CO2, water, and sunlight to hydrocarbon fuels: A sustained sunlight to fuel (Joule-to-Joule) photoconversion efficiency of 1%. Energy Environ. Sci. 2019, 12, 2685–2696.
- Ajayan, P.M.; Ebbesen, T.W. Nanometre-size tubes of carbon. Rep. Prog. Phys. 1997, 60, 1025–1062.
- Ruoff, R.S.; Lorents, D.C. Mechanical and thermal properties of carbon nanotubes. Carbon 1995, 33, 925–930.
- Dong, X.; Zhang, H.-B.; Lin, G.-D.; Yuan, Y.-Z.; Tsai, K. Highly Active CNT-Promoted Cu–ZnO–Al2O3 Catalyst for Methanol Synthesis from H2/CO/CO2. Catal. Lett. 2003, 85, 237–246.
- Huang, J.; Zhang, Q.; Zhao, M.; Wei, F. A review of the large-scale production of carbon nanotubes: The practice of nanoscale process engineering. Chin. Sci. Bull. 2012, 57, 157–166.
- Schnorr, J.M.; Swager, T.M. Emerging Applications of Carbon Nanotubes. Chem. Mater. 2011, 23, 646–657.
- Zhang, Q.; Zuo, Y.-Z.; Han, M.-H.; Wang, J.-F.; Jin, Y.; Wei, F. Long carbon nanotubes intercrossed Cu/Zn/Al/Zr catalyst for CO/CO2 hydrogenation to methanol/dimethyl ether. Catal. Today 2010, 150, 55–60.
- Ozden, S.; Tiwary, C.S.; Hart, A.H.C.; Chipara, A.C.; Romero-Aburto, R.; Rodrigues, M.-T.F.; Taha-Tijerina, J.; Vajtai, R.; Ajayan, P.M. Density Variant Carbon Nanotube Interconnected Solids. Adv. Mater. 2015, 27, 1842–1850.
- Ozden, S.; Narayanan, T.N.; Tiwary, C.S.; Dong, P.; Hart, A.H.C.; Vajtai, R.; Ajayan, P.M. 3D Macroporous Solids from Chemically Cross-linked Carbon Nanotubes. Small 2015, 11, 688–693.
- Guiderdoni, C.; Estournes, C.; Peigney, A.; Weibel, A.; Turq, V.; Laurent, C. The preparation of double-walled carbon nanotube/Cu composites by spark plasma sintering, and their hardness and friction properties. Carbon 2011, 49, 4535–4543.
- Ozden, S.; Brunetto, G.; Karthiselva, N.S.; Galvão, D.S.; Roy, A.; Bakshi, S.R.; Tiwary, C.S.; Ajayan, P.M. Controlled 3D Carbon Nanotube Structures by Plasma Welding. Adv. Mater. Interfaces 2016, 3, 1500755.
- Al-Hakami, S.M.; Khalil, A.B.; Laoui, T.; Atieh, M.A. Fast Disinfection of Escherichia coli Bacteria Using Carbon Nanotubes Interaction with Microwave Radiation. Bioinorg. Chem. Appl. 2013, 2013, 458943.
- Khalid, N.; Majid, A.; Tahir, M.B.; Niaz, N.; Khalid, S. Carbonaceous-TiO2 nanomaterials for photocatalytic degradation of pollutants: A review. Ceram. Int. 2017, 43, 14552–14571.
- Chen, M.-L.; Zhang, F.-J.; Oh, W.-C. Synthesis, characterization, and photocatalytic analysis of CNT/TiO2 composites derived from MWCNTs and titanium sources. New Carbon Mater. 2009, 24, 159–166.
- Jauris, I.M.; Fagan, S.B.; Adebayo, M.A.; Machado, F.M. Adsorption of acridine orange and methylene blue synthetic dyes and anthracene on single wall carbon nanotubes: A first principle approach. Comput. Theor. Chem. 2016, 1076, 42–50.
- Zhang, W.; Li, G.; Liu, H.; Chen, J.; Ma, S.; An, T. Micro/nano-bubble assisted synthesis of Au/TiO2@CNTs composite photocatalyst for photocatalytic degradation of gaseous styrene and its enhanced catalytic mechanism. Environ. Sci. Nano 2019, 6, 948–958.
- Wang, W.; Wang, S.; Ma, X.; Gong, J. Recent advances in catalytic hydrogenation of carbon dioxide. Chem. Soc. Rev. 2011, 40, 3703–3727.
- Wang, D.; Xie, Z.; Porosoff, M.D.; Chen, J.G. Recent advances in carbon dioxide hydrogenation to produce olefins and aromatics. Chem 2021, 7, 2277–2311.
- Duyar, M.; Ramachandran, A.; Wang, C.; Farrauto, R.J. Kinetics of CO2 methanation over Ru/γ-Al2O3 and implications for renewable energy storage applications. J. CO2 Util. 2015, 12, 27–33.
- Mutz, B.; Carvalho, H.W.; Mangold, S.; Kleist, W.; Grunwaldt, J.-D. Methanation of CO2: Structural response of a Ni-based catalyst under fluctuating reaction conditions unraveled by operando spectroscopy. J. Catal. 2015, 327, 48–53.
- Younas, M.; Kong, L.L.; Bashir, M.J.K.; Nadeem, H.; Shehzad, A.; Sethupathi, S. Recent Advancements, Fundamental Challenges, and Opportunities in Catalytic Methanation of CO2. Energy Fuels 2016, 30, 8815–8831.
- Rönsch, S.; Schneider, J.; Matthischke, S.; Schlüter, M.; Götz, M.; Lefebvre, J.; Prabhakaran, P.; Bajohr, S. Review on methanation—From fundamentals to current projects. Fuel 2016, 166, 276–296.
- Potocnik, P. Natural Gas; BoD–Books on Demand; IntechOpen: London, UK, 2010.
- Thampi, K.R.; Kiwi, J.; Grätzel, M. Methanation and photo-methanation of carbon dioxide at room temperature and atmospheric pressure. Nature 1987, 327, 506–508.
- Koschany, F.; Schlereth, D.; Hinrichsen, O. On the kinetics of the methanation of carbon dioxide on coprecipitated NiAl(O)x. Appl. Catal. B Environ. 2016, 181, 504–516.
- Fujiwara, M.; Satake, T.; Shiokawa, K.; Sakurai, H. CO2 hydrogenation for C2+ hydrocarbon synthesis over composite catalyst using surface modified HB zeolite. Appl. Catal. B Environ. 2015, 179, 37–43.
- Zhang, J.; Qian, Q.; Cui, M.; Chen, C.; Liu, S.; Han, B. Synthesis of ethanol from paraformaldehyde, CO2 and H2. Green Chem. 2017, 19, 4396–4401.
- Le Duff, C.S.; Lawrence, M.J.; Rodriguez, P. Role of the Adsorbed Oxygen Species in the Selective Electrochemical Reduction of CO2 to Alcohols and Carbonyls on Copper Electrodes. Angew. Chem. 2017, 129, 13099–13104.
- Climent, V.; Feliu, J.M. Cyclic Voltammetry. In Encyclopedia of Interfacial Chemistry: Surface Science and Electrochemistry; Elsevier: Amsterdam, The Netherlands, 2018; pp. 48–74.
- Ezenarro, J.J.; Párraga-Niño, N.; Sabrià, M.; Del Campo, F.; Muñoz-Pascual, F.-X.; Mas, J.; Uria, N. Rapid Detection of Legionella pneumophila in Drinking Water, Based on Filter Immunoassay and Chronoamperometric Measurement. Biosensors 2020, 10, 102.
- Ali, A.; Oh, W.-C. Preparation of Nanowire like WSe2-Graphene Nanocomposite for Photocatalytic Reduction of CO2 into CH3OH with the Presence of Sacrificial Agents. Sci. Rep. 2017, 7, 1867.
- Biswas, R.U.D.; Ali, A.; Cho, K.Y.; Oh, W.-C. Novel synthesis of WSe2-Graphene-TiO2 ternary nanocomposite via ultrasonic technics for high photocatalytic reduction of CO2 into CH3OH. Ultrason. Sonochem. 2018, 42, 738–746.
- Dinh, C.-T.; Burdyny, T.; Kibria, M.G.; Seifitokaldani, A.; Gabardo, C.M.; de Arquer, F.P.G.; Kiani, A.; Edwards, J.P.; De Luna, P.; Bushuyev, O.S.; et al. CO2 electroreduction to ethylene via hydroxide-mediated copper catalysis at an abrupt interface. Science 2018, 360, 783–787.
- Ma, Z.; Porosoff, M.D. Development of Tandem Catalysts for CO2 Hydrogenation to Olefins. ACS Catal. 2019, 9, 2639–2656.
- Huan, T.N.; Corte, D.A.D.; Lamaison, S.; Karapinar, D.; Lutz, L.; Menguy, N.; Foldyna, M.; Turren-Cruz, S.-H.; Hagfeldt, A.; Bella, F.; et al. Low-cost high-efficiency system for solar-driven conversion of CO2 to hydrocarbons. Proc. Natl. Acad. Sci. USA 2019, 116, 9735–9740.
- Wu, Y.; Jiang, Z.; Lu, X.; Liang, Y.; Wang, H. Domino electroreduction of CO2 to methanol on a molecular catalyst. Nature 2019, 575, 639–642.
- Ramirez, A.; Ould-Chikh, S.; Gevers, L.; Chowdhury, A.D.; Abou-Hamad, E.; Aguilar-Tapia, A.; Hazemann, J.; Wehbe, N.; Al Abdulghani, A.J.; Kozlov, S.M.; et al. Tandem Conversion of CO2 to Valuable Hydrocarbons in Highly Concentrated Potassium Iron Catalysts. Chemcatchem 2019, 11, 2879–2886.
- Tada, S.; Otsuka, F.; Fujiwara, K.; Moularas, C.; Deligiannakis, Y.; Kinoshita, Y.; Uchida, S.; Honma, T.; Nishijima, M.; Kikuchi, R. Development of CO2-to-Methanol Hydrogenation Catalyst by Focusing on the Coordination Structure of the Cu Species in Spinel-Type Oxide Mg1–xCuxAl2O4. ACS Catal. 2020, 10, 15186–15194.
- Tada, S.; Kinoshita, H.; Ochiai, N.; Chokkalingam, A.; Hu, P.; Yamauchi, N.; Kobayashi, Y.; Iyoki, K. Search for solid acid catalysts aiming at the development of bifunctional tandem catalysts for the one-pass synthesis of lower olefins via CO2 hydrogenation. Int. J. Hydrogen Energy 2021, 46, 36721–36730.
- Rahmani, A.; Aubert, X.; Fagnon, N.; Nikravech, M. Liquid oxygenated hydrocarbons produced during reforming of CH4 and CO2 in a surface dielectric barrier discharge: Effects of steam on conversion and products distribution. J. Appl. Phys. 2021, 129, 193304.
- Islam, H.; Burheim, O.S.; Hihn, J.-Y.; Pollet, B. Sonochemical conversion of CO2 into hydrocarbons: The Sabatier reaction at ambient conditions. Ultrason. Sonochem. 2021, 73, 105474.
- Tian, G.; Wu, Y.; Wu, S.; Huang, S.; Gao, J. Influence of Mn and Mg oxides on the performance of In2O3 catalysts for CO2 hydrogenation to methanol. Chem. Phys. Lett. 2022, 786, 139173.
- Chernyak, S.A.; Kustov, A.L.; Stolbov, D.N.; Tedeeva, M.A.; Isaikina, O.Y.; Maslakov, K.I.; Usol’Tseva, N.V.; Savilov, S.V. Chromium catalysts supported on carbon nanotubes and graphene nanoflakes for CO2-assisted oxidative dehydrogenation of propane. Appl. Surf. Sci. 2022, 578, 152099.
