1. High Fat Diet, Gut Microbiota and Dyslipidemia
High fat diets (HFD), such as the WD, are comprised by increased amounts of animal proteins, saturated fats and sugars, with low levels of fibers and phenols. Additionally, chronic HFD intake results in decreased total microbiota richness with a shift towards overgrowths of lipopolysaccharides (LPS) containing, Gram-negative enteric bacteria, which are shown to contribute to metabolic endotoxemia
[1][2][106,107]. More specifically, elevated concentrations of circulating LPS induced by a chronic HFD facilitates increased binding to its receptor, Toll-like receptor 4 (TLR4), to activate innate and adaptive immunity triggering a pro-inflammatory cascade
[3][108]. TLR4 activation has been implicated in the development of dyslipidemia and associated atherosclerosis, through enhanced release of pro-inflammatory cytokines like IL-8, IL-1beta and TNF-alpha
[4][109]. Results
from th
ereis study also showed TLR-4 mediated oxidation of low-density lipoproteins, which are known to accumulate in unstable plaques that deposit in the vasculature. As such, the combination of altered lipid metabolism as seen through increased LDL combined with LPS/TLR-4 mediated oxidization, worsens dyslipidemia leading to more severe associated clinical sequelae (
Figure 12). It is also shown that lipoproteins, particularly HDL, has a protective effect against LPS-mediated metabolic endotoxemia by facilitating clearing of LPS from circulation
[3][108]. Therefore, decreases in HDL and increases in relative concentrations of unfavorable Gram-negative bacteria resulting from HFD-induced dyslipidemia, further worsens hypercholesterolemic states through inability to clear excess LPS.
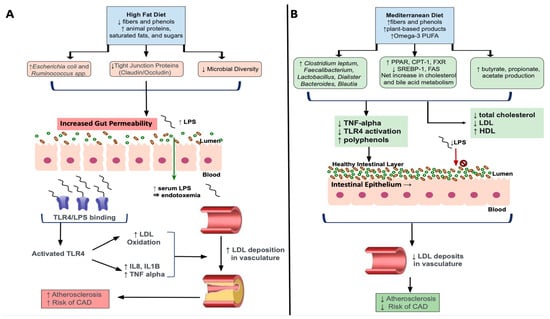
Figure 12. Effects of a High-Fat Diet and a Mediterranean Diet on Dyslipidemia and Atherosclerosis. (A) The High-Fat Diet is composed of large quantities of animal proteins, saturated fats and sugars with decreased fibers and phenols. HFD feeding increase Gram-negative bacteria Ruminococcus and Escherichia spp. while intestinal tight junction proteins occludin and claudin and microbial diversity are reduced. This leads to increased gut permeability, LPS enters the blood stream, resulting in metabolic endotoxemia. Lipopolysaccharides bind TLR-4 on circulating host cells to increase pro-inflammatory cytokines, increased inflammation and reactive oxygen species. Oxidized LDL builds up, causing plaques and increased risk of atherosclerosis. (B) The Mediterranean diet is rich in fibers and phenols, plant-based products, and omega-3 poly-unsaturated fatty acids. The MD increases Clostridium leptum and key genera Faecalibacterium, Lactobacillus, Dialister, Bacteroides, Dialister, Bacteroides and Blautia. It increases SCFA, while upregulating cholesterol and bile acid metabolism by increasing PPAR, CPT-1, FXR activity and decreasing SREBP-1 and FAS activity. This lead to decreased TNF-alpha, TLR4 activation, total cholesterol and LDL, increase HDL and supports intestinal barrier integrity. Less LDL builds up, lessening atherosclerosis and CAD risk. Abbreviations: LPS, Lipopolysaccharides; TLR4, Toll-like receptor 4; LDL, Low-density lipoprotein; IL, interleukin; TNF, Tumor necrosis factor; CAD, coronary artery disease; PUFA, poly-unsaturated fatty acids; PPAR, Peroxisome proliferator activated receptor; CPT-1, Carnitine palmitoyl transferase I; SREBP-1, sterol-regulatory element-binding protein 1; FAS, fatty-acid synthase; HDL, High-density lipoprotein.
Further, it has been shown that microbial species like
Escherichia coli and
Ruminococcus spp., that are elevated after HFD feeding, contribute to increased intestinal barrier permeability, allowing for LPS to translocate into the bloodstream and cause systemic low-grade inflammation characteristic of dyslipidemia
[5][110]. In turn, high-fat diets and associated dyslipidemia chronically activate the NLRP3 inflammasome, which plays a role in inducing macrophage activity and release of proinflammatory cytokines like interleukin-1 (IL-1)
[6][111]. In particular, sterol regulatory element binding protein 2 (SREBP-2), a transcription factor that facilitates lipogenesis, complexed with SREBP cleavage-activating protein (SCAP) contributes to NLRP3 inflammasome assembly
[7][112]. This, in turn, affects cholesterol biosynthesis signaling with NLRP3 induced inflammation in macrophages. Excessive NLRP3-mediated release of proinflammatory cytokines has been associated with downregulation or decreased expression of the LDL-receptor
[8][113]. The LDL-receptor has been shown to be downregulated in chronic high fat intake
[9][114]. Taken together, these findings show how chronic high-fat diets can contribute to metabolic endotoxemia and dyslipidemia through upregulation of key pro-inflammatory factors including NLRP3.
2. Mediterranean Diet, Gut Microbiota and Dyslipidemia
The Mediterranean Diet (MD) consists of plant-based ingredients including wheats, cereals, nuts, fruits, vegetable, omega-3 polyunsaturated fatty acids, with high amounts of fibers and polyphenols
[10][8]. Collectively, these ingredients have been shown to have beneficial effects on lipid imbalances through their antioxidant and anti-inflammatory effects
[11][12][115,116] as shown via reduction in TNF-alpha and LPS concentrations
[13][14][117,118]. Further, diets high in polyphenols, known as indigestible phytochemicals, that are abundant in plant-based foods, increase production of beneficial SCFAs because of their interaction with colonic microbiota
[15][119]. For example, the polyphenol, resveratrol, specifically has been found to increase the SCFA-producing bacterial genera,
Allobaculum,
Bacterioides, and
Blautia while also inhibiting TLR-4
[16][17][18][120,121,122]. Further, the rich plant-based MD increases fiber-derived SCFA by the gut microbiota, such as acetate, propionate, and butyrate
[15][119] and has been shown to decrease total cholesterol and LDL cholesterol
[19][123] (
Figure 12). As described in the previous sections, SCFA exert a myriad of beneficial effects including attenuating the progression, or preventing against, the onset of dyslipidemia.
Several studies have shown that low carbohydrate (LC) diets significantly improve the Bacteroidetes/Firmicutes ratio, as well as important metabolic markers of dyslipidemia
[20][124]. More specifically, the LC diet reduced fatty acids associated with de novo lipogenesis pathways, while increasing omega-3 PUFA that are shown to exert anti-inflammatory and anti-hypertriglyceridemia effects
[21][125]. Importantly, microbiota changes after LC were also associated with increased HDL and decreased triglycerides. This is supported by findings showing that vegetable oils particularly omega-3 PUFA and phytonutrients, had beneficial effects on serum lipid profiles including HDL, TG, and apolipoprotein B in hypercholesterolemic patients
[22][126]. Hypocholesterolemia has been associated with increased levels of
Clostridium leptum, known to be involved in promoting increased cholesterol and bile acid metabolism
[23][127]. Therefore, vegetable oils such as omega-3 PUFA, a key component of the MD has an important role in maintaining a healthy lipid balance.
Further, oatmeal, a food option within the umbrella of the MD, improve cardiometabolic parameters in patients with dyslipidemia through key changes in gut microbiota profile
[24][128]. These changes include increased beneficial bacteria like
Akkermansia along with SCFA-producing
Faecalibacterum,
Dialister,
Lactobacillus, with decreases within the
Rumminococcacae family.
Akkermansia spp. are shown to improve metabolic parameters through reducing oxidative stress, reducing fat mass, insulin resistance and dyslipidemia in a rodent model
[25][26][129,130]. Therefore, these findings of elevated
Akkermansia and SCFA-producing bacterial species after oatmeal were associated with decreased total cholesterol and LDL with increased amounts of serum antioxidant capacity. Further, Sun et al. showed that oat-based foods (OF) rich in beta-glucans, when compared to high-fat diets (HFD) and control diets had significant reductions in plasma total cholesterol (TC), low-density lipoprotein (LDL), and triglycerides (TG), along with increased concentrations in several SCFAs including butyrate, propionate, and acetate
[27][131]. Similarly, it has recently been shown that flavonoids, a component of whole-grain oat, regulates bile acid pathways to reduce hyperlipidemia induced by chronic HFD feeding
[28][132]. Flavonoids upregulate expression of PPAR, carnitine palmitoyl transferase I (CPT-1) and FXR, while down-regulate SREBP-1 and fatty acid synthase (FAS). As mentioned, PPAR contributes to the breakdown of fatty acids via beta-oxidation by inducing CPT-1 activity while FXR regulates bile acid synthesis and transport by promoting efflux to feces to improve dyslipidemia. On the other hand, SREBP-1 induces lipogenesis in the liver and promotes fat storage in the form of triglycerides
[29][133], therefore down-regulating this transcription factor is beneficial in states of dyslipidemia. Additionally,
Akkermansia is increased in flavonoid treated mice, while unfavorable species associated with a HFD like
Desulfovibrio was decreased. As such,
Akkermansia can serve an important role in improving dyslipidemia not only through dietary interventions, but as a potential next-generation probiotic, discussed in a future subsection. Taken together, these findings provide strong evidence for MD as a lifestyle intervention that contributes to generalized and specific favorable changes in the gut microbiota composition to improve metabolic parameters.
3. Current Pharmacologic Treatments for Dyslipidemia and Relations to Gut Microbiota
Pharmacologic interventions of dyslipidemia may include inhibition of cholesterol synthesis, increasing use of cholesterol for bile acid production, or conversion of cholesterol in the gut to non-absorbable metabolites. Interestingly, the current pharmacological treatment modalities are associated with changes in gut microbiota, indicating the potential for synergistic effects of medications and targeted gut microbiota therapy for dyslipidemia.
Statin drugs are the first-line agents to reduce cholesterol synthesis by inhibition of HMG CoA Reductase. Use of statins has been demonstrated to improve the composition and function of gut microbiota
[30][134] and has been associated with lower occurrence of gut microbiota dysbiosis
[31][135]. For example, statin responsive patients showed increased concentrations of SCFA-producing genera including
Lactobacillus,
Eubacterium,
Faecalibacterium and
Bifidobacterium, all of which are characteristically decreased in dyslipidemia patients
[32][136]. On the other hand, statin resistant patients did not exhibit similar changes. Further, other studies support the changes that statins can exert on gut microbiota, particularly elevations in
Blautia and
Bifidobacterium longum, which has specifically been correlated with decreased triglycerides and overall body weight
[30][134]. As such, these combined findings suggest that response to statin treatment may, to some extent, be mediated or predicted by alterations in gut microbiota
Bile acid sequestering agents in the treatment of dyslipidemia are used to partially remove bile acids from enterohepatic cycling to increase use of cholesterol in the synthesis of new bile acids
[33][137]. As mentioned earlier, bile acids have been shown to have favorable effects on gut microbiota. Therefore, it is possible that bile sequestering agents such as Cholestyramine, can mediate beneficial effects on dyslipidemia through changes in gut microbiota. For example, HFD and cholecystectomy mice treated with cholestyramine exhibit improved lipid profiles, beneficial effects on PPARδ and SREBP1 concentrations while concurrently elevating concentration of favorable gut bacteria including
Blautia,
Alistipes and
Eubacterium [34][138]. It has also recently been shown that cholestyramine increased SCFA and enriched concentrations of SCFA-producing
Lachnospiraceae spp. in treatment responsive groups
[35][139]. More generally, cholestyramine increased the Bacteroidetes/Firmicutes ratio, indicating a shift towards a more favorable gut composition profile with overall reductions of inflammatory markers. These anti-inflammatory effects are supported by results showing that cholestyramine can reduce inflammatory signaling in HFD-induced mice
[36][140]. Therefore, bile acid sequestrants are able to influence gut microbiota via bile acid-microbiota crosstalk to improve dyslipidemia, though more studies are needed to elucidate the specific mechanisms by which they do so.
Although not first-line for dyslipidemia treatment, metformin can also improve dyslipidemia in patients with T2DM through mechanisms that increase insulin sensitivity to reducing LDL and TG
[37][141]. Recent findings have shown that metformin exerts favorable changes in gut microbiota, most notably through increases in
Blautia and
Faecalibacterium, which were associated with lipid homeostasis and improvements in serum triglyceride levels
[38][142]. Further, metformin has been shown to increase concentrations of
Akkermansia while improving total cholesterol levels in a rodent model of metabolic syndrome
[39][143]. Therefore, these changes in gut microbiota can provide more insight into mechanisms by which pharmacological therapy can augment microbiota mediated pathways to exert their effects.