Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Motasem Alazaiza and Version 2 by Camila Xu.
Biochar is a carbonaceous substance that is created from algal biomass by thermal breakdown in an oxygen-absence environment.
- microalgae
- value-added products
- biochar
- biopolymer
1. Introduction
On prehistoric Earth, microalgae were the first photosynthetic organisms. By reducing significant amounts of carbon dioxide through photosynthesis, unicellular microorganisms help to lower greenhouse gases in the atmosphere. Consequently, algae are considered a feasible carbon capture technique [1]. Microalgae can be divided into prokaryotic cyanobacteria, which come in blue and green colors, and eukaryotic microalgae, such as the brown Phaeophyta, green Chlorophyta, and gold Chrysophyceae [2]. Additionally, they are divided into groups based on various metabolic processes (e.g., heterotrophic, photoautotrophic, photoheterotrophic, and mixotrophic) [3]. Algae’s unique metabolisms contribute to a highly diverse variety of biological settings, including habitats with severe temperatures and pH levels, freshwater or saltwater, and effluents with a high concentration of organic and inorganic substances [4]. Microalgal biomass is produced at a rapid rate because of its brief life cycle. Many studies investigated the potential uses of algae for bioremediation and the creation of added-value products such as, e.g., bio-oil, biochar, syngas, and biopolymers [5].
Biochar is a carbonaceous substance that is created from algal biomass by thermal breakdown in an oxygen-absence environment. The biomass content (such as cellulose, lignin, protein, etc.) and its thermochemical process (e.g., hydrothermal liquefaction, pyrolysis, torrefaction, gasification, direct combustion) have a significant impact on the structure and physicochemical features of biochar [6][7][6,7]. By modifying heating conditions (final temperature and heating rate) and biomass precursors, biochar with high physicochemical characteristics, porous structure, and structural stability, as well as having enough surface functional groups and ash, may be particularly developed [8]. Biochar has recently gained popularity due to its ease of production, low cost, and high sustainability [9]. As algal biochar has high adsorption capacity, the use of conventional coal-based carbons in the wastewater treatment filed may be reduced or eliminated. Biochar is comparatively stable, renewable, cost-effective, and ecologically sustainable, due to lower manufacturing costs than non-renewable activated carbons [10][11][12][13][10,11,12,13]. Various studies have examined the use of algal biochar as a low-cost, environmentally friendly biochar technology in wastewater remediation and other useful applications [14][15][14,15].
2. Biochar
2.1. Formation and Characterization of Microalgal-Based Biochar
Many processes for producing biochar from microalgae using thermochemical reactions have been developed. Hydrothermal carbonization (HTC), pyrolysis, and torrefaction are the main studied methods. However, post-treatment have recently received a great deal of attention. Biochemical methods such as anaerobic digestion and fermentation are also discussed in the literature. However, thermochemical methods are preferable due to their high efficiency, quality, and yield [16][17][18][24,25,26]. Biochemical methods are efficient when high moisture content microalgae, above 50%, are converted to biochar [19][27].2.1.1. Torrefaction
Torrefaction is used to remove volatiles before pyrolysis; in other words, it is used as a pre-treatment step prior to the pyrolysis process. Torrefaction can be used in both wet and dry environments. Wet torrefaction is conducted at temperatures between 180 and 260 °C under higher pressures (200–700 psi) and a residence time of 5 min, while dry torrefaction conditions are: temperature (200–300 °C), pressure (atmospheric pressure), and a reaction time of 80 min. The type of torrefaction has a significant impact on the quality of the biochar produced. Wet torrefactions produce high yields with low ash content and great hydrophobicity, which significantly increase the adsorption capacity of produced biochar [20][28]. Moreover, moisture content increases the heat transfer rate, which decreases the cost of heating. Furthermore, hydrolysis attack, which leads to biomass decomposition, is easily triggered in a wet environment [21][29]. Recently, microwave-assessed wet torrefaction has gained more attention as microwave heating has advantages such as uniform heat distribution, instantaneous start/stop, and rapid heating rate [22][23][30,31]. Gan et al. [21][29] investigated the performance of microwave-assessed wet torrefaction in water and acid media for biochar production from microalgae (Chlorella sp.). The findings revealed that acid media combined with microwave-assisted torrefaction produced a high solid yield. The use of sulfuric acid could produce solids suitable for bioethanol production, while using organic acid could produce biochar applicable for solid fuel. High-yield biochar could be produced by a torrefaction system with a short time, low temperature, and low heating rate [24][32]. In addition, hydrophobic biochar could be produced as a torrefaction process that could destroy the hydroxyl groups of microalgal biomass by heating [25][33]. In contrast, some studies reported that high temperature and reaction time could decrease the H and O content in biochar [26][34].2.1.2. Pyrolysis
Pyrolysis is the most common thermochemical process for converting algae biomass into value-added products such as biochar and bio-oil. The old version of pyrolysis is known as slow pyrolysis, while the other advanced pyrolysis processes are fast pyrolysis, catalytic pyrolysis, microwave-assisted pyrolysis, and hydro-pyrolysis [27][28][35,36]. Before algae undergo the pyrolysis process, pretreatment using acid is needed to remove inorganic chemicals such as Ca, Na, Mg, and K from the biomass. Differently, these chemicals could increase the ash content of biochar, which would decrease the biochar yield during pyrolysis [29][37]. Slow pyrolysis is the old version for high-yield biochar production thermochemically from algae biomass [17][25]. The slow pyrolysis process conducts at temperatures of 550–950 °C with more than a 5 min reaction time and a heating rate less than 60 °C/min [30][38]. One of the main advantages of slow pyrolysis is the ability to convert a wide range of microalgae sizes (5–50 mm) to biochar [31][39]. Optimization of pyrolysis operating parameters such as temperature, pyrolysis time, sweeping gas flow rate, and heating rate could enhance the quantity and quality of produced biochar [32][40]. For example, a long residence time of 450–550 days coupled with a low heating rate of 0.1–1 K/S boosts the production of biochar. This is due to restrained vapors, which undergo more reactions with solid material to produce more biochar. The microalgae strain species also significantly affects the biochar yield produced by slow pyrolysis; for example, Chaetoceros muelleri, Dunaliella tertiolecta, and Synechococcus produce high yields reaching up to 60%, whereas microalgae species such as Tetraselmis chui, Spirulinaplatensis, Spirulina sp., and Chlorella vulgaris produce moderate biochar yields [33][34][41,42]. Contrarily, fast pyrolysis produces a lower biochar yield (24–54% as a higher heating rate and shorter reaction time 1.5–3.3 s) are applied [35][43]. Catalyst pyrolysis is a new technology that aims to improve the quality and yield of biochar produced by the traditional pyrolysis process. Catalyst pyrolysis includes adding catalysts to promote the production of value-added products. Catalysts such as bases, acids, metals or even mixtures of these could be used as catalysts. Primary catalytic pyrolysis and secondary catalytic pyrolysis are the two main types of catalytic pyrolysis. Primary catalysis is applied in situ when the catalyst is blended with algae biomass, while secondary catalysis is an ex situ method where catalysts are fixed at the bed of the reactor and biomass is separated inside the reactor. With the help of heat from sand, catalyst activation occurs before the thermal degradation of biomass takes place. Microalgae species are an important factor influencing the type of catalyst pyrolysis; for example, Nanochloropsis and Spirulina produce high yields when the ex situ method is used, while Pavlova biomass is more suitable for the in situ process [36][37][44,45]. The ex situ method separates catalysts and biomass, which means more control of catalytic activities and pyrolysis. Reusability of catalysts is another advantage of ex situ catalyst pyrolysis, as fewer minerals (Ca, Na, Mg, K, etc.) are deposited, which enhances the process in terms of cost. In contrast, the in situ catalyst process forms a layer of cake on the catalyst, varying its activity; thus, liquid products rather than solid products are promoted to generate. In a study, Aysu et al. [36][44] reported high biochar yields (35–48%) of pavlova biomass using in situ catalyst pyrolysis, while Jia et al. [37][45] examined the ex situ catalyst pyrolysis for Spirulina and Nanochloropsis biomass transfer to biochar and found that the solid yield was around 20%. Compared to conventional pyrolysis, catalyst pyrolysis has many advantages, such as low pyrolytic temperature, low energy consumption, and impurity removal ability.2.1.3. Hydrothermal Carbonization
One more advanced thermochemical method for producing biochar from microalgal biomass is hydrothermal carbonization (HTC). The main benefit of such a method is the ability to directly convert moist biomass to biochar or hydrochar [17][25]. Hydrothermal carbonization includes heating carbon-rich content, for example microalgal biomass, in a moist environment at a temperature of 175–250 °C for a reaction time of 30–120 min under an applied pressure of 20–60 kPa [38][46]. Comparing this to other thermochemical techniques, there is no need to dry the biomass for the HTC application, which decreases the cost of heating. Moreover, the combination of water and heat inside the reactor functions as a milder environment of pressure and temperature [39][40][47,48]. These coins make HTC a cost-effective choice for value-added product production from microalgal biomass, especially hydrochar production, which differentiates HTC from pyrolysis [17][25]. The main parameters that control production yield through HTC are retention time and temperature. Khoo et al. [41][49] reported that the yield production of hydrocar decreased from 41.8% to 26.3% when the temperature and retention time of the HTC process were changed from 180 °C and 30 min to 250 °C and 4 h, respectively. The same result was reported by Yao et al. [42][50], who investigated the hydrochar production yield using the HTC process. The results showed that 190 °C produced the highest yield (36.7%), while 210 °C produced the lowest yield (27.2%). These two studies showed that increasing pressure and temperature reduced the yield of hydrochar. Recently, the same results were reported by Castro et al. [39][47], who found that a retention time of 10 min and temperature of 170 °C resulted in a high solid yield of 77.72% from microalgal biomass. Low carbonization temperature yields high-yield hydrochar with high-quality properties such as controlled porosity, electronic properties, regulated surface chemistry, and functional surfaces (e.g., -C=O, -COOH, -OH) [7]. In addition, Castro et al. [39][47] found that during the HTC process, the level of hydrogen and carbon was increased while the level of nitrogen and oxygen was decreased, and therefore a lower oxygen-to-carbon ration was found during the HTC process, resulting in solids with high hydrophobicity properties.2.1.4. Post-Treatment/Activation
The biochar produced from microalgal biomass needs post-treatment, either physical or chemical, before it can be used [43][51]. This treatment is crucial to activate biochar before its application. The post-treatment process improves the physiochemical properties of the produced biochar, such as pore area or volume, specific surface area, surface chemistry, and functional agents. Magnetization and ball milling are two physical modification processes. Magnetization includes allocating magnetic iron oxides such as Fe2O4, Fe2O3 or Fe3O4 on biochar. Magnetizations make biochar recovery from solutions easier and enhance the cation exchange capacity of biochar [44][45][52,53]. Moreover, ball milling breaks chemical bonding in biochar by using kinetic energy, leading to an enhancement in the shape and size of biochar at the nanoscale. Regarding chemical modification, the oxidation process, and acid and alkali post-treatment, the chemical modification changes the biochar’s surface chemistry. Examples of chemical agents used for biochar chemical modification are H2O2, KMnO4, KOH, NaOH, HNO3, and HCl.2.2. Characteristics of Microalgal-Based Biochar
The performance of biochar produced from microalgal biomass is characterized by surface charge and special pH, specific surface area, mineral components, and surface functional groups [46][54]. In this section, the relation between microalgae-based biochar characteristics and adsorption capacity is discussed.2.2.1. Surface Charge and pH
In general, the pH of biochar derived from microalgal biomass is alkaline (pH above 7). Examples of microalgae strains that produce alkaline biochar are marine Chlorella sp., Lacustrine algae, Spirulina sp., and Scenedesmus dimorphus. Nevertheless, acidic biochar is produced from Chlorella sp. [47][55]. The surface charge of biochar is highly influenced by solution pH. To be more specific, the pH at zero charge (pHpzc) refers to the solution pH at which the surface net charge is zero. The importance of such parameters is attributed to their effects on the electrostatic attraction between charged contaminants such as high-energy minerals and biochar [48][56]. When pH is lower than pHpzc, biochar is positively charged and binds to negatively charged metals. On the other hand, when the solution pH is higher than pHpzc, biochar is negatively charged and binds with positively charged metals [49][57].2.2.2. Physical Properties
The physical properties include pore area, surface area, bulk density, microspores, pore volume, etc. The specific surface area of biochar produced from microalgal biomass is considered low (>3 m2/g), especially the biochar derived via pyrolysis and hydrothermal liquefaction from Spirulina [50][58]. However, the surface area could be increased as the pyrolytic temperature is increased. Ge et al. [51][59] reported high surface area biochar (15 m2/g) derived from Spirulina when the pyrolytic temperature was increased from 300 to 700 °C. Remarkably, Amin and Chetpattananondh [52][60] reported extra-high surface area biochar (266 m2/g) produced from Chlorella via sonication. High surface area and pore volumes increase biochar affinity and uptake of pollutants [53][54][61,62].2.2.3. Chemical Properties
The chemical properties include ash content, moisture content, fixed carbon, and volatile matter. According to Yu et al. [7], biochar produced from Gracilaria sp. have more moisture content than biochar derived from Chlorella vulgaris. Microalgae-based biochar has a low moisture content (10 wt.%), while volatile matter contents and ash depend on the microalgae species. In addition, microalgae-derived biochar has a high fixed carbon content ranging from 40 to 70%. As O/C and H/C fractions significantly affect the aromaticity degree and the stability of biochar, the ultimate analysis to estimate chemical elements such as (hydrogen, carbon, sulfur, nitrogen, and oxygen) is crucial [55][56][63,64]. Biochar’s high hydrophilicity could be figured out by its high O/C ratio, meaning that such biochar is useful for heavy meatal removal [52][60]. Higher O/C ratios contribute to high oxygen functional groups that enhance the adsorption of heavy metals. On the other hand, low H/C or O/C ratios revealed high hydrophobicity and aromaticity, which led to the favorable removal of non-polar organic compounds [48][56].2.2.4. Mineral Elements
Microalgae-derived biochar could contain mineral elements such as K, P, Na, Ca, etc.; these elements promote the formation of the oxygen functional group. Moreover, alkali metals could increase the pH of biochar [57][65]. The mineral elements may perform as natural pore-forming agents, which generate a hierarchically porous structure on the biochar. Furthermore, contaminants could be adsorbed to mineral elements [58][66].2.2.5. Surface Functional Groups
Adsorptive mechanisms, hydrophobicity, and hydrophilicity are important properties that could be determined by the surface functional groups of biochar. These surface functional groups could interact with metallic ions and interact with heavy metals through mechanisms such as complexation, surface precipitation, and electrostatic attraction [59][67]. Nevertheless, important acidic functional groups such as CH and OH could be destroyed by high pyrolytic temperatures. However, high pyrolytic temperatures could produce basic functional groups, pH, carbon stability, ash content, and gaseous yield [60][68]. Therefore, low pyrolytic temperatures could produce biochar with an acidic functional group, which improved heavy metal removal, while high pyrolytic temperatures resulted in biochar with high hydrophobicity, which enhanced organic pollutants’ removal [56][59][64,67]. Furthermore, high hydrophobicity leads to humidity resistance [38][46].2.3. Application of Microalgal-Based Biochar
2.3.1. Inorganic Contaminants Removal
Toxic heavy metals could be absorbed and accumulated in organisms, thus presenting a severe threat to both human health and natural water [38][61][46,69]. Biochar derived from microalgae could eliminate and adsorb heavy metals such as Zn (II), Cr (VI), Co (II), Ni (II), and Hg (II) from aqueous solutions. Many studies in the literature have reported the performance of biochar derived from microalgae for heavy metal removal. In a study, Daneshvar et al. [62][70] produced biochar from Scenedesmus quadricauda using the pyrolysis process at a temperature of 500 °C. The authors evaluated the performance of derived biochar for Cr (VI) removal. The results showed complete removal of Cr (VI) when the initial concentration was in the range of 1–10 mg/L. In addition, the authors reported that the mechanisms of removal of Cr (VI) were electrostatic interaction, ionic exchange, and Cr (VI) reduction. Previously, Bordoloi et al. [63][71] performed many equilibrium studies such as Langmuir and Freundlich adsorption isotherms to examine the application of biochar derived from Scenedesmus microalgae for Co (II) ion removal. The results revealed that the adsorption capacity of the produced biochar was 0.672 mg/g. Ge et al. [51][59] reported that biochar derived from high-salinity Spirulina microalgae was able to remove Hg (II) ions by immobilization and reach a sorption capacity of 6–12.7 mg/g for long-term uptake. Microcystis sp. biochar is capable of successfully removing chelated nickel from alloy electroplating wastewater [45][53]. In another study, biochar derived from marine algae such as Sargassum fusiforme and Saccharina japonica was used to remove Zn, Cd, and Cu from aqueous solutions. The results reported that a high number of oxygen functional groups was the main mechanism for heavy-metal removal. In addition, cation exchange was another removal mechanism by biochar as marine algae have a high number of minerals such as Na, K, Mg, Ca, etc. [64][72].2.3.2. Organic Contaminants Removal
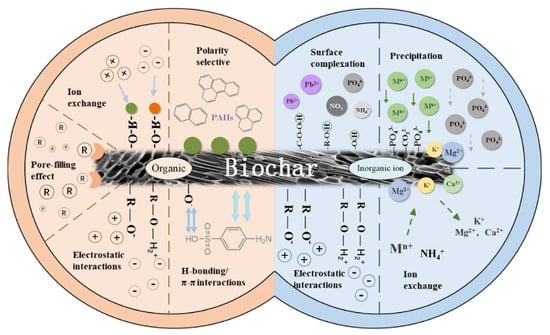
Organic contaminants such as personal care products, antibiotics, pharmaceuticals, plasticizers, and flame retardants are environmentally hazardous, societally ubiquitous, and structurally diverse chemicals [10]. Conventional wastewater treatment cannot remove such undegradable compounds; thus, more effective remediation methods such as adsorption have been developed. Microalgae-based biochar is a promising adsorbent that efficiently removes organic pollutants [65][73]. The removal mechanism of organic pollutants by biochar is due to one or a combination of the following mechanisms: electrostatic attractions, polar-selective interactions, pore filling, hydrophobic interactions, and p-p interactions. Ho et al. [66][74] investigated the removal of sulfamethoxazole (antibiotics) by N-doped graphitic biochar produced from Spirulina. The results showed that the derived biochar could absorb sulfamethoxazole via electron transfer. In another study, Zheng et al. [67][75] reported p-nitrophenol removal using biochar derived from chlorella. Nautiyal et al. [68][76] examined acidic Congo red dye removal using biochar produced from Spirulina. The removal efficiency of the investigated dye was 82% at pH of 2, initial dye concentration of 90 mg/L, and 0.2 g/100 mL biochar dosage. Figure 1 shows the biochar adsorption mechanism for both organic and inorganic pollutants.
2.3.3. Carbon Dioxide Removal
Human and industrial activities release a huge amount of CO2 every day, causing disastrous environmental problems. Among CO2 mitigation measures, adsorption is considered a promising technology to decrease the CO2 concentration through chemical and physical processes. Microalgae-based biochar has a high specific surface area (SSA), active surface functional groups, and a highly porous structure, which make this type of biochar an ideal adsorbent for CO2 [70][71][78,79]. High N content and large SSA are crucial for CO2 adsorption. Creamer et al. [72][80] produced biochar from sugarcane bagasse at 600 °C. The author reported high CO2 adsorption efficiency due to the presence of N-functional groups and high SSA. In addition. The author suggested that the enhancement in CO2 adsorption was due to Lewis’s acid–base interaction, which is increased in the presence of an N-functional group [73][81]. Another factor that affects CO2 adsorption is the size of the pores, as it controls the rate of gas transport through the pore system [74][82]. Plaza et al. [75][83] reported that microalgae-based biochar pyrolyzed at 600 °C under N2 flow had a high CO2 capacity due to its micro-porous structure. For high CO2 absorptivity, the optimal biochar pore diameter was between 0.5 nm and 0.8 nm, as reported by Creamer and Gao [76][84].2.3.4. Other Applications
Using microalgae-based biochar to produce coal fuel is a promising method to produce green energy. The torrefaction process is used to convert biochar into coal fuel. Higher heating value (HHV), O/C, and H/C ratios all have an impact on coal-based biochar. Congyu Zhang et al. [77][85] examined the production of coal fuel-based biochar using the torrefaction process in the absence and presence of O2. The results revealed that oxidative torrefaction (the presence of O2) could produce biochar with a large SSA, high HHV, and high hydrophobicity that could be used for industrial applications as coal fuel. Non-oxidative torrefaction, on the other hand, produced biochar with good storage and transportation characteristics. Supercapacitors are emerging energy storage devices with many advantages such as high-power density, fast charge–discharge, low cost, low environmental impact, and a long life cycle. The use of biochar produced from algae as a supercapacitor has been investigated in many studies. To enhance the supercapacitors’ specific capacity, characteristics such as the hierarchical porosity and SSA of the produced biochar should be improved [78][86]. Wang et al. [45][53] produced KOH-activated biochar derived from Enteromorpha prolifera with a high capacitance of 440 F/g at A/g and an SSA of 3345 m2/g. Subsequently, Wang et al. [79][87] confirmed that carbonaceous materials derived from Nostoc flagelliforme were characterized by lower internal resistance, a more porous structure, and high specific capacitance (283 F/g). Table 1 presents the process parameters, microorganisms, and synthesis techniques used for biochar production.Table 1.
Application of biochar derived from microalgae.
| Microalgae Strain | Biochar Yield | Biochar Production Method | Synthesis Conditions | Biochar Characterization | Biochar Application | Reference |
|---|---|---|---|---|---|---|
| Gongolaria barbata | 40 wt.%. | Microwave assisted method |
|
|
Aniline removal. | [80][88] |
| Three types of marine biomass waste (kelp, undaria pinnatifida, and Enteromorpha prolifer) | - | Pyrolysis |
|
|
Electrochemical energy storage | [81][89] |
| Agardhiella subulata | 67.6 wt.%. | Pyrolysis |
|
|
4-Nonylphenol (4-NP), a phenolic endocrine disruptor chemical (EDC) removal | [82][90] |
| Chlorella sp. | 57% | Pyrolysis |
|
|
Heavy metal removal (Cr (VI), Zn (II), Ni (II)) | [52][60] |
| Sargassum sp. | 70% | Pyrolysis |
|
|
Agronomy as a soil ameliorant | [83][91] |
