The Coronavirus disease 2019 (COVID-19) pandemic has negatively affected the treatment of malignant diseases and the development of new prodrugs worldwide. Patients with cancer are more susceptible to a higher risk of coronavirus infection and its severe complications than the general population. The lungs are the most strongly affected organs in SARS-CoV-2 infection. Additionally, within the lungs, as in other human organ tissues, angiotensin-converting enzyme 2 (ACE2) was proven to be the main host cell receptor for the binding of SARS-CoV-2. ACE2 expression is also elevated in tumor and tumor-adjacent normal tissues in patients with lung cancer, which might partially explain why patients with lung cancer are potentially at a higher risk of severe COVID-19.
1. COVID-19 and Lung Cancer
Studies show that patients with a variety of comorbidities (e.g., hypertension, diabetes, and obesity) are at a higher risk of hospitalization due to COVID-19 compared to those without such conditions
[1][7]. Cancer patients are also considered a particularly vulnerable population during this global pandemic due to their systemic immunosuppressive state caused by malignancy and anticancer treatments. The earliest studies indicated that patients who have other underlying chronic conditions were at a higher risk than cancer patients of becoming critically ill if infected with severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2)
[2][8]. However, the early data also showed that patients with cancer might have a higher risk of COVID-19 than individuals without cancer, as well as a higher risk of severe events (intensive care unit admission, invasive ventilation, or death) compared to patients without cancer
[3][4][5][9,10,11].
Diagnosing COVID-19 in patients with cancer is another challenge due to multiple factors
[6][14]. One of these factors is that cancer patients might have atypical radiographic features
[7][15] or radiographic findings similar to those of a SARS-CoV-2 infection, which can be misleading
[8][16]. Moreover, it is important to note that due to similar symptoms between the infection and the underlying disease, the diagnosis of COVID-19 may be delayed, particularly in lung cancer patients and patients with pulmonary metastasis. Additionally, some clinical and biological characteristics can mask COVID-19 presentation in cancer patients
[9][17]. On the other hand, COVID-19 may delay the initial diagnosis and treatment of lung cancer. The immune activation required for antitumor immune responses sometimes triggers autoimmunity and immunorelated adverse events (irAE)
[10][11][12][18,19,20]. Pneumonitis is a rare but serious irAE complication in patients receiving immune checkpoint inhibitors (ICIs). The radiological discovery of SARS-CoV-2 infection in lung cancer may overlap and be confused with lung cancer progression or immune-related pneumonitis as a complication of anticancer therapies
[13][21]. Thus, interpreting lung cancer’s initial diagnosis or clinical status may, in some situations, be challenging and harbor the possibility of misdiagnosis
[14][15][16][17][18][19][22,23,24,25,26,27].
The lungs are the most strongly affected organs in SARS-CoV-2 infection. Additionally, within the lungs, as in other human organ tissues, angiotensin-converting enzyme 2 (ACE2) was proven to be the main host cell receptor for the binding of SARS-CoV-2
[20][36]. ACE2 expression is also elevated in tumor and tumor-adjacent normal tissues in patients with lung cancer
[21][22][37,38], which might partially explain why patients with lung cancer are potentially at a higher risk of severe COVID-19. ACE2 expression patterns and levels are closely associated with a susceptibility to and symptoms of COVID-19
[23][39]. Moreover, tumor cells are more susceptible to viral replication due to defects in innate antiviral immunity associated with transformation
[24][40]. However, there is still a need to clarify the factors affecting SARS-CoV-2 replication in lung cancer cells, as well as the possible impact of COVID-19-induced inflammation on lung cancer development and its pathophysiology (
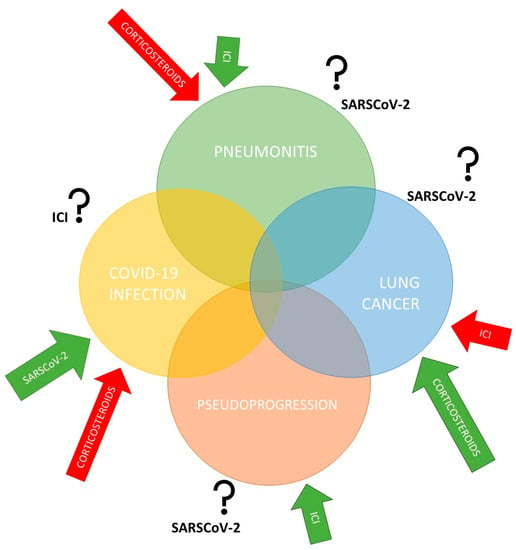
Figure 1). The positive impact of immunotherapy on enhancing the antitumor response was clearly demonstrated, as was the negative impact of corticosteroids in the same process. For this reason, the concomitant application of corticosteroids with ICIs is avoided
[10][18]. The influence of corticosteroids in the treatment of the COVID-19 infection and serious side effects such as pneumonitis were also shown to have significant effects and is an integral part of the COVID-19 treatment protocol
[13][16][17][21,24,25]. The simultaneous mutual effects between all these factors, however, have not been sufficiently investigated or have provided conflicting results, especially with anti-COVID-19 vaccination. Cancer cells require high energy to survive and can recapture mitochondria from the microenvironment, including from immune cells. The abundance of mitochondria potentially represents a good environment for high replication of SARS-CoV-2
[25][26][41,42].
Figure 1. The interplay between COVID-19 infection, lung cancer, pneumonitis, and pseudoprogression. Lung cancer is characterized by an increased risk of pulmonary complications, as is COVID-19 infection, due to pathophysiological, clinical, and treatment-related risk factors. In addition, there remains concern regarding overlapping pneumonitis from immune checkpoint inhibitors (ICIs) and COVID-19-induced pneumonia. Therefore, the influence of specific treatments and SARS-CoV-2 virus on each segment still needs to be clarified.
2. COVID-19 and Anticancer Treatment with Immune Checkpoint Inhibitors
Over the last decade, immune-oncology (IO), specifically ICIs, has become one of the most promising areas of cancer research, as well as one of the fastest-growing areas of drug development, with the approval of more than 20 agents globally
[27][43]. Data strongly support the concept that the immune system can identify, locate, and control tumor cells in a process called cancer immunosurveillance. In addition, the immune system can cooperate with the tumor microenvironment and promote tumor progression through chronic inflammation, the immunoselection of poorly immunogenic variants, and immunosuppression
[28][29][44,45].
Since ICIs can change immune competence, it is important to clarify whether the immune response to SARS-CoV-2 is influenced by patients receiving ICIs or other immunomodulating treatments, including steroid therapy
[30][46]. To date, most studies have not found an association between cancer therapy and increased mortality among patients with cancer and COVID-19
[31][47].
There is a concern about the possible negative interference of ICIs in the severity of COVID-19. It is known one of the most important mechanisms underlying the progression of COVID-19 is cytokine storm. It is very important to recognize cytokine storm because it has negative prognostic and therapeutic implications and can lead to acute respiratory distress syndrome, multiple organ failure, and deterioration of cancer treatment outcomes
[32][49]. Cytokine storm and cytokine release syndrome (CRS) are life-threatening systemic inflammatory syndromes involving elevated levels of circulating cytokines and immune-cell hyperactivation that can be triggered or worsened by various therapies, including ICIs, chemotherapy, other pathogens, autoimmune conditions, and comorbidity. Cytometric analyses of COVID-19 patients showed reduced counts and a hyperactivated status of peripheral CD4 and CD8 T cells. Furthermore, studies found an increased concentration of proinflammatory T cells. Additionally, CD8 T cells were found to harbor high concentrations of cytotoxic granules, suggesting that the hyperactivation of T cells worsens autoimmune injury
[33][50]. Considering this immunological interplay, the hypothesis of a correlation between ICIs mechanisms, tumor immunology, and irAE alongside COVID-19 pathogenesis should be considered. Notably, ICIs-induced CRS is rare, and a cytokine storm is a late event in the COVID-19 pathogenesis. Moreover, patients still receive ICIs during the early stages of COVID-19 infection
[30][46].
3. COVID-19 Vaccines in Patients with Cancer
Studies on more than 1000 cancer patients have investigated the COVID-19 vaccine’s safety and effectiveness, including the early safety profile of the BNT162b2 vaccine in 134 patients with cancer (including lung cancer) under immune checkpoint blockage [34][35][36][37][38][39][40][51,52,53,54,55,56,57]. The most common side effects were mild and similar to those of other common vaccines [41][58]. Initial data suggest that SARS-CoV-2 vaccines are effective in patients with cancer. However, most studies only assessed seroconversion in patients with cancer, and only some studies performed neutralization assays to measure neutralizing antibody responses against variants of concern [42][43][59,60].
Moreover, patients with solid and hematologic cancer were not included in pivotal clinical trials conducted to demonstrate the efficacy and safety of COVID-19 vaccines. Patients with cancer appear to be more likely to develop a less efficient immune response following vaccination against COVID-19. Prior to the predominance of the Omicron variant, this ability was assessed to be lower than that in immunocompetent individuals [39][44][45][46][47][48][49][50][56,68,70,71,72,73,74,75].
Risk factors that can impair seroconversion in patients with solid tumors include older age, male sex, vaccine type, and chronic corticosteroid use. The influence of chemotherapy and immunotherapy on the efficacy of COVID-19 vaccines is of special interest. Recent chemotherapy has been repeatedly identified as a strong risk factor for lower seroconversion and neutralizing responses [42][51][52][59,76,77]. Oosting et al. recently reported the impact of immunotherapy, chemotherapy, and chemoimmunotherapy on the immunogenicity and safety of the COVID-19 vaccination in patients treated for solid tumors.
Data from studies involving patients with cancer so far indicate that the adverse events did not differ based on registration with the various vaccine platforms. The most common adverse events reported were soreness or pain at or around the injection site (63% of vaccines), local swelling (9%), muscle pain (34%), fatigue (34%), headache (16%), fever (10%), chills (10%), and gastrointestinal events (10%). Of course, there is still no knowledge of the long-term adverse effects of COVID-19 vaccines in individuals with and without cancer due to the very short observation time [40][43][57,60]. Patients receiving immunocheckpoint inhibitors were at possible additional risk of developing irAEs after the COVID-19 vaccination, but this was not confirmed [40][57]. Radiation phenomena, such as pneumonitis or dermatitis, have also been described following the COVID-19 vaccination and can sometimes significantly complicate discernment; awareness of this complication is important to avoid mistaking such phenomena as adverse effects of cancer therapy [53][54][89,90].